Perspective - Interventional Cardiology (2013) Volume 5, Issue 6
Should endovascular therapy be recommended for descending thoracic aortic dissections?
- Corresponding Author:
- Eric E Roselli
Department of Thoracic and Cardiovascular Surgery
Cleveland Clinic, 9500 Euclid Avenue
Desk J4-1, Cleveland, OH 44195-5108, USA
Tel: +1 216 444 0995
Fax: +1 216 636 1393
E-mail: roselle@ccf.org
Abstract
Descending aortic dissection is a lifelong disease with a high morbidity and mortality. Current management is based on presentation. In the acute setting, the initial approach is medical therapy to reduce the aortic impulse (D pressure/D time) with intravenous antihypertensive medications. Intervention is recommended when complications, such as rupture and end-organ malperfusion, are present. In the chronic state, dissections are managed medically with close imaging surveillance until complications, such as aneurysmal degeneration, warrant surgical intervention.
Keywords
acute dissection, aortic dissection, chronic dissection, complicated dissection, descending aorta, endovascular, surgery, thoracic aorta, thoracic endovascular aortic repair, uncomplicated dissection
Descending aortic dissection is a lifelong disease with a high morbidity and mortality. Current management is based on presentation. In the acute setting, the initial approach is medical therapy to reduce the aortic impulse (D pressure/D time) with intravenous antihypertensive medications. Intervention is recommended when complications, such as rupture and end-organ malperfusion, are present. In the chronic state, dissections are managed medically with close imaging surveillance until complications, such as aneurysmal degeneration, warrant surgical intervention [1,2].
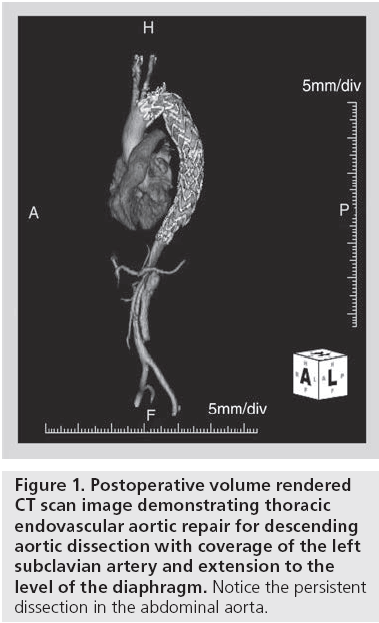
Thoracic endovascular aortic repair (TEVAR) has emerged as a suitable, low-risk and less invasive alternative to surgery for treatment of degenerative aortic aneurysms. The devices used are the same as those indicated for degenerative aneurysms. The delivery and deployment is usually performed from a transfemoral approach under fluoroscopic guidance. The primary objective is to cover the proximal entry tear and expand the dissected true lumen. This usually requires positioning of the device in the distal aortic arch, with or without left subclavian artery coverage (Figure 1).
The indications and understanding of the outcomes for endovascular therapy for aortic dissection are still evolving [3-5]. Each treatment modality for aortic dissection (i.e., medical, surgical and endovascular) offers unique advantages, and they should be considered complementary to, instead of competitive with, one another.
In this article, we focus on the current role of TEVAR in treating distal aortic dissection in its current state and discuss its potential role in the future. This is presented in terms of the timing (acute vs chronic) and presentation (complicated vs uncomplicated) of the disease since these characteristics are the most important determinants of therapy.
Acute descending thoracic aortic dissection
▪ Complicated acute dissection
Acute complicated descending thoracic aortic dissection is associated with high mortality (20-50%) if left untreated, especially in the subset of patients presenting with rupture or organ ischemia [3,6,7]. By contrast, mortality is typically less than 10% when the presentation is uncomplicated [6]. Prompt intervention is the treatment of choice in all patients presenting with acute complicated type B dissection. Options include open surgical graft replacement, endovascular stent grafting, open or endovascular fenestration, or extra-anatomic bypass [8].
Although excellent results have been reported for a highly selected population treated with open surgical repair, this experience has not been the norm [9]. Even large-volume highly experienced centers reported operative mortality of 22.4%, paraplegia of 6.6%, respiratory failure requiring tracheostomy of 13.6% and renal failure of 19.7% [10]. Open repair mortality was 29.3% in a similar study from the International Registry of Aortic Dissection. Age greater than 70 years and presence of preoperative shock or hypotension have been found to be independent predictors of surgical mortality [11].
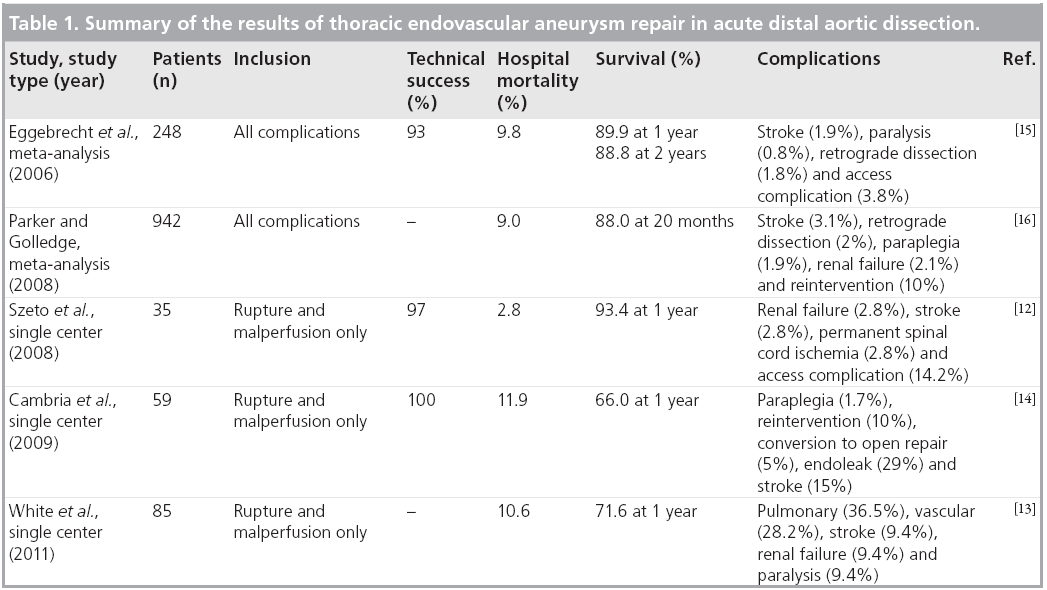
Less than ideal outcomes with open repair for acute distal dissections inspired the use of TEVAR for this indication. The effectiveness of emergency TEVAR in acute aortic dissection complicated by rupture or malperfusion has been evaluated in several nonrandomized studies (Table 1). In a series of 35 patients, which included 18 ruptures and 17 patients with malperfusion, technical success was achieved in 97%. Operative mortality was 2.8% and 1-year survival was 93.4% [12]. In another study, 85 patients with acute complicated dissection were included; 31.8% presented with rupture and 71.8% with malperfusion. Early major complications occurred in 37.6% of patients, including mortality (10.6%), stroke (9.4%), renal failure (9.4%) and paralysis (9.4%). In a subgroup analysis, patients with rupture had higher early and late adverse events than patients with malperfusion [13]. In another study of 59 patients with highrisk complicated acute dissection (79% had either rupture or malperfusion syndromes at presentation), stent grafts were successfully deployed in all patients. Combined 30-day mortality and paraplegia rate was 13.6% and stroke rate was 15%. Endovascular reintervention was required in 10% of patients and 5% required conversion to open surgery. Survival at 1 year was 66%. Age at treatment and chronic obstructive pulmonary disease were predictive of death at 1 year [14].
