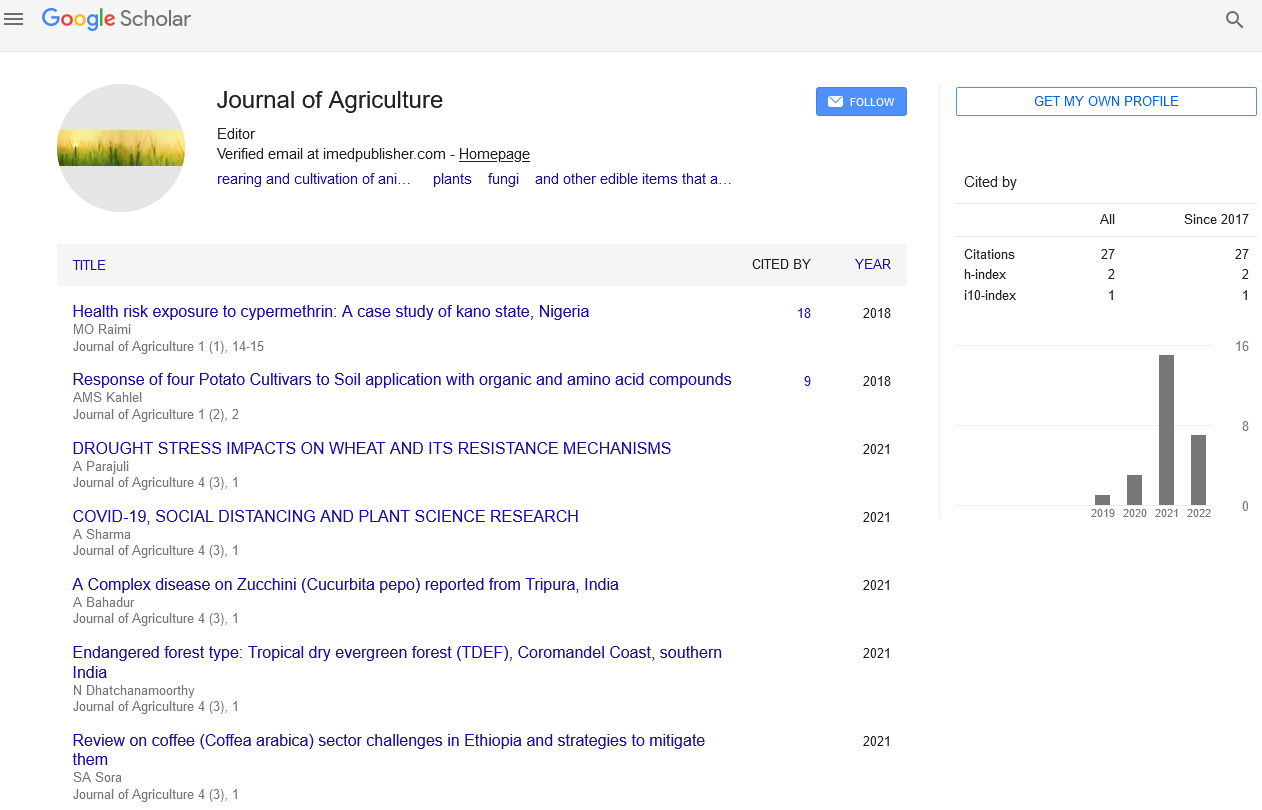
Editorial - Journal of Agriculture (2023) Volume 6, Issue 4
Unlocking the 'Effector Toolkit' of Plant-Parasitic Nematodes: A Path to Alter Physiology and Development
Zobaer Ahmed*
College of Forestry, Nanjing Forestry University, Jiangsu, China
College of Forestry, Nanjing Forestry University, Jiangsu, China
E-mail: Zobae76@gmail.com
Received: 01-Aug-2023, Manuscript No. jagri-23-108630; Editor assigned: 03-Aug-2023, Pre-QC No. jagri-23-108630 (PQ); Reviewed: 17- Aug-2023, QC No. jagri-23-108630; Revised: 22-Aug-2023, Manuscript No. jagri-23-108630 (R); Published: 29-Aug-2023; DOI: 10.37532/ jagri.2023.6(4).109-111
Abstract
Plant parasites take advantage of host developmental plasticity to elicit profound developmental and physiological changes. In the case of plant-parasitic nematodes (PPNs), these changes can result in the development of new plant organs. Despite the importance of the development- and physiology-altering abilities of these parasites in pathology, research has historically focused on their abilities to suppress immunity. We argue that, given the dramatic changes involved in feeding site establishment, it is entirely possible that development- and physiology-altering abilities of PPNs may, in fact, dominate effector repertoires – highlighting the need for novel high-throughput screens for development- and physiology-altering ‘tools’. Uncovering this portion of the nematode ‘toolbox’ can enable biotechnology, enhance crop protection, and shed light on fundamental host biology itself.
Introduction
Plant-parasitic nematodes are microscopic worms that cause substantial damage to various crops, resulting in significant economic losses worldwide. These tiny pests possess a specialized arsenal of proteins known as “effectors,” which play a crucial role in their ability to manipulate host plants, suppress immune responses, and secure a favourable environment for their own development. Understanding and unlocking the secrets of this effector toolkit holds tremendous potential for developing innovative strategies to control nematode infestations and improve agricultural productivity [1-3].
Deciphering the effector toolkit
The effector toolkit of plant-parasitic nematodes comprises an array of proteins that are secreted into the host plant during infection. These effectors function as molecular keys, enabling the nematodes to manipulate host cellular processes to their advantage. By gaining access to the host’s resources and suppressing its defenses, nematodes ensure their survival and successful reproduction.
