Research Article - International Journal of Clinical Rheumatology (2020) Volume 15, Issue 5
The clinical efficacy of artificial saliva using four point ordinal scale and visual analogue scale in patients of sjogrens syndrome with xerostomia
- Corresponding Author:
- Saba Samreen
Fauji Foundation Hospital, Rawalpindi, Pakistan
E-mail: sabasamreen@hotmail.com
Abstract
Objective: To evaluate the efficacy of artificial saliva for relief of xerostomia in patients with Sjogrens syndrome. Place/duration of study: This is a quasi-experimental trial of 6 months’ duration from 1st October 2019 till 30th march 2020 conducted at Fauji Foundation Hospital, Rawalpindi. Patients and methods: We enrolled 50 patients with xerostomia due to Sjogrens syndrome. The clinical efficacy of Xerostomia was evaluated by means of a patient reported score on a 10-point visual analog scale (VAS) and assessment of the oral tissue condition by means of a 4-point ordinal scale at 0, 2, 4 and 6 weeks. Mean ± standard deviation of quantitative variables was calculated and level of significance was determined using paired-t-test. An improvement in xerostomia was measured by comparing patient reported VAS and a physician recorded 4-point ordinal score at baseline, 2nd, 4th and 6th week. Results: All of the patients were female with a mean age (years ± SD) of 48.48 ± 10.8.Four-point ordinal score at screening visit was 25.30 ± 5.21(mean ± SD) whereas patient VAS was 57.92 ± 12.03 (mean ± SD). The mean change in both four-point ordinal score and patients VAS was statistically significant at 2 weeks, 4 weeks and 6 weeks (p value 0.000 in each). It was well tolerated in most of the patients (only 7 patients discontinued ;5 due to mucositis ,1 due to respiratory tract infection and one had mouth bleed due to dental issues). Conclusions: Artificial saliva is a safe and efficacious option for patients with xerostomia.
Keywords
sjogren syndrome • artificial saliva • dry mouth
Introduction
Sjogrens syndrome is a slowly progressive chronic disorder that leads to immune mediated damage targeting exocrine glands as well as multiple organs [1]. The resultant infiltration of lymphocytes leads to dryness of mouth (xerostomia) and dryness of eyes (keratoconjunctivitis sicca). In 1933 Dr. Henrik Sjogren first elaborated sicca symptoms to be a hallmark of Sjogrens syndrome along with polyarthritis [2]. Clinical spectrum varies from mild symptoms like dry eyes and dry mouth to severe systemic symptoms, involving multiple organ systems [3].Various autoantibodies are associated with Sjogrens syndrome; Ro and La autoantibody are also detected in biopsy specimens from salivary gland [4].
Primary Sjögren syndrome occurs in the absence of any underlying autoimmune disease, whereas secondary Sjögren syndrome is associated with underlying disease, such as Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA), or scleroderma. Multiple studies have shown the presence of secondary Sjogren syndrome in RA and SLE in variable rates [5]. The Prevalence of Primary Sjogren Syndrome in United States is around 0.1-4% of the population. This wide range reflects the lack of uniform diagnostic criteria [6].
Saliva plays an important role to keep the mouth wet. Xerostomia is defined as feeling of dryness of the mouth which can be caused by hypo-salivation and/or hyper-evaporation of saliva. Saliva also plays an important role in food digestion, protects teeth from decay, prevents infections and makes chewing and swallowing easy. If not managed properly, dryness of mouth can lead to discomfort in chewing and swallowing food, dental caries, tooth sensitivity, candidiasis and other oral diseases [7].
The nature of symptoms as well as the chronicity of the disease leads to significant adverse impact on patient’s life. Hence, a comprehensive management strategy [8] should be the aim that involves patient education and lifestyle modification (smoking cessation, a good oral hygiene and frequent intake of water) as well as palliative therapies [9] like sugar free gums are giving as a first line. The treatment of xerostomia is more of symptomatic nature. Though there are systemic sialagogues with anticholinesterasic and cholinergic action which can help to alleviate the symptoms of oral dryness, however due to their side effect profile they are infrequently used. Patients with severe xerostomia may still need pharmacotherapy that usually includes muscarinic agents like pilocarpine or civemilline [10,11]. Depletion of B cells using rituximab has also been employed for control of severe systemic symptoms [12]. Nonetheless, preparations like saliva substitutes tend to be good alternatives to be tried first especially in patients reluctant to use excessive medications. Recently there has been an expansion of the number of saliva substitutes available for alleviation of xerostomia. So far no single product adequately mimics the properties of natural saliva. Hence it is imperative to have an understanding of the advantages and disadvantages of such products. There exists absolutely no local data that demonstrates safety and effectiveness of saliva substitutes in Pakistani population. The rationale of the study was firstly to determine the safety and effectiveness of such a saliva substitute in patients of Sjogrens syndrome with xerostomia presenting to a tertiary care rheumatology setup in Pakistan. The artificial saliva used in this study had neutral pH which contained potassium chloride, magnesium chloride hexahydrate, calcium chloride dehydrate, potassium phosphate dibasic, potassium dihydrogen orthophosphate, methyl paraben, and purified water. None of the ingredients are animal derived.
Patients and methods
This was a quasi-experimental study. Approval for this study was taken from Ethical review committee, Fauji foundation hospital, Rawalpindi. The sample size of 50 patients was selected and non-probability consecutive sampling technique was used. This study was carried out in the Department of Rheumatology, Fauji Foundation Hospital; Rawalpindi. The mean duration of study was 06 months from 1st October 2019 to 30th march, 2020 in which the patients were randomly selected from the outpatient department.
A total of 50 patients (18 to 80 years of age) were included in the study. Patients with Sjogrens (either primary or secondary) [1] were enrolled fulfilling American College of Rheumatology (ACR) criteria [13]. Subjects must have oral sicca symptoms defined as xerostomia (dry mouth) for at least 3 months.
Out of these, patients with any other disease of oral cavity disease (such as candidiasis), dental infection, gross intra-oral neglect or those in needs for extensive dental therapy were excluded. Patients taking any anticholinergic agents or other medications known to affect salivation were excluded. Patients with a known allergy to active ingredient of artificial saliva, a history of alcohol or substance abuse in previous six months or severe metabolic disease (porphyria, uremia, hypokalemia, and myeloid neuropathy) unless controlled by adequate therapy were also excluded. Patients who had already participated in an investigational product research within 1 month prior to study entry were not a part of the study. Apart from these exclusion criteria patients with any other condition or disease detected during medical interview considered unsuitable for study were not enrolled.
Patients with Sjogrens syndrome (primary or secondary) fulfilling the inclusion criteria were enrolled in the study. A voluntary signed and dated written informed consent to participate in the study was taken from all the patients. A detailed oral cavity and dental examination was done at the screening visit. Patient’s biodata was entered into a proforma along with identity card number and a contact number for follow up. Patient’s biodata was recorded as age, gender, marital status and occupation. The duration of disease since the symptom onset was also noted. Anti-Ro/SSA and anti-La/SSB titers were determined at baseline.
The xerostomia (oral dryness) severity of each patient was calculated at the first visit by noting down the patient reported visual analogue scale [14] (from 0 to 10) where 0 means no xerostomia and 10 means maximum sicca symptoms.
A blinded assessment of xerostomia by a physician was carried out as a 4-point ordinal score [15] at baseline. This four point ordinal score included assessment of the lips, tongue, hard and soft palate, gingiva, muco-buccal fold areas, buccal mucosa, and floor of the mouth and gauged the clinical severity of xerostomia as none, mild, moderate or severe.
After recording baseline profile, patients were provided artificial saliva in a spray format for a convenient administration. Artificial saliva came in as a spray preparation. This spray had to be taken as 3 graduated sprays in right, left and center of buccal cavity three times a day. Patients were followed up and re-evaluated using the above mentioned tools at 2 weekly intervals i.e. 15days, 30 days and 45 days respectively.
Any patient who developed the adverse effects (either patient or physician reported) was noted down at each follow up visit. Adverse effect was defined as any sign, symptom, syndrome, or illness that appeared during the study period, and that might have impaired wellbeing of the subject. Any adverse effect considered life threatening or serious eventually lead to discontinuation of the therapy. The drug combination was with-held temporarily or discontinued depending upon the adverse effects experienced; the decision to do so was left upon the evaluating physician.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 20. Mean ± standard deviation of quantitative variables like age and duration of disease was calculated. Paired t-test was used for quantitative variables to evaluate the level of significance and a comparison of xerostomia at baseline was made with both 30 and 45 day assessments. An improvement in a patient reported VAS or a physician recorded 4 point ordinal score was calculated. A p- value of <0.05 was considered significant. A confidence interval of 95% was used. Frequency and percentages were presented for categorical variables like gender, taste disturbance or other adverse events. Also percentages% of patients who continued, temporarily stopped or discontinued the therapy were noted.
Results
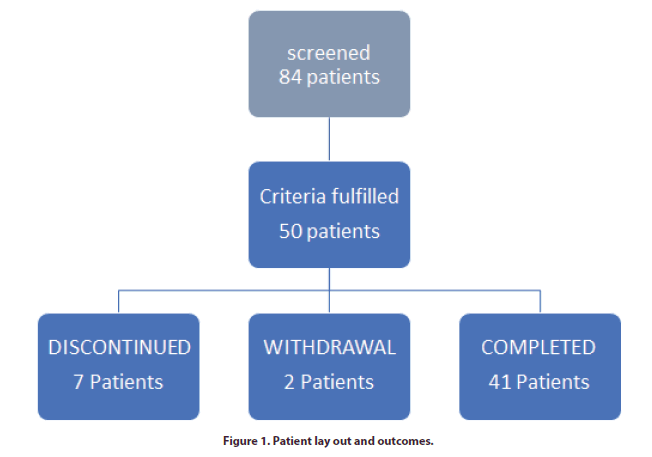
Of 84 patients screened, 50 patients met the inclusion criteria.7 patients discontinued, 2 withdrew and 41 patients completed the study (Figure 1). All of the patients were female. The mean age of the patients at the screening visit was 48.48 ± 10.81 mean ± SD (years). The rest of baseline characteristics are as shown in Table 1.
Table 1. Baseline clinical and demographic features of the patients included in the study.
| Variables | |
|---|---|
| Total patient | 50 (100%) |
| Gender | Female |
| Age mean ± SD (years) | 48.48 ± 10.81 |
| Duration of symptoms (months) | 46.70 ± 58.71 |
| Duration of treatment (months) | 37.59 ± 51.88 |
| Anti SSA | 10 (20%) |
| Anti SSB | 7 (14%) |
| Mean four point score at visit 1 | 25.30 ± 5.21 |
| Mean VAS at visit 1 | 57.92 ± 12.03 |
At four weeks, the mean change in VAS score was -24.9 ± 14.6 whereas the mean change in the four point ordinal scale from the baseline score was -10.24 ± 5.59. The mean change in both VAS and four point ordinal scale was statistically significant (p-value ≤ 0.05) as shown in Table 2.
Table 2. Efficacy Profile of patients at baseline and on subsequent visits.
| Variables | 0 _visit1 | 15 days-visit2 | 30 days-visit3 | 45 days-visit4 | p-Value * | p-value |
|---|---|---|---|---|---|---|
| Visual analogue scale | 57.92 ± 12.03 | 49.6 ± 13.87 | 39.02 ± 12.14 | 32.59 ± 13.2 | 0 | 0 |
| Four point ordinal scale | 25.30 ± 5.21 | 21.4 ± 5.34 | 17.9 ± 4.59 | 14.63 ± 3.47 | 0 | 0 |
P ≤ 0.05: significant (mentioned in bold); p- value*: comparison between baseline and 30 days; p -value: comparison between baseline and 45 days
Dose titration in all of the patients prescribed at the screening visit was 3 graduated sprays three times a day. At 15 days dose was up titrated to 4 graduated sprays three times daily in only one patient and at 30 days, in 11 patients due to partial response (physician discretion). No dose titrations were done after 30 days.
During the course of treatment, 7 patients discontinued the saliva substitute. Amongst them, 5 patients developed mucositis. One patient developed upper respiratory tract infection which was unrelated to the treatment product and another patient had mouth bleed due to ongoing dental issues.2 patients withdrew from the study protocol; one due to lack of any response at 15 days and the other did not specify any particular reason.
Discussion
Sjögren syndrome is characterized by xerostomia, keratoconjunctivitis sicca and polyarthritis along with a cluster of symptoms involving other vital organs. Out of the symptom complex of the syndrome xerostomia and keratoconjuctivitis sicca tend to be the most cumbersome and chronic features that add to debility of the patient [16]. Xerostomia is one of the underestimated symptoms both by patients and physicians firstly because of poor adherence to the treatment modalities and partly because of uncertain nature of the response [17]. Hence, symptomatic treatment is necessary specifically for the sicca symptoms [18].
Amongst the pharmacological agents available, two most commonly employed are pilocarpine and civemilline (muscarinic agonists) [11]. Nonetheless many patients face frequent adverse events with these agents or are reluctant to take these medications on long term basis. Therefore physiological stimulation with sugar free gums [19] is an effective alternate for patients who retain some residual salivary gland function. For patients who have no residual salivary gland function, artificial saliva or saliva substitutes may be employed. There are trials conducted on the use of B-cell depletion therapies in Sjögren syndrome. The role of rituximab in improving xerostomia remains controversial. One such study demonstrates no improvement [20]. The TEARS trial also supports the fact that though mean VAS of xerostomia symptoms in better in patient who took rituximab but the efficacy is not sufficient enough to advocate it in every patient of Sjögren syndrome [21].
Saliva substitutes are preparations that have a viscosity and electrolyte composition that approximates whole saliva and are meant to provide longer relief and lubrication of the oral cavity. Saliva substitutes are based on either Carboxy-Methyl-Cellulose (CMC) or mucin [22] .In many trials [22,23] conducted previously various saliva substitutes have been evaluated for their efficacy and also compared with each other. But many patients as well as physicians still have reserved reviews about saliva substitutes; firstly because of non-standardized preparations and secondly because of improper guidance and instructions given to the patients. In our study, we used a CMC based artificial saliva preparation containing mineral salts and ingredients needed for buffer and lubricant effect and saliva-like properties which according to Moore and Guggenheimer [24] are the pre-requisites for the palliative management. We also utilized a standardized graduated spray bottle and adherence to dosing protocol was given as a written hand out and also confirmed on each subsequent visit.
In this study the end results were remarkable with a statistically significant improvement in patient reported VAS as well as physician reported four point ordinal scale (p<0.05). Approximately 80% patients in our study showed improvement with the saliva spray .On the contrary, in the study by F.J. Silvestre [25] and his colleagues only 54% patients showed improvement and a lower proportion of respondents was explained by the concomitant use of psychotropic in his study whereas patients on such medications were excluded in our study. There were only seven discontinuations; mostly attributable to mucositis which could be partly explained by already altered oral flora of these patients [26-28].
Our study showed that:
• The patients of Sjögren syndrome with xerostomia felt symptomatic relief with the saliva substitute
• Patients also reported improved oral functions such as chewing and swallowing
Conclusion
Artificial saliva appears to be an effective and safe treatment option for symptomatic relief of xerostomia in patients with Sjogren syndrome.
References
- Huang Y, Cheng Q, Jiang C et al. The Immune Factors Involved in the Pathogenesis, Diagnosis, and Treatment of Sjogren’s syndrome. Clin. Dev. Immunol. 2013, 1–6 (2013).
- Ghafoor M. Sjögren’s Before Sjögren: Did Henrik Sjögren (1899-1986) Really Discover Sjögren’s Disease? J. Maxil. Oral. Surg. 11(3), 373–374 (2012).
- Rischmueller M, Tieu J, Lester S. Primary Sjögren's syndrome. Best. Pract. Res. Clin. Rheumatol. 30 (1), 189–220 (2016).
- Haga HJ, Naderi Y, Moreno AM et al. A study of the prevelance of sicca symptoms and secondary Sjogren’s Syndrome in patients with rheumatoid arthritis, and its association to disease activity and treatment profile. Int. J. Rheum. Dis. 15(3), 284–288 (2012).
- Kosrirukvongs P, Ngowyutagon P, Pusuwan P et al. Prevalence of dry eye syndrome and Sjogren's syndrome in patients with rheumatoid arthritis. J. Med. Assoc. Thailand. 95(Suppl 4), S61–S69 (2012).
- Helmick CG, Felson DT, Lawrence RC et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis. Rheum. 58(1), 15–25 (2008).
- Mittal S, Bansal V, Garg S et al. The diagnostic role of Saliva-A reivew. J. Clic. Exp. Dent. 3, e314–e320 (2011).
- Plemons JM, Al-Hashimi I, Marek CL. Managing xerostomia and salivary gland hypofunction: executive summary of a report from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 145(8), 867–873 (2014).
- Routsias JG, Tzioufas AG. Sjögren’s syndrome-study of autoantigens and autoantibodies. Clin. Rev. Allerg. Immu. 32(3), 238–251 (2007).
- Furness S, Worthington HV, Bryan G et al. Interventions for the management of dry mouth: topical therapies. The Cochrane Library. (2011).
- Fife RS, Chase WF, Dore RK et al. Cevimeline for the treatment of xerostomia in patients with Sjögren syndrome: a randomized trial. Arch. Intern. Med. 162(11), 1293–1300 (2002).
- Meijer JM, Meiners PM, Vissink A et al. Effectiveness of Rituximab treating in primary sjogren syndrome:a randomized , double-blind, placebo-controlled trial. Arthritis. Rheum. 62(4), 960–968 (2010).
- Shiboski SC, Shiboski CH, Criswell LA et al. American College of Rheumatology classification criteria for Sjögren's syndrome: A data‐driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance Cohort. Arthritis. Care. Res. 64(4), 475–487 (2012).
- Pai S, Ghezzi EM, Ship JA. Development of a Visual Analogue Scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 91(3), 311–316 (2001).
- Salom M, Hachulla E, Bertolus C et al. Efficacy and safety of a new oral saliva equivalent in the management of xerostomia: a national, multicenter, randomized study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 119(3), 301–309 (2015).
- Sreenby LM, Valdini A. Xerostomia. A neglected symptom. Arch. Intern. Med. 147, 1333–1337 (1987).
- Stefanski AL, Tomiak C, Pleyer U et al. The diagnosis and treatment of Sjögren’s syndrome. Deutsches. Ärzteblatt. Int. 114(20), 354–361 (2017).
- MoutsopoulosHM.Sjögren's syndrome.Fauci AS, Martin JB, Braunwald E et al.edsHarrison's Principles of Internal Medicine. 14th ed. New York, NY McGraw-Hill Co pp, 1901–1904 (1998).
- Davies AN. A comparison of artificial saliva and chewing gum in the management of xerostomia in patients with advanced cancer. Palliat. Med. 14(3), 197–203 (2000).
- van der Reijden WA, van der Kwaak H, Vissink A et al. Treatment of xerostomia with polymer‐based saliva substitutes in patients with Sjögren's syndrome. Arthritis. Rheumatol. 39(1), 57–63 (1996).
- St Clair EW, Levesque MC, Luning Prak ET et al. Rituximab therapy for primary Sjogren Syndrome: An open-label Clinical trial and mechanistic analysis. Arthritis. Rheumatol. 65(4), 1097 (2013).
- Roukema PA, Panders AK. The effect of mucin-containing artificial saliva on severe xerostomia. Int. J. Oral. Surg. 3(6), 435–439 (1974).
- Vissink A, De Jong HP, Busscher HJ et al. Wetting properties of human saliva and saliva substitutes. J. Dent. Res. 65(9), 1121–1124 (1986).
- Vissink A, Schaub RM, Rijn LV et al. The Efficacy of Mucin‐Containing Artificial Saliva in Alleviating Symptoms of Xerostomia. Gerodontology. 6(3), 95–101 (1987).
- Aagaard A, Godiksen S, Teglers PT et al. Comparison between new saliva stimulants in patients with dry mouth: a placebo‐controlled double‐blind crossover study. J. Oral. Pathol. Med. 21(8), 376–380 (1992).
- Atkinson JC, Wu AJ. Salivary gland dysfunction: causes, symptoms, treatment. J. Am. Dent. Assoc. 125(4), 409–416 (1994).
- Moore PA, Guggenheimer J. Medication-induced hyposalivation: etiology, diagnosis, and treatment. Compend. Contin. Educ. Dent. 29(1), 50–55 (2008).
- Silvestre FJ, Minguez MP, Suñe-Negre JM. Clinical evaluation of new artificial saliva in spray form for patients with dry mouth. Med. Oral. Patol. Oral. Cir. Bucal. 14(1), E8–E11 (2009).