Meta-analyses have been published in the last several years. The first of these studies included 609 patients. Technical success was 98%, major complications occurred in 11%, neurological complications in 2.6% and hospital mortality rate was 9.8% [15]. Another meta-analysis of 29 studies comprised 942 patients with reported operative mortality of 9%. Other complications included stroke (3.1%), retrograde type A dissection (2%), paraplegia (1.9%) and renal failure (2.1%). Estimated survival at a mean of 20 months was 88% and rupture was reported in only 0.8%. However, the reintervention rate was 7.6%, and this was managed surgically in 2.8% of patients [16]. In a third meta-analysis of 39 studies comprised of 1304 Chinese patients, the technical success was 99.2%. The 30-day mortality was 2.6%, survival at 5 years was 95.2% and 0.6% suffered neurologic complications [17]. These studies included mixed populations of patients but the results are similar.
No randomized trials have been performed directly comparing TEVAR with open repair in acute complicated dissection, but several nonrandomized head-to-head comparisons have been published. In a report from the International Registry of Aortic Dissection comparing different treatment modalities for acute complicated dissection, mortality after surgical repair was 33.9 versus 10.6% in those treated with TEVAR. After multivariable adjustment, surgical repair was found to be an independent risk factor of in-hospital mortality [18]. In another study comparing outcomes after TEVAR, surgery or medical therapy, hospital mortality was 4, 40 or 33%, respectively. Estimated survival at 1, 3 and 5 years after TEVAR was 82, 79 and 79% versus 58, 52 and 44% for the open repair and medical therapy groups, respectively [19].
In a meta-analysis comparing outcomes after TEVAR or open repair in acute complicated descending aortic dissection, 1951 patients were included from 76 studies. Early mortality and paraplegia were significantly reduced in patients who underwent TEVAR. Aortic-related complications were also reduced after TEVAR compared with open repair. There were no differences in late mortality, reintervention, renal dysfunction and stroke [20]. Another meta-analysis of five studies comparing TEVAR and open repair for the treatment of acute type B aortic dissection showed improved 30-day mortality in the TEVAR group, but there was no difference in complications or long-term outcome [21].
The use of thoracic stent grafts has supplanted open surgical repair for acute distal aortic dissections complicated by rupture or malperfusion. Superior outcomes with regard to operative mortality have been reproducibly demonstrated and eliminate the equipoise necessary for a randomized trial.
The disease process is quite variable, however, and the endovascular approach is not applicable to all morphologies and anatomy. In patients presenting with rupture, it is critical that the primary intimal entry tear is covered in order to eliminate direct flow into, and reduce pressurization of, the false lumen. This often requires coverage of the left subclavian artery and a more distal extension to the level of the diaphragm to address all the intimal tears in the thoracic aorta.
For patients who present with malperfusion, the objective is to optimize the true lumen flow through the thoracic aorta and into the affected branch vessels. This is usually achieved by covering the primary intimal entry tear and stenting open compressed portions of the true lumen with the use of a single stent graft device. The length of coverage needed is patient specific and guided by intraoperative imaging (angiography and/or intravascular ultrasonography), but is often less than is required for a patient presenting with rupture.
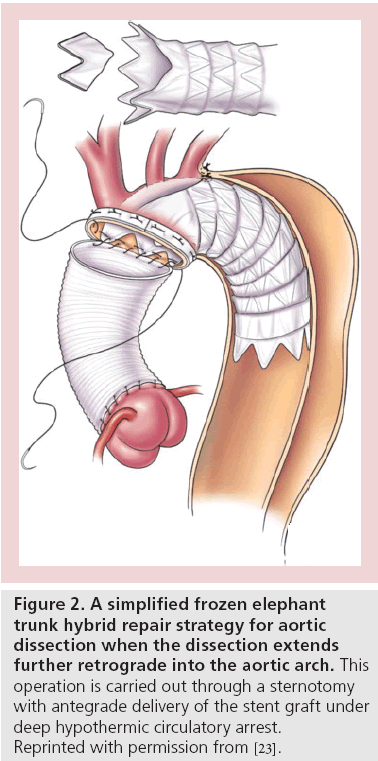
Occasionally, additional endovascular procedures, such as branch vessel stenting (in case of static vessel occlusion) or fenestrating the dissection flap (to decompress the false lumen), may be necessary [22]. In an even smaller subset of patients with arch extension where purely endovascular stent grafting is not feasible, a hybrid approach involving sternotomy and direct antegrade stent graft delivery represents another safe alternative to classic open repair (Figure 2) [23-25].
Figure 2: A simplified frozen elephant trunk hybrid repair strategy for aortic dissection when the dissection extends further retrograde into the aortic arch. This operation is carried out through a sternotomy with antegrade delivery of the stent graft under deep hypothermic circulatory arrest. Reprinted with permission from [23].
▪ Uncomplicated acute dissection
Medical treatment is the current therapy for uncomplicated acute dissection [2,3]. Early results of medical treatment in acute type B dissection are encouraging, but there is a high incidence of long-term complications resulting from progressive aneurysmal degeneration during the chronic phase. An International Registry of Aortic Dissection study reported a 60% incidence of progressive aortic dilatation in patients treated medically after acute type B dissection. A total of 20% developed fatal aortic rupture after surviving the acute phase [26]. In another study, the actuarial survival at 1, 5, 10 and 15 years was 85, 71, 38 and 20% for the medically treated patients, respectively, and aortic-related mortality accounted for 34% of the deaths [7].
Prophylactic use of TEVAR to treat acute uncomplicated dissection is controversial but slowly gaining traction. The potential benefit of early TEVAR in these patients includes a better chance of achieving aortic remodeling than when intervention is deferred. In a retrospective study comparing the use of TEVAR in acute complicated and uncomplicated dissection, there was no perioperative mortality in the uncomplicated group, with false lumen obliteration occurring in 12 out of 14 patients [27]. Currently, there are no published trials evaluating the role of TEVAR versus medical therapy in acute uncomplicated dissection.
The groundbreaking INSTEAD trial is often misrepresented as such a trial, but all of these patients were studied during the period ranging from 2 to 52 weeks after the acute event [28]. By standard definition, that makes this a study of patients with chronic dissection. More details regarding the latest findings of the INSTEAD trial are included in the section ‘Chronic descending thoracic aortic dissection’.
The encouraging results of TEVAR in acute complicated descending aortic dissection and lack of level I evidence for the usefulness of TEVAR in acute uncomplicated dissections necessitated the design of a randomized trial. The ADSORB trial is the first randomized trial comparing TEVAR and medical therapy in acute uncomplicated aortic dissection. Aortic remodeling, reintervention and aneurysmal degeneration will be compared in 61 patients during a 36-month follow-up period [29]. The results from this study shall be published soon. Furthermore, the STABLE trial is ongoing and evaluates the use of a novel strategy for aortic dissection repair by adding a long uncovered stent to the proximal conventional stent graft repair and extending the uncovered segment across the visceral segment of the aorta [30].
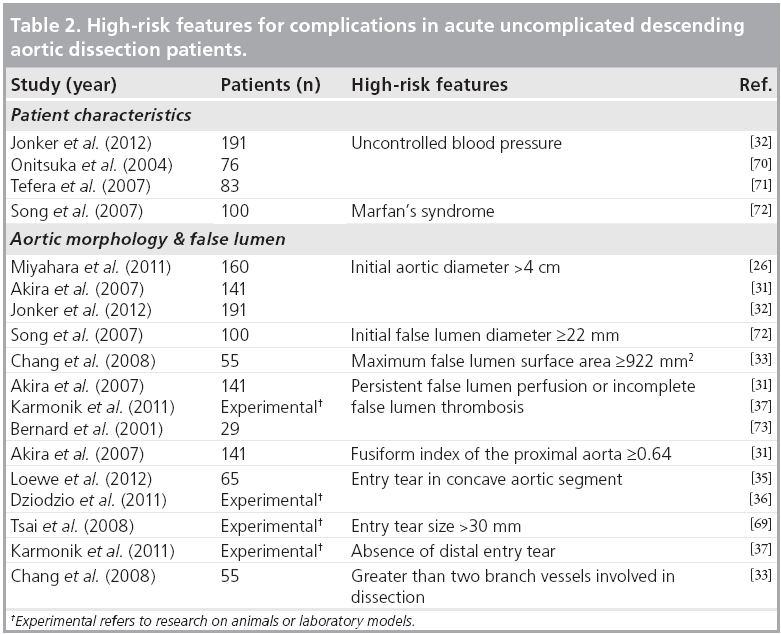
▪ Uncomplicated but high-risk acute dissection
Although many centers are now pre-emptively treating all patients with uncomplicated acute distal dissections, there are currently no definitive data to support this practice. A subset of patients with uncomplicated dissection is at higher risk of developing complications, such as impending rupture, rapid dilatation or malperfusion [2,3,6,8]. These patients are classified as acute uncomplicated but high risk. Since TEVAR can be performed safely, it has been postulated that high-risk patients with uncomplicated acute aortic dissection may benefit from early intervention. Several studies have shown that aortic diameter [26,31,32], false lumen morphology [33,34], site and number of entry tears [35-37], and morphology of the descending aorta can predict prognosis [8,31]. Based on these risk factors, a method for subclassification of uncomplicated distal dissection into low- and high-risk groups has been proposed [38]. Further studies are required to better define the high-risk criteria and clarify the best treatment option for these patients. Table 2 summarizes several candidate features that may help to categorize patients into this uncomplicated but high-risk acute dissection group.
Chronic descending thoracic aortic dissection
▪ Complicated chronic dissection
In most patients, chronic dissection is managed medically after the initial recovery. A subset will develop complications due to disease progression, manifesting as aneurysmal degeneration, rupture or end-organ malperfusion [2,3,39]. One study reported a 60% incidence of progressive aortic dilatation, and a risk of rupture as high as 20% at a mean (± standard deviation) follow-up of 44.6 ± 25.4 months [26]. These patients are categorized as complicated chronic descending thoracic dissection, and most should be managed surgically or with interventional therapy [2,3]. The goal of any of the treatment modalities is to prevent late aorta-related deaths.
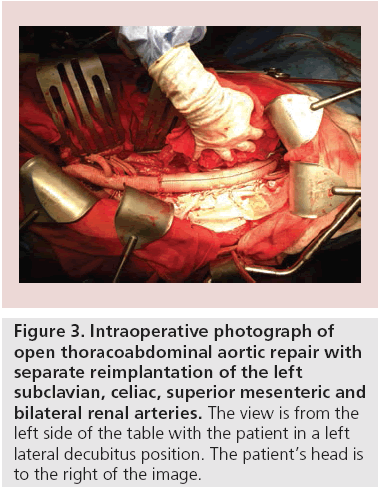
An early series of open repair for distal aortic dissection reported high mortality and morbidity, but subsequent studies showed better results attributable to improvement in care. Svensson et al. analyzed Crawford’s experience with aortic dissection and reported an overall hospital mortality of 7.4% with improving results over time. In total, 56% of patients underwent repairs of the descending or thoracoabdominal aorta, and operative mortality in those patients improved over time from 13 to 5%. Survival at 1 and 3 years was 84 and 71%, respectively, but the late follow-up was limited. Despite improvement in operative mortality, open repair is complex and carries a significant risk for morbidity (Figure 3). Neurologic dysfunction remained in 14% of patients at hospital discharge and 14% had renal failure. With a limited resection strategy of repair, it is not surprising that 28% of patients required additional aortic surgery in late follow-up [40].
Figure 3: Intraoperative photograph of open thoracoabdominal aortic repair with separate reimplantation of the left subclavian, celiac, superior mesenteric and bilateral renal arteries. The view is from the left side of the table with the patient in a left lateral decubitus position. The patient’s head is to the right of the image.
As the experience with TEVAR has grown, its use has been expanded to patients with complicated chronic dissections, although the devices are not approved for that indication. This is mostly due to the lower acute risk of the endovascular procedure compared with open repair [41]. Currently, the use of TEVAR in patients with chronic dissections is controversial since there has not been a fair comparison to surgical repair [2,3,42]. The nature of the disease in its chronic state is varied and so the population is heterogeneous, making a fair comparison difficult to perform. In the meantime, data are accruing from many large centers (Table 3).
In a more recent study of our experience at the Cleveland Clinic (OH, USA), we evaluated the results of open repair in 169 patients with descending aortic dissection who were also deemed to be anatomically suitable for TEVAR. Operative mortality was 8% and neurological complications occurred in 2.4%. Many of these patients had undergone previous ascending, arch and first-stage elephant reconstruction. Survival at 1, 2 and 3 years was 82, 78 and 75%, respectively, and 14% required reintervention [43]. In a concurrent study, we published our outcomes after TEVAR in patients with complicated chronic type B dissection [44]. Open surgical patients were younger compared with those treated with TEVAR, but were also more likely to have extensive dissection extending through the abdominal aorta, while the TEVAR series included many patients with the dissection limited to the thoracic aorta. Operative mortality in our TEVAR patients was 5% and survival at 1, 2 and 3 years was 86, 82 and 80%, respectively, but the rate of reintervention was 22% [44]. In both studies, the freedom from adverse events (i.e., death or vascular reoperation) was approximately 55% at 5 years. Patients in whom the dissection was limited to the thoracic aorta and underwent TEVAR had the best results, with greater than 80% experiencing false lumen thrombosis and complete aortic remodeling. In the patients with more extensive dissection undergoing TEVAR, only 13% had complete remodeling. A predictor of late adverse events in both studies was a larger distal aortic diameter at the time of treatment, suggesting that patients may benefit from earlier intervention.
Two studies have directly compared open repair to TEVAR for chronic dissection using propensity scoring [45,46]. Mortality following open repair was higher in emergency cases but not significantly different than TEVAR in elective cases. Open repair was associated with a higher risk for complications and longer length of hospital stay than TEVAR. Another interesting finding was that centers that performed 30 or more TEVAR cases annually had better survival for emergency cases [46]. A metaanalysis of 17 studies evaluating outcomes after TEVAR for chronic distal dissection including 567 patients showed an overall technical success of 89.9% (ten studies had 100% success). Mortality was 3.7% with survival ranging from 60 to 100% at 2 years. Reintervention rates ranged from 0 to 60% and type I endoleaks occurred in 8%. Overall, 8% of patients developed aneurysms of the distal aorta and 15% demonstrated continued false lumen perfusion with subsequent aneurysmal dilatation [47].
Chronicity poses additional challenges in patients undergoing TEVAR as they are less likely to have thromboexclusion and remodeling of the treated segment compared with those undergoing early treatment. In the chronic state, multiple entry tears have matured and the dissection flap has thickened. Several studies have demonstrated that relative immobility of the septum lowers the likelihood of false lumen thrombosis and undermines success with TEVAR compared with acute dissection [48-51].
Morphological changes in the dissected aorta after medical therapy or TEVAR have been studied both in the acute and chronic setting. Blount and Hagspiel reported that medical treatment of descending dissection was associated with progressive increase in maximal aortic and false lumen diameter, and this negatively affected the long-term results of the medically treated patients [52]. However, whether or not false lumen thrombosis after TEVAR is a true marker for treatment success is unclear. In the publication of the initial INSTEAD trial, more false lumen thrombosis and aortic remodeling occurred in TEVAR patients, but there was no impact on survival during 2-year follow-up [28].
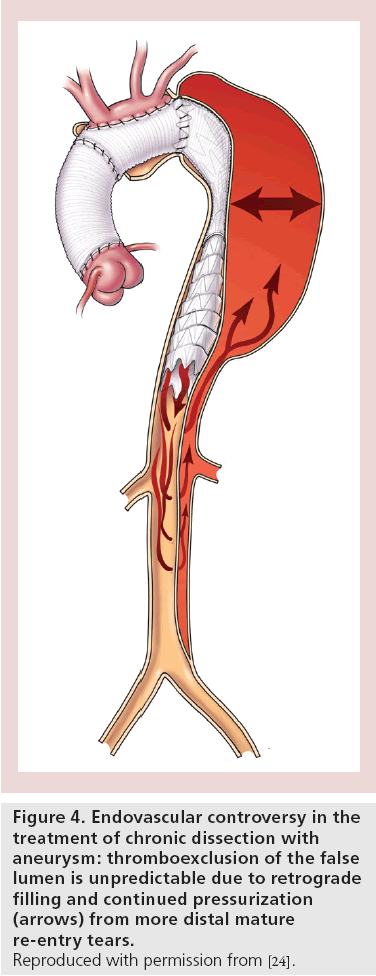
Categorical determination of false lumen thrombosis may not be an entirely satisfactory end point when assessing patients after TEVAR for dissections. The thrombosis that occurs is a continuous process and so accurate assessment requires evaluation over time. Andacheh and colleagues reported that following TEVAR, true lumen expansion occurred in patients with chronic complicated aortic dissection at a rate of 38, 46, 71 and 114% over baseline at 1, 3, 6 and 12 months of follow-up, respectively. This was accompanied by subsequent regression of the false lumen by 65, 68, 84 and 84% for the same periods, respectively [53]. Many of these patients continue to have false lumen flow and pressurization provided by indirect flow through distal re-entry tears (Figure 4). Partial thrombosis of the false lumen is associated with increased pressure in the false lumen and may be related to increased late aortic related events [37]. Contrast in the false lumen may not be detectable in the early arterial phase of a CT scan and only seen on the delayed venous-phase imaging. Many reports probably overestimate the degree of false lumen thrombosis unless the analysis included delayedphase contrast-enhanced imaging [44]. Surgeons from Guy’s and St Thomas’ Hospital (London, UK) demonstrated that shrinkage of the false lumen is critical to treatment success. In their series, survival at 4 years was 89% in the group of patients with a reduction in false lumen size, while it was only 39% in those with either stable or increased aortic size [54].
Good results have been demonstrated in recent series of both open repair and TEVAR for chronic complicated dissection [41,43,44,47,53,54]. Although there is equipoise in several centers lending support for a randomized trial, outcomes depend upon specific details of a patient’s disease and the operator’s experience with either approach. Given the current controversy, establishment of a prospective multicenter registry could provide a great deal of information as to how best to treat these particularly complex patients.
▪ Uncomplicated chronic dissection
Along with others, we have shown that the larger the aorta when it is treated for complicated chronic dissection, the worse the outcome [43,44]. The chance of inducing remodeling is better in the early than the late phase of disease. Because of these factors, earlier treatment for aortic dissection has been increasingly considered as a reasonable strategy.
TEVAR performed before aneurysm formation may be associated with improved aortic remodeling and resolution of dissection [49]. The INSTEAD trial is a randomized controlled study in which patients undergo TEVAR during the early chronic phase of disease, or optimal medical therapy alone. In total, 146 patients with distal aortic dissection were enrolled and randomized at a median of 5 weeks after onset of dissection. At 2 years, there was no survival benefit of TEVAR over medical therapy alone, and this is mostly due to an early hazard for patients receiving stent grafts. However, 16% of patients in the medical arm crossed over to require endovascular intervention. Furthermore, after the initial TEVAR, 8% required stent graft extension as an additional intervention. False lumen thrombosis, true lumen expansion and overall aortic shrinkage were better in TEVAR than with the medical therapy alone arm [28]. The initial design of the INSTEAD trial was probably underpowered. The INSTEAD XL trial, however, has allowed for continued follow-up of the cohorts for at least 5 years. Early reports of this analysis have demonstrated early decreasing hazard and a later slowly increasing hazard with the TEVAR patients doing worse during the early hazard phase but better during the later hazard phase. The survival curves of the two groups cross at approximately 3 years [55]. This study suggests that if we can predict the risk for poor outcome during the early hazard, TEVAR in select patients may improve late outcomes in patients presenting with uncomplicated aortic dissection.
Complications of TEVAR in patients with dissection
Despite the morbidity advantages in high-risk populations, TEVAR is associated with the complications of conventional aortic surgery, as well as others specific to the endovascular approach. Some of these additional complications can be lifethreatening. Management mandates long-term surveillance and reintervention as needed [44].
Endoleak after TEVAR for dissection ranges from 6 to 26%. Unlike degenerative aneurysms where type II endoleaks are most common, type I endoleaks are most frequent for dissection [44,47,56]. Kim et al. compared the true lumen volume among patients who developed (n = 8) or did not develop (n = 33) endoleak after TEVAR and showed that endoleaks adversely affect remodeling. In the nonendoleak group, increase in true lumen was 29, 51 and 80%, at 1 month, 1 year and 5 years, respectively, compared with the endoleak group in which no change was observed without intervention [56].
It is important to differentiate endoleak from persistent filling of the false lumen. The former is due to poor sealing of the device within the lumen of the aorta, and the latter is related to the limitations of TEVAR in aortic dissections where distal entry tears often remain patent. Persistent retrograde flow and pressurization of the false lumen is not a complication of TEVAR, as much as it is a failure of this technique in its current form to treat dissection in the chronic state [44].
Retrograde ascending aortic dissection is one of the most feared complications after TEVAR. It requires immediate surgical repair and the mortality is as high as 42% [57-59]. Fortunately, it is not too frequent with a reported incidence of 1-3%. As TEVAR use has expanded, this complication is being increasingly recognized [60]. Eggebrecht et al. reported retrograde dissection occurring intraoperatively in 15%, during hospitalization in 21% and at follow-up in 64% of patients [57]. Risk factors include unstable aortic pathology, such as dissection or intramural hematoma, proximal landing zone in the arch or ascending aorta, use of stent grafts with bare springs, disease progression, a diagnosis of connective tissue disease and inadequate conformability or poor approximation of the stent graft with the native aorta [44,57-59].
Patients with connective tissue disorders
Aortic-related events are the major cause of morbidity and mortality in patients with Marfan’s syndrome and other connective tissue disorders. Risk of major aortic-related event, such as dissection or death, is at least 1.3% per year with aortic diameter above 5 cm [61]. Many of these patients will suffer dissection without aneurysm.
Use of TEVAR in patients with connective tissue disorders is a matter of controversy. The majority of these patients are young. The impact of radial force from stent grafts on weakened aortic tissue is unknown and the durability of endovascular repair in these patients is less wellknown than in any other group. Current guidelines recommend against the use of TEVAR, and the preferred treatment in this population is surgical repair [3]. Since the disease is one of continuous degeneration and it usually affects several aortic segments, many of these patients will require multiple interventions. Ultimately, many patients with a dissection and connective tissue disorder require staged replacement of their entire aorta.
The role of TEVAR as primary repair is not well established in patients with distal aortic dissection and connective tissue disease. Only a few descriptive studies have been published and include a limited number of patients who were high risk for open repair. Results were mediocre at best [62-64]. Despite this fact, many aortic surgeons agree that TEVAR options provide an important adjunct to open repair for urgent indications and may prove durable when placed into graft material at the landing zones [65]. Some studies advocate the use of TEVAR for subsequent operations, since the mortality with reoperative open repair is high [66].
Conclusion
Endovascular therapy should be recommended for descending thoracic dissections in select patients. As our understanding of the natural history of dissection after TEVAR evolves, we can expect to see the development of more disease- specific devices and techniques, and broader application of these technologies to more patients with dissection.
Executive summary
Acute descending thoracic dissection
▪ Complicated dissection:
- Several reports of real-world data show good results after thoracic endovascular aneurysm repair (TEVAR) for acute complicated dissection.
- Superiority of TEVAR over open surgery has been demonstrated in nonrandomized studies, especially in patients presenting with malperfusion or rupture.
▪ Uncomplicated dissection:
- Current management is medical therapy and the use of TEVAR in the acute uncomplicated setting is still controversial since comprehensive data are lacking. ADSORB is the first randomized trial comparing medical therapy to TEVAR in this group.
▪ Uncomplicated but high-risk dissection:
- A subset of patients with uncomplicated acute dissection are more prone to late complications. Several studies have addressed some of the potential risk factors. More studies are required to properly define this subset of patients and to investigate the effect of early endovascular therapy in preventing late complications.
Chronic descending aortic dissection
▪ Complicated dissection:
- Recent series of both TEVAR and open therapy for chronic complicated dissection have demonstrated good results. With the current controversy regarding the best approach for these patients, establishment of a prospective multicenter registry could provide a great deal of information about how best to treat these particularly complex patients.
▪ Uncomplicated dissection:
- There is no superiority of the early results of TEVAR over medical therapy in chronic uncomplicated dissection regarding survival, while TEVAR patients show better remodeling. Late results have suggested improved outcome in TEVAR patients. Proper selection of patients for TEVAR is required. Late INSTEAD trial results will be important.
▪ Complications of TEVAR in patients with dissection:
- Despite the less invasiveness and lower risk of TEVAR compared with open repair, TEVAR has potential for serious complications. Proper patients selection and identification of risk factors are required to avoid the complications.
▪ Patients with connective tissue disorders:
- Definitive management with TEVAR for patients with connective disorders is not recommended; however, there may be a role in patients requiring emergency intervention or multiple-staged interventions.
Future perspective
As most proximal entry tears occur in close proximity to the left subclavian artery, it is common for the stent grafts to extend proximally into the aortic arch. This means that the device must lie in a curved position and the procedure commonly requires coverage of the left subclavian artery. Serious complications related to these factors include the occurrence of retrograde dissections and injury to the brain or spinal cord.
Newer stent graft devices to address these shortcomings include those that are more flexible. The CTAG device from Gore Medical (AZ, USA) was redesigned to be more conformable in a curved position. Recent modifications to the delivery systems of both the Zenith (Cook Inc., IN, USA), Valiant™ (Medtronic Inc., CA, USA) and Relay® (Bolton Medical, FL, USA) systems allow for a controlled proximal release to facilitate conformation of the arch to the curve. Several companies are trialing devices with branches to accommodate the left subclavian artery.
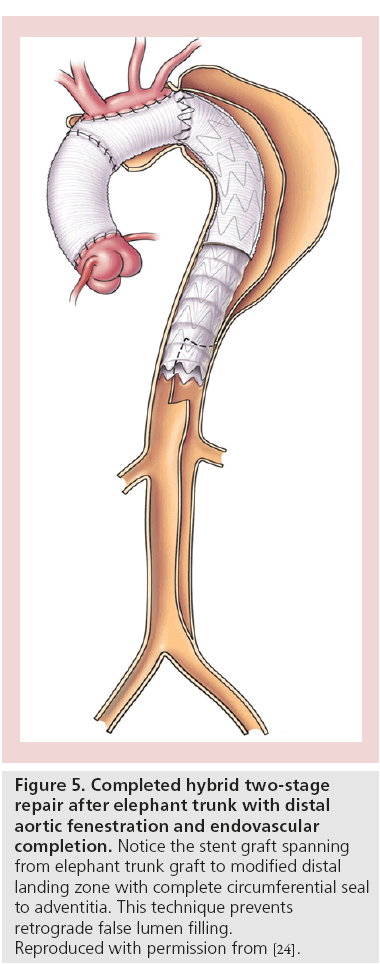
For patients in whom the tear is too proximal to allow for safe endovascular fixation and seal, hybrid options provide for a durable alternative that may be less invasive than a two-stage open repair (Figure 5) [25,65].
Figure 5: Completed hybrid two-stage
repair after elephant trunk with distal
aortic fenestration and endovascular
completion. Notice the stent graft spanning
from elephant trunk graft to modified distal
landing zone with complete circumferential seal
to adventitia. This technique prevents
retrograde false lumen filling.
Reproduced with permission from [24].
To reduce the risk of retrograde false lumen filling and improve the prospect of aortic remodeling, newer techniques have included the use of distal bare stenting in the abdominal aorta, stent grafting into a broadly fenestrated portion of the distal aorta and false lumen embolization [5,24,67,68].
Financial and competing interests disclosure
E Roselli has a consulting agreement with Medtronic Inc., Cook Inc. and Terumo Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Subramanian S, Roselli E. Thoracic aortic dissection: long-term results of endovascular and open repair. Semin. Vasc. Surg. 22(2), 61–68 (2009).
- Hiratzka LF, Bakris GL, Beckman JA et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/ SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Anesth. Analg. 111(2), 279–315 (2010).
- Svensson LG, Kouchoukos NT, Miller DC et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent grafts. Ann. Thorac. Surg. 85(1 Suppl.), S1–S41 (2008).
- Makaroun MS, Dillavou ED, Kee ST et al. Endovascular treatment of thoracic aortic aneurysms: results of the Phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J. Vasc. Surg. 41, 1–9 (2005).
- Brozzi NA, Roselli EE. Endovascular therapy for thoracic aortic aneurysms: state of the art in 2012. Curr. Treat. Options Cardiovasc. Med. 14(2), 149–163 (2012).
- Trimarchi S, Tolenaar JL, Tsai TT et al. Influence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J. Cardiovasc. Surg. (Torino) 53(2), 161–168 (2012).
- Umaña JP, Lai DT, Mitchell RS et al. Is medical therapy still the optimal treatment strategy for patients with acute type B aortic dissections? J. Thorac. Cardiovasc. Surg. 124(5), 896–910 (2002).
- Winnerkvist A, Lockowandt U, Rasmussen E, Radegran K. A prospective study of medically treated acute type B aortic dissection. Eur. J. Vasc. Endovasc. Surg. 32(4), 349–355 (2006).
- Lansman SL, Hagl C, Fink D et al. Acute type B aortic dissection: surgical therapy. Ann. Thorac. Surg. 74(5), S1833–S1835 (2002).
- Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann. Thorac. Surg. 85, 965–971 (2008).
- Trimarchi S, Nienaber CA, Rampoldi V et al. Role and results of surgery in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD). Circulation 4(1 Suppl.), I357–I364 (2006).
- Szeto WY, McGarvey M, Pochettino A et al. Results of a new surgical paradigm: endovascular repair for acute complicated type B aortic dissection. Ann. Thorac. Surg. 86(1), 87–93 (2008).
- White RA, Miller DC, Criado FJ et al. Report on the results of thoracic endovascular aortic repair for acute, complicated, type B aortic dissection at 30 days and 1 year from a multidisciplinary subcommittee of the society for vascular surgery outcomes committee. J. Vasc. Surg. 53(4), 1082–1090 (2011).
- Cambria RP, Crawford RS, Cho JA et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J. Vasc. Surg. 50, 1255–1264 (2009).
- Eggebrecht H, Nienaber CA, Neuhäuser M et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur. Heart J. 27, 489–498 (2006).
- Parker JD, Golledge J. Outcome of endovascular treatment of acute type B aortic dissection. Ann. Thorac. Surg. 86, 1707–1712 (2008).
- Xiong J, Jiang B, Guo W et al. Endovascular stent graft placement in patients with type B aortic dissection: a meta-analysis in China. J. Thorac. Cardiovasc. Surg. 138, 865–872 (2009).
- Fattori R, Tsai TT, Myrmel T et al. Complicated acute type B dissection: is surgery still the best option? A report from the international registry of acute aortic dissection. JACC Cardiovasc. Interv. 1(4), 395–402 (2008).
- Zeeshan A, Woo EY, Bavaria JE et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional open surgical and medical therapy. J. Thorac. Cardiovasc. Surg. 40(Suppl. 6), S109–S115 (2010).
- Luebke T, Brunkwall J. Outcome of patients with open and endovascular repair in acute complicated type B aortic dissection: a systematic review and meta-analysis of case series and comparative studies. J. Cardiovasc. Surg. (Torino) 51(5), 613–632 (2010).
- Zhang H, Wang ZW, Zhou Z et al. Endovascular stent-graft placement or open surgery for the treatment of acute type B aortic dissection: a meta-analysis. Ann. Vasc. Surg. 26(4), 454–461 (2012).
- Patel HJ, Williams DM, Meerkov M et al. Long-term results of percutaneous management of malperfusion in acute type B aortic dissection: implications for thoracic aortic endovascular repair. J. Thorac. Cardiovasc. Surg. 138(2), 300–308 (2009).
- Roselli EE, Rafael A, Soltesz EG, Canale L, Lytle BW. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J. Thorac. Cardiovasc. Surg. 145(3 Suppl.), S197–S201 (2013).
- Roselli EE, Sepulveda E, Pujara AC et al. Distal landing zone open fenestration facilitates endovascular elephant trunk completion and false lumen thrombosis. Ann. Thorac. Surg. 92, 2078–2084 (2011).
- Lima B, Roselli EE, Soltesz EG et al. Modified and ‘reverse’ frozen elephant trunk repairs for extensive disease and complications after stent grafting. Ann. Thorac. Surg. 93, 103–109 (2012).
- Miyahara S, Mukohara N, Fukuzumi M et al. Long-term follow-up of acute type B aortic dissection: ulcer-like projections in thrombosed false lumen play a role in late aortic events. J. Thorac. Cardiovasc. Surg. 142, e25–e31 (2011).
- Uchida N, Katayama A, Tamura K et al. Early entry closure for acute type B aortic dissection by open stent grafting. Gen. Thorac. Cardiovasc. Surg. 59(5), 329–334 (2011).
- Nienaber CA, Zannetti S, Barbieri B et al. INvestigation of STEnt grafts in patients with type B aortic dissection: design of the INSTEAD trial – a prospective, multicenter, European randomized trial. Am. Heart J. 149(4), 592–599 (2005).
- Brunkwall J, Lammer J, Verhoeven E, Taylor P. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur. J. Vasc. Endovasc. Surg. 44(1), 31–36 (2012).
- Lombardi JV, Cambria RP, Nienaber CA et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J. Vasc. Surg. 55(3), 629–640 (2012).
- Akira A, Mochizuki T, Koyama T, Mitsui N. Degree of fusiform dilatation of the proximal descending aorta in type B acute aortic dissection can predict late aortic events. J. Thorac. Cardiovasc. Surg. 134, 1163–1170 (2007).
- Jonker F, Trimarchi S, Rampoldi V et al. Aortic expansion after acute type B aortic dissection. Ann. Thorac. Surg. 94(4), 1223–1229 (2012).
- Chang CP, Liu JC, Liou YM et al. The role of false lumen size in prediction of in-hospital complications after acute type B aortic dissection. J. Am. Coll. Cardiol. 52, 1170–1176 (2008).
- Akutsu K, Nejima J, Kiuchi K et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur. J. Cardiothorac. Surg. 26(2), 359–366 (2004).
- Loewe C, Czerny M, Sodeck G et al. A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes complicated, or remains uncomplicated. Ann. Thorac. Surg. 93, 1215–1222 (2012).
- Dziodzio T, Juraszek A, Reineke D et al. Experimental acute type B aortic dissection: different sites of primary entry tears cause different ways of propagation. Ann. Thorac. Surg. 91, 724–727 (2011).
- Karmonik C, Bismuth J, Shah DJ et al. Computational study of haemodynamic effects of entry-and exit-tear coverage in a DeBakey type III aortic dissection: technical report. Eur. J. Vasc. Endovasc. Surg. 42(2), 172–177 (2011).
- Augoustides JG, Szeto WY, Woo EY et al. The complications of uncomplicated acute type-B dissection: the introduction of the penn classification. J. Cardiothorac. Vasc. Anesth. 26(6), 1139–1144 (2012).
- Sayer D, Bratby M, Brooks M et al. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur. J. Vasc. Endovasc. Surg. 36(5), 522–529 (2008).
- Svensson LG, Crawford ES, Hess KR et al. Dissection of the aorta and dissecting aortic aneurysms. Improving early and long-term surgical results. Circulation 82(5 Suppl.), S24–S38 (1990).
- Patel HJ, Shillingford MS, Williams DM et al. Survival benefit of endovascular descending thoracic aortic repair for the high-risk patient. Ann. Thorac. Surg. 83(5), 1628–1633 (2007).
- Grabenwoger M, Alfonso F, Bachet J et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Society of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 33, 1558–1563 (2012).
- Pujara AC, Roselli EE, Hernandez AV et al. Open repair of chronic distal aortic dissection in the endovascular era: implications for disease management. J. Thorac. Cardiovasc. Surg. 144(4), 866–873 (2012).
- Kang WC, Greenberg RK, Mastracci TM et al. Endovascular repair of complicated chronic distal aortic dissections: intermediate outcomes and complications. J. Thorac. Cardiovasc. Surg. 142(5), 1074–1083 (2011).
- Sachs T, Pomposelli F, Hagberg R et al. Open and endovascular repair of type B aortic dissection in the nationwide inpatient sample. J. Vasc. Surg. 52(4), 860–866 (2010).
- Brunt ME, Egorova NN, Moskowitz AJ. Propensity score-matched analysis of open surgical and endovascular repair for type B aortic dissection. Int. J. Vasc. Med. 3640–3646 (2011).
- Thrumurthy SG, Karthikesalingam A, Patterson BO et al. A systematic review of mid-term outcomes of thoracic endovascular repair (TEVAR) of chronic type B aortic dissection. Eur. J. Vasc. Endovasc. Surg. 42(5), 632–647 (2011).
- Stanley GA, Murphy EH, Knowles M et al. Volumetric analysis of type B aortic dissections treated with thoracic endovascular aortic repair. J. Vasc. Surg. 54(4), 985–992 (2011).
- Kusagawa H, Shimono T, Ishida M. Changes in false lumen after transluminal stent-graft placement in aortic dissections: six years’ experience. Circulation 111(22), 2951–2957 (2005).
- Resch TA, Delle M, Falkenberg M et al. Remodeling of the thoracic aorta after stent grafting of type B dissection: a Swedish multicenter study. J. Cardiovasc. Surg. 47(5), 503–508 (2006).
- Schoder M, Czerny M, Cejna M. Endovascular repair of acute type B aortic dissection: long-term follow-up of true and false lumen diameter changes. Ann. Thorac. Surg. 83(3), 1059–1066 (2007).
- Blount KJ, Hagspiel KD. Aortic diameter, true lumen, and false lumen growth rates in chronic type B aortic dissection. Am. J. Roentgenol. 192(5), 222–229 (2009).
- Andacheh ID, Donayre C, Othman F et al. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J. Vasc. Surg. 56(3), 644–650 (2012).
- Mani K, Clough RE, Lyons OT et al. Predictors of outcome after endvascular repair for chronic type B dissection. Eur. J. Vasc. Endovasc. Surg. 43(4), 386–391 (2012).
- Ulug P, McCaslin JE, Stansby G, Powell JT. Endovascular versus conventional medical treatment for uncomplicated chronic type B aortic dissection. Cochrane Database Syst. Rev. 14(11), CD006512 (2012).
- Kim KM, Donayre CE, Reynolds TS et al. Aortic remodeling, volumetric analysis, and clinical outcomes of endoluminal exclusion of acute complicated type B thoracic aortic dissections. J. Vasc. Surg. 54(2), 316–324 (2011).
- Eggebrecht H, Thompson M, Rousseau H et al. Retrograde ascending aortic dissection during or after thoracic aortic stent graft placement: insight from the European registry on endovascular aortic repair complications. Circulation 120(11 Suppl.), S276–S281 (2009).
- Williams JB, Andersen ND, Bhattacharya SD et al. Retrograde ascending aortic dissection as an early complication of thoracic endovascular aortic repair. J. Vasc. Surg. 55(5), 1255–1262 (2012).
- Dong ZH, Fu WG, Wang YQ et al. Retrograde type A aortic dissection after endovascular stent graft placement for treatment of type B dissection. Circulation 119(5), 735–741 (2009).
- Idrees J, Arafat A, Johnston D, Svensson L, Roselli EE. Repair of retrograde ascending dissection after descending stent grafting. doi:10.1016/j.jtcvs.2013.08.075 2013 (2013) (Epub ahead of print).
- Jondeau G, Detaint D, Tubach F et al. Aortic event rate in the Marfan population: a cohort study. Circulation 125(2), 226–232 (2012).
- Marcheix B, Rousseau H, Bongard V et al. Stent grafting of dissected descending aorta in patients with Marfan syndrome: mid-term results. JACC Cardiovasc. Interv. 1(6), 673–680 (2008).
- Geisbüsch P, Kotelis D, von Tengg-Kobligk H et al. Thoracic aortic endografting in patients with connective tissue diseases. J. Endovasc. Ther. 15, 144–149 (2008).
- Waterman AL, Feezor RJ, Lee WA et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J. Vasc. Surg. 55(5), 1234–1240 (2012).
- Aortic Surgery Symposium 2012 discussions – panel 3 (sessions V and VI): descending thoracic aorta, thoracoabdominal aorta, and trauma. J. Thorac. Cardiovasc. Surg. 145(3 Suppl.), S159–S164 (2013).
- Akin I, Kische S, Rehders TC et al. Current role of endovascular therapy in Marfan patients with previous aortic surgery. Vasc. Health Risk Manag. 4, 59–66 (2008).
- Roselli E. Commentary: hybrid modifications to the aorta provide a more complete endovascular treatment for chronic dissection. J. Endovasc. Ther. 20(1), 32–33 (2013).
- Melissano G, Bertoglio L, Rinaldi E et al. Volume changes in aortic true and false lumen after the ‘PETTICOAT’ procedure for type B aortic dissection. J. Vasc. Surg. 55(3), 641–651 (2012).
- Tsai T, Schlicht M, Khanafer K et al. Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. J. Vasc. Surg. 47, 844–851 (2008).
- Onitsuka S, Akashi H, Tayama K et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann. Thorac. Surg. 78, 1268–1273 (2004).
- Tefera G, Acher CW, Hoch JR et al. Effectiveness of intensive medical therapy in type B aortic dissection: a single-center experience. J. Vasc. Surg. 45(6), 1114–1118 (2007).
- Song JM, Kim SD, Kim JH et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J. Am. Coll. Cardiol. 50(8), 799–804 (2007).
- Bernard Y, Zimmermann H, Chocron S et al. False lumen patency as a predictor of late outcome in aortic dissection. Am. J. Cardiol. 87, 1378–1382 (2001).
▪ Describes the current recommendations for the management of thoracic aortic disease.
▪▪ Describes the current recommendations for the management of thoracic aortic disease, with a focus on endovascular therapy.
▪▪ Describes the current recommendations for the management of thoracic aortic disease, with a focus on endovascular therapy.
▪▪ First randomized trial comparing medical and endovascular therapy in chronic uncomplicated type B dissection.
▪▪ Description of the trial design for the first randomized trial comparing medical and endovascular therapy in acute uncomplicated type B dissection.
▪ Recent study in the era of endovascular therapy showing the results of open repair of chronic distal aortic dissection in patients anatomically fit to undergo endovascular therapy.
▪▪ Propensity score-matched analysis in nationwide sample comparing open and endovascular repair for chronic type B dissection.