Effector function and host interactions
Plant-parasitic nematodes use their effectors to interact with various components of the host’s immune system and physiological pathways. Some effectors interfere with the plant’s signaling networks, while others target specific plant cells to create a suitable feeding site for the nematode. By modulating gene expression, altering hormone levels, and reprogramming cellular responses, these effectors effectively subvert the plant’s defenses and redirect its resources for the nematode’s benefit.
Unravelling effector diversity
One of the most challenging aspects of studying the effector toolkit lies in its remarkable diversity. Different species of plant-parasitic nematodes deploy distinct effectors, and even individual populations of the same species may carry unique repertoires of effectors. Unraveling this diversity is a complex task that requires cutting-edge genomic and bioinformatics approaches.
CRISPR-Cas9 technology and functional analysis
Advancements in genome-editing technologies, such as CRISPR-Cas9, have revolutionized the study of plant-parasitic nematodes. Researchers can now target specific nematode genes involved in effector production, delivery, or function. By analyzing nematodes with altered effector profiles, scientists can gain insights into the precise roles of individual effectors during the infection process.
Implications for nematode control
Understanding the effector toolkit provides a strategic advantage for developing effective nematode control measures. By identifying key effectors responsible for virulence and pathogenicity, researchers can explore novel approaches to disrupt their function. This might include designing genetically modified crops with enhanced resistance against nematode effectors or developing targeted treatments that inhibit effector activity.
Biotechnological applications
Apart from agricultural benefits, the knowledge of nematode effectors may have broader biotechnological applications. Some effector proteins show promise as carriers for delivering beneficial compounds, such as enzymes or antimicrobial peptides, directly into plant cells. These carriers could aid in enhancing plant defenses against various pathogens, not just nematodes.
Nematode infection is a connection that alters bilateral development
The interaction between nematode and plant is remarkable in that, during infection, a cross kingdom dialogue between the parasite and the host determines the developmental outcomes of both parties. Upon perception of hostspecific diffusates in the soil, nematodes hatch as sexless second-stage juveniles which migrate towards the growing root [4-8]. To penetrate host cells, infective juveniles secrete effectors which allow them to degrade the plant cell wall and move to the vascular system [9]. Here, the nematodes initiate their association with the plant by delivering a mixture of effector proteins and metabolites into host cells using a needlelike structure called a stylet. Crucially, these effectors are secreted from two sets of specialised pharyngeal gland cells: the sub ventral glands and the dorsal gland. It is thought that the sub ventral gland is primarily active during the early life-stages, whereas the dorsal gland is primary active during the sedentary phase
Upon interaction with the cell, nematodes evoke a major reprogramming of host biology to establish the feeding site. The specific morphological and subcellular changes elicited by the nematode depend on species. Cyst nematodes (Heterodera spp. and Globodera spp.) induce the formation of a feeding structure called a syncytium. They do so by injecting effectors into a single initial syncytial cell concurrent with numerous changes in subcellular architecture. The initial cell is arrested in G2 stage of the cell cycle, the nucleus enlarges, the vacuole reduces in size, and there is extensive proliferation of subcellular organelles including the smooth endoplasmic reticulum (ER), ribosomes, mitochondria, and plastids. Plasmodesmata between adjacent cells enlarge, the middle lamella is digested, and protoplasts are incorporated into the syncytium via plasma membrane fusion [10]. These changes are essential for nematode infection as, in this way, hundreds of cells form a nutrient sink capable of sustaining the nematode as it matures and reproduces. Comparatively, root-knot nematodes (Meloidogyne spp.) select several feeding site-progenitor cells, which all undergo successive rounds of mitosis without cytokinesis, resulting in a group of giant, multinucleate cells up to 100 times larger than their neighbours. As these giant cells develop, the cells surrounding them proliferate, forming a gall within which the nematode resides
Conclusion
Unraveling the ‘effector toolkit’ of plant-parasitic nematodes represents a significant milestone in understanding the molecular basis of hostparasite interactions. With this knowledge, researchers are better equipped to develop sustainable and effective strategies to mitigate nematode-induced crop losses. By altering nematode physiology and development through targeted disruption of specific effectors, the potential for novel agricultural practices and biotechnological advancements is promising. Ultimately, this research paves the way for a future where crop plants can better defend themselves against these notorious pests, leading to increased global food security.
References
- Maarten K, Stam P. Changing paradigms in plant breeding. Plant Physiol. 125,156-159(2001).
- Rajeev K, Yu J, Mark E et al. Designing future crops: genomics-assisted breeding comes of age.Trends Plant Sci. 26, 631-649 (2021).
- Morrell PL, Edward S, Ibarra JR et al. Crop genomics: advances and applications.Nat Rev Genet. 13, 85-96 (2012).
- Zamir D. Improving plant breeding with exotic genetic libraries.Nat Rev Genet.2,983-989(2001).
- Matin Q. Role of new plant breeding technologies for food security and sustainable agricultural development.Applied Economic Perspectives and Policy. 42, 129-150 (2020).
- Muthamilarasan M, Prasad M. Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses.TheorApplGenet.128, 1-14(2015).
- Dempewolf H, Baute G, Anderson J et al. Past and future use of wild relatives in crop breeding.Crop Sci.57, 1070-1082(2008).
- Moose SP, Mumm RH. Molecular plant breeding as the foundation for 21st century crop improvement.Plant physiology,147,969-977(2008).
- Sunny A. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: recent advances and future outlook.IntJMolSci. 21, 2590 (2020).
- Davies W, Paul J. An historical perspective from the green revolution to the gene revolution. NutrRev. 61, 124-134(2003).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref