Review Article - Interventional Cardiology (2015) Volume 7, Issue 2
Role, risk and benefit of interventional cardiology procedures during pregnancy
- Corresponding Author:
- Patrizia Presbitero
Humanitas Clinical & Research Institute, Rozzano
Milan, Italy
E-mail: patrizia.presbitero@humanitas.it
Abstract
Pregnancy is associated with significant hemodynamic, tissue and hematic changes in the maternal cardiovascular system, which could result in worsening some previously asymptomatic cardiac conditions. Since the last 30 years, new techniques and devices have been introduced in the field of structural and coronary heart disease. In this review, we describe the balance between the risk and benefit of percutaneous therapy and the possibility of transcatheter approach to the most common heart disease during pregnancy.
Keywords
Cardiac intervention, fetus, percutaneous, pregnancy, radiation, transcatheter
Cardiovascular diseases complicate 0.2–4.0% of all pregnancy and this rate is increasing in western countries [1]. When cardiac disease occurs during pregnancy or peripartum period, the mortality rate can reach up to 10% especially if the mother requires intensive care assistance [2].
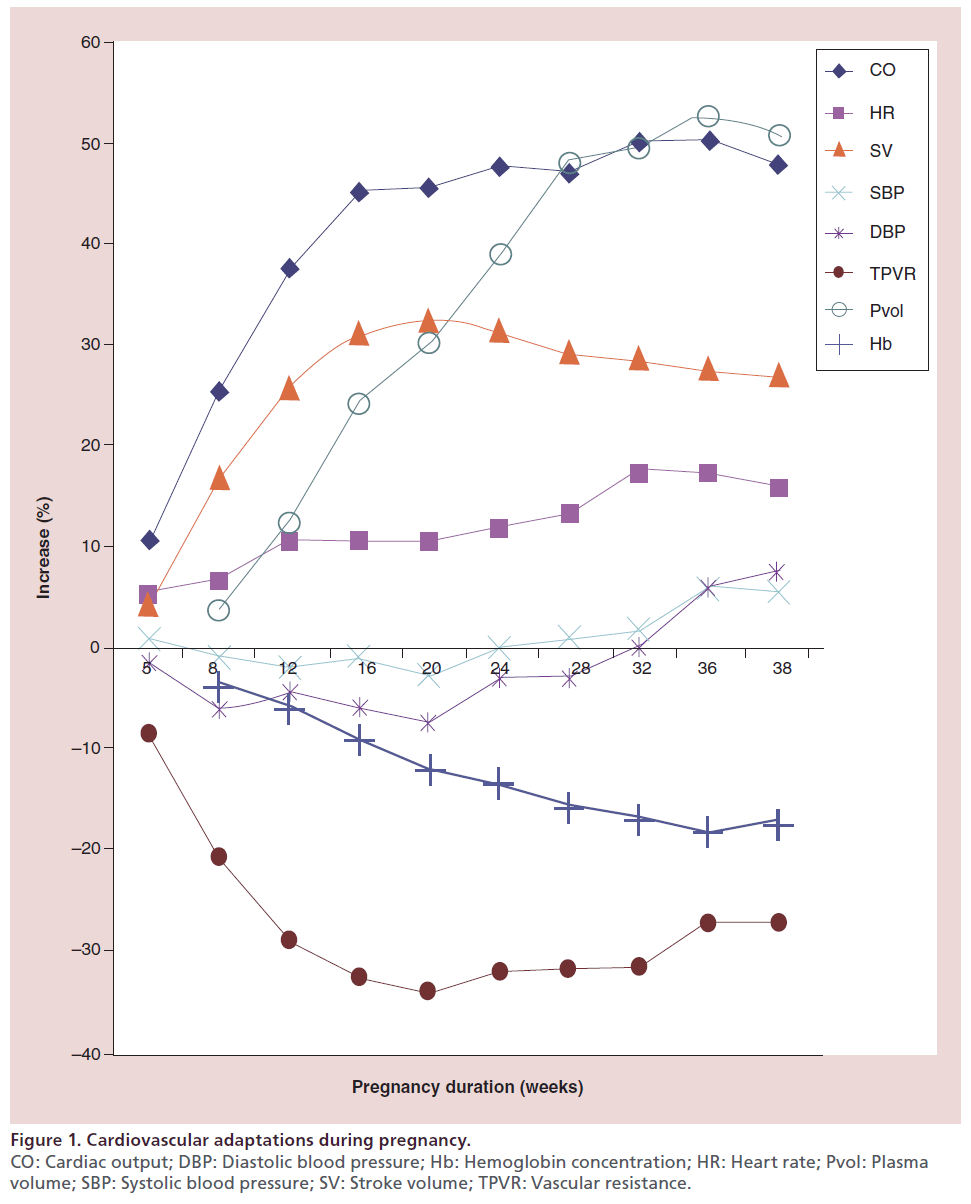
Gestational period is associated with significant hemodynamic changes in the maternal cardiovascular system, which increases the work load of the heart [3]. Figure 1 & Table 1 summarize cardiovascular adaptations according to gestational periods. As a result of these hemodynamic changes, an asymptomatic patient with cardiovascular disease who is well in the nonpregnant state may develop cardiac failure as a result of increased cardiac output.
Figure 1: Cardiovascular adaptations during pregnancy. CO: Cardiac output; DBP: Diastolic blood pressure; Hb: Hemoglobin concentration; HR: Heart rate; Pvol: Plasma volume; SBP: Systolic blood pressure; SV: Stroke volume; TPVR: Vascular resistance.
Over the last 25 years, interventional cardiology has emerged as a new therapeutic tool and as an effective alternative to surgical therapy in several cardiac diseases [4]. Even though interventional cardiology, particularly mitral valvuloplasty, has been performed in the pregnant patient over the last two decades, randomized prospective study are absent and guidelines are mainly based on experts’ consensus [1].
The treatment of pregnant women should take care of two subjects: the mother and the fetus. The possible adverse effects on the fetus of diagnostic examination and treatments should be always considered. After delivery, the possible drug interactions on breastfeeding should be taken in account. Interventional procedure could represent an invasive low-risk strategy compared with cardiac surgery which is related to 50% of fetal lost and about 10% of maternal mortality [5].
This article provides an overview on the risk–benefit ratio of the interventional treatment: first, balancing the radiation exposure risk and the event risk related to specific cardiac condition; and second, the role of percutaneous intervention in the most common cardiac disease during pregnancy.
Fetus risk during mother cardiac catheterization
Fetal risk during cardiac catheterization in pregnant patients is related to radiation exposure and contrast media. The effect of radiation on the fetus depends on two factors: the maternal radiation dose and the gestational age. With regard to the first, the safe threshold of radiation dose is below 50 mGy, while there is an uncertain risk of malformation between 50 and 100 mGy, and a certain increased risk above a dose of 100 mGy [6,7]. The majority of diagnostic procedures includ-ing cardiac catheterization do not expose the fetus to such high levels of radiation. Percutaneous interventional procedures imply more radiation exposure but always within those limits (Table 1).
| Procedure | Fetal exposure (mGy) | Maternal exposure (mGy) |
|---|---|---|
| Chest | <0.01 | 0.1 |
| radiograph | ||
| CT chest | 0.3 | 7 |
| Coronary | 1.5 | 7 |
| angiography | ||
| PCI | 3 | 15 |
Table 1. Fetal and maternal radiological doses for different procedures.
As far as gestational age concerned, the effect of radiation during pregnancy can be divided into three phases: preimplantation period (1–2 weeks), during this stage the consequence of irradiation has only two outcomes: loss of embryo or healthy birth, following the rule of ‘all-or-nothing’; organogenesis period (2–12 weeks), during this time radiation can cause congenital malformations, growth restriction and intellectual disability (for this reason, it has been suggested to delay radiation exposure until after completion of organogenesis); and second and third trimester, in this period radiation risk is primarily related to the development of childhood leukemia and other malignancies.
As general rule of each cardiac catheterization during pregnancy, a radial approach, a very low frame rate and time of fluoroscopy (up to 2 fps), a small collimated beam sizes, a minimal angulation of x-ray source and echo-guided procedure, when feasible as balloon valvuloplasty, will minimize the radiation exposure [8]. In some specific case, lowering the radiation exposure has to be balance with sufficient quality of imaging. The use of pelvic and abdominal shield could reduce the fetal exposure; however, the incorrect or inadequate position of the shield could even increase uterus exposure. For this reason, the recommendation to shield the abdomen is controversial during pregnancy.
Concerning the risk secondary to the use of iodine contrast media, this is due to a possible fetal hypothyroidism, after 25 weeks when the fetal thyroid becomes active.
The ideal time for performing interventional procedures is considered the fourth month, when organogenesis is completed, the fetal thyroid is still inactive, the risk of cancer is lower than in the third trimester and there is a greater distance between the fetus and the chest than in later months [1,9]. Moreover, another reason to intervene in the second trimester if possible is the lower risk of provoking labor than during the third trimester.
Mother risk
Although the treatment of pregnant women should take care of mother and fetus, their relationship changes during gestation in close connection with fetal vitality. In the early stages of pregnancy, since the fetus is not vital, the achievement of good clinical conditions in the mother is the only way to guarantee possible fetal survival. When the fetus is vital, especially during the last 2 months of pregnancy, an early delivery should always be considered because the survival rate of both is higher if the fetus is outside, rather than inside a critically ill mother [3].
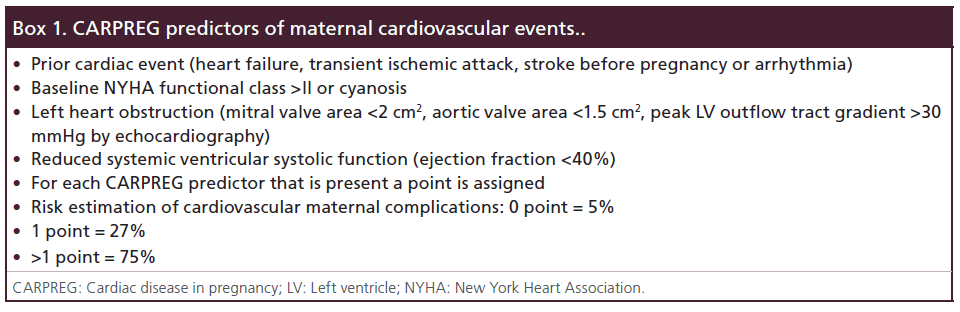
In order to assess the risk of maternal cardiac events, several risk scores have been reported, whose Cardiac Disease in Pregnancy (CARPREG) risk score is the most known and used [10]. This score has been developed and validated in several studies, and it predicts quite accurately the rate of cardiovascular events, though overestimation can occur (Box 1) [11,12]. Beside the CARPREG risk score, an experts task force defined the ‘modified WHO risk classification’, which represents a more comprehensive scoring system, including all known maternal cardiovascular risk factors, underlying heart disease, any other comorbidity and contraindications for pregnancy. WHO risk classification is not simple to handle due to his complexity; however, it fits better to each specific cardiac disease during pregnancy [13].
Box 1. CARPREG predictors of maternal cardiovascular events..
Mitral stenosis
Mitral stenosis, commonly rheumatic, remains a major problem in developing countries and is still present in western countries, especially in immigrants [14,15].
Valvular gradients may increase during pregnancy because of the physiological increase in cardiac output and heart rate. Cardiac output, because of fixed obstruction, is unable to increase during effort, leading to increase of atrial and pulmonary pressure. Even women with moderate mitral stenosis may become severely symptomatic during the second and third trimester of pregnancy. This risk remains during labor and delivery, as a result of uterine contraction and with the increasing of venous return from the limb, as the emptied uterus is no longer blocking venous return.
Percutaneous balloon mitral valvotomy during pregnancy should be considered in patients with symptomatic (New York Heart Association class III/IV) moderate- to-severe mitral stenosis or systolic pulmonary pressure greater than 50 mmHg, despite optimal medical treatment [1]. Surgical valve replacement or commissurotomy is generally not necessary, because young women often present with noncalcified valves without subvalvular thickening or significant mitral regurgitation, suitable for percutaneous approach [16]. Percutaneous balloon valvotomy, since the initial description by Inoue in 1984 [17], has been shown to be successful in large studies of patients with symptomatic mitral stenosis [18]. Dilatation of the stenotic mitral valve results in immediate hemodynamic improvement. The mitral gradient generally decreases from 33 to 50% of its initial value, and the cross-sectional area almost doubles [19]. Both pulmonary capillary wedge pressure and pulmonary artery pressure decrease immediately, with the latter dropping further the week after valvuloplasty [20]. There are some potential complications associated with this procedure, including atrial perforation, cardiac tamponade, arrhythmias, emboli and mitral regurgitation. Mortality has been reported to be around 0.5% [4]. Mitral regurgitation is the most common complication and varies from 0 to 50%; however, severe mitral regurgitation is uncommon and can occur when the valve is heavily calcified [21]. Currently, 3D transesophageal or even transthoracic ultrasound, when a good echo window is achievable, can be used to guide percutaneous balloon valvuloplasty in order to minimize radiation exposure [22]. Balloon mitral valvuloplasty in the pregnant patient is a technically complex procedure that needs to be done quickly with a surgical backup and should only be performed in center with an extensive experience in this field.
In the largest series reported a procedural success was achieved in 100% of cases, fetal loss is extremely rare and pregnancy was accomplished in 100% of cases. Mid- and long-term follow-up has been shown favorable for both mother and child [23,24].
Aortic stenosis
Severe aortic stenosis is rare during pregnancy [25]. Nevertheless, even in moderate aortic stenosis, during pregnancy, aortic gradient can double and patient can become symptomatic for hypotension, syncope, angina or EKG can show signs of left ventricular hypertrophy or ischemia.
When symptoms occur in optimal medical therapy, valve repair or replacement should be attempted either by percutaneous balloon valvuloplasty or surgery. Percutaneous balloon valve valvuloplasty has been performed in AS, obtaining reduction of transvalvular aortic gradient and acute clinical improvement. Mild undersized balloon-anulus or maximum 1:1 ratio has been used in order to avoid aortic regurgitation. In general, it is sufficient to halve the gradient in order to obtain a significant clinical improvement, which allows ending the pregnancy. Transient maternal hypotension and a decrease in fetal heart rate may occur because of balloon inflation or prolonged supine position and can be treated with crystalloid infusion. Postprocedural hemodynamic results are not sustained after ballooning; moreover, even though aortic balloon angioplasty enables the pregnancy to continue, the patient has to be followed up strictly for a possible setback of the valvular disease [26]. Aortic balloon valvuloplasty should be performed in hospital where transcatheter aortic valve implantation in available as backup strategy. No cases of transcatheter aortic valve implantation have been reported so far, but it can be an option in specific cases, and a recent percutaneous implantation of pulmonary valve has demonstrated the feasibility of transcatheter valve treatment during pregnancy [27].
Myocardial infarction during pregnancy
Acute coronary syndrome occurs in 3–10 of 100,000 deliveries, with a peak of incidence during the third trimester and in the puerperium, with mortality rates ranging from 5 to 7% in the mother and 13 to 17% in the fetus [28,29]. The increasing age of women having babies together with changes in lifestyle and a higher prevalence of obesity and the hypercoagulable state of pregnancy, may also explain the increasing rates of acute myocardial infarction (AMI) observed in the last decade during pregnancy [30,31]. An underlying atherosclerotic disease is present in only half of cases. The other cause of AMI is spontaneous coronary dissection, probably secondary to hormonerelated changes in composition of vessel walls and paradoxical thromboembolism due to the physiological increased right-to-left shunting through a PFO or atrial septal defects (ASD). These pathogenetic mechanisms do not allow adequate development of compensatory coronary collateral circulation, with subsequent large size infractions and evolution toward dilated cardiomyopathy. Left anterior descending artery is the vessel most frequently involved [30]. Urgent percutaneous revascularization has to be considered the treatment of choice if an AMI is ongoing; however, the interventional approach to this syndrome has to be cautious because of the arteries of these patients are friable. Because the propagation of dissection occurs very frequently during percutaneous coronary intervention, it is very important to place the guide-wire in the true lumen very distally, then to check with echo or OCT the position of the wire in the true lumen and finally to stent all dissected segments; sometimes also diagonal and marginal brunch can be dissected; in this case, a conservative approach can be considered if a small peripheral vessel is involved and symptoms are well controlled by medical therapy. Surgery has to be considered only if complication of percutaneous coronary intervention occurs, as well as wire into the false lumen and propagation of dissection to the aorta.
A radial access and abdominal shielding may reduce fetal radiation exposure. Furthermore, the use of contrast should be limited to avoid fetal dysthyroidism. A major issue is the management of dual antiplatelet therapy, when coronary stents are implanted. Aspirin is considered safe but data regarding the use of dual antiplatelet therapy are scant. Case reports show no adverse outcomes relating to either clopidogrel or prasugrel [32,33]. Experiences with thrombolysis in this subset are scarce, but data from its use in pulmonary embolism report major complications in up to 15% of cases with a 6% of fetal loss [34,35].
Peripartum cardiomyopathy
Peripartum cardiomyopathy (PPC) is defined as an idiopathic cardiomyopathy presenting with heart failure secondary to systolic dysfunction toward the end of pregnancy or in the months following delivery, where no other cause of heart failure is found [36]. The true PPC incidence is unknown, but it is estimated in 1 of 3000–4000 live births in western countries [37,38]. It occurs in the last month of pregnancy in 10% of patients, after delivery in 80% of patients, and either in the last month antepartum and during the 5 months postpartum in the remaining 10%. Although PPC remains a diagnosis of exclusion, several underlying etiological mechanisms have been proposed, such as inflammation- oxidative stress-mediated mechanisms and the nursing- hormone prolactin seem to be involved [39–42].
Clinical diagnosis is based on development of heart failure signs in absence of other causes of heart failure and lack of evidence of heart disease before the last month of pregnancy. Although recent data have shown good outcome at 6 months with left ventricle function improvement in 85% of the cases [43], prognosis depends on left ventricular function recovery with mortality rates up to 25% of cases in patients with persistent low EF [38]. It must be stressed that natural history may be characterized by a sudden deterioration of left ventricular function with acute cardiac failure and shock. Therefore, patients with PPC require hospitalization in referral center where Intensive Care Unit and Cardio-Thoracic Surgery Unit are available. When heart failure is refractory to medical treatment, intra-aortic balloon pump, insertion of a left ventricular assist device, and ECMO may be performed as a bridge to recovery, in most cases, or to heart transplantation [44,45]. Recently, a successful percutaneous left ventricular assistance with Impella 2.5 microaxial pump (Abiomed, Inc.) was reported; however, this patient was complicated by device thrombosis after 5 days of treatment, underlying the necessity of adequate anticoagulation regimen during this period [46]. Currently, Impella CP (Abiomed, Inc., Danvers, MA, USA) is available for percutaneous use (needing 14-Fr sheath), and it represents a new device available in our equipment for treatment of severe heart failure patients.
Atrial septal defects
Atrial septal defects are the most frequent congenital cardiac defect of adulthood and they are most common in women than in men. For these reason, it is not uncommon to detect an ASD during pregnancy. However, pregnancy is well tolerated by most women with an ASD. The decreasing in systemic vascular resistance counterbalances the increasing in right ventricle volume-load, remaining the hemodynamic condition stable. When ASD is hemodynamically significant, a percutaneous closure should be performed before pregnancy [1]. A closure of ASD or of persistent foramen can be considered in case of paradoxical embolism during pregnancy, particularly if deep venous thrombosis is present despite medical treatment. The percutaneous procedure can be performed under the guidance of intracardiac echocardiography and limited fluoroscopy. Few successful cases have been previously reported, the patient have been treated safely and effectively under local anesthesia using intracardiac echocardiography and with trivial fetal radiation exposure [47,48].
Conclusion
There are several cardiac conditions that can benefit from transcatheter interventions during pregnancy. Since the last 30 years new techniques and devices have been introduced in the field of structural and coronary heart disease. All these innovations can increase the success rate of severe condition during pregnancy, as well as mitral and aortic stenosis, coronary dissection and severe left ventricle failure. Furthermore, bi- and tridimensional transesophageal echocardiography and radial approach can minimize the amount of fetal radiation exposure.
However, it has to be pointed out that such procedures need to be evaluated in high volume centers and they should be performed only in presence of lifethreatening situations by teams with high experience in pregnancy disease.
Future perspective
In the next 10 years, transcatheter technology development will lead to minimize and bioabsorbable devices, which allow to treat more and more conditions currently unapproachable due to anatomical limitation in both mother and fetus. The raising of echo use during interventionalist procedure is making the treatment not harmful for fetal malformation.
There are several cardiac conditions that can benefit from transcatheter interventions during pregnancy. Since the last 30 years new techniques and devices have been introduced in the field of structural and coronary heart disease.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
• Cardiovascular diseases complicate 0.2–4.0% of all pregnancy.
• The treatment of pregnant women should take care of two subjects: the mother and the fetus.
• The ideal time for performing interventional procedures is considered the fourth month.
• In order to assess the risk of maternal cardiac events, cardiac disease in pregnancy risk score is the most known and used.
• Mitral stenosis remains a major problem in developing countries and is still present in western countries, especially in immigrants.
• Percutaneous balloon mitral valvotomy during pregnancy should be considered in patients with symptomatic moderate to severe mitral stenosis or systolic pulmonary pressure greater than 50 mmHg.
• In the largest series reported a procedural success was achieved in 100% of cases, fetal loss is extremely rare and pregnancy was accomplished in 100% of cases.
• Acute coronary syndrome occurs in 3–10 of 100,000 deliveries with mortality rates ranging from 5 to 7% in the mother and 13 to 17% in the fetus.
• An underlying atherosclerotic disease is present in only half of cases. The other cause of acute myocardial infarction is spontaneous coronary dissection or paradoxical thromboembolism.
• Urgent percutaneous revascularization has to be considered the treatment of choice if an acute myocardial infarction is ongoing.
• The peripartum cardiomyopathy incidence is estimated in 1 of 3000–4000 live births.
• Clinical diagnosis is based on heart failure signs in absence of other causes of heart failure and lack of evidence of heart disease before the last month of pregnancy.
• Recent data have shown good outcome at 6 months with left ventricle function improvement in 85% of the cases.
• Prognosis depends on left ventricular function recovery with mortality rates up to 25% of cases in patients with persistent low EF.
• Percutaneous left ventricular assistance with Impella 2.5 micro-axial pump can be considered as bridge to left ventricle recovery or transplantation.
• Atrial septal defects (ASD) are the most frequent congenital cardiac defect during pregnancy.
• When ASD is hemodynamically significant, a percutaneous closure should be performed before pregnancy. A closure of ASD or of persistent foramen can be considered in case of paradoxical embolism during pregnancy.
References
- European Societyof Gynecology (ESG), Association forEuropean Paediatric Cardiology (AEPC), German Societyfor Gender Medicine (DGesGM) et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 32(24), 3147–3197 (2011).
- Al-Suleiman SA, Qutub HO, Rahman J, Rahman MS. Obstetric admissions to the intensive care unit: a 12-year review. Arch. Gynecol. Obstet. 274(1), 4–8 (2006).
- Presbitero P, Zavalloni D, Agnoli B, Tubaro M. Cardiac emergency in pregnancy. In: The ESC Textbook of Intensive and Acute Cardiac Care. Oxford University Press, NY,USA, 625–636 (2011).
- Presbitero P, Prever SB, Brusca A. Interventional cardiology in pregnancy. Eur. Heart J. 17(2), 182–188 (1996).
- Elassy SM, Elmidany AA, Elbawab H. Urgent cardiac surgery during pregnancy: a continuous challenge. Ann. Thorac. Surg. 97(5), 1624–1629 (2014).
- Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin. Oncol. 16(5), 347–368 (1989).
- ACOG Committee Opinion. Number 299, September 2004 (replaces No. 158, September 1995). Guidelines for diagnostic imaging during pregnancy. Obstet. Gynecol. 104(3), 647–651 (2004).
- Orchard E, Dix S, Wilson N, Mackillop L, Ormerod O. Reducing ionizing radiation doses during cardiac interventions in pregnant women. Obstet. Med. 5(3), 108–111 (2012).
- Timins JK. Radiation during pregnancy. N. J. Med. 98(6), 29–33 (2001).
- Siu SC, Sermer M, Colman JM et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation 104(5), 515–552 (2001).
- Drenthen W, Boersma E, Balci A et al. Predictors of pregnancy complications in women with congenital heart disease. Eur. Heart J. 31(17), 2124–2132 (2010).
- Jastrow N, Meyer P, Khairy P et al. Prediction of complications in pregnant women with cardiac diseases referred to a tertiary center. Int. J. Cardiol. 151(2), 209–213 (2011).
- Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart 92(10), 1520–1525 (2006).
- Iung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 24(13), 1231–1243 (2003).
- Ralph AP, Carapetis JR. Group a streptococcal diseases and their global burden. Curr. Top Microbiol. Immunol. 368, 1–27 (2013).
- Block PC. Who is suitable for percutaneous balloon mitral valvotomy? Int. J. Cardiol. 20(1), 9–14 (1988).
- Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J. Thorac. Cardiovasc. Surg. 87(3), 394–402 (1984).
- Nobuyoshi M, Arita T, Shirai S et al. Percutaneous balloon mitral valvuloplasty: a review. Circulation 119(8), E211–E219 (2009).
- Hung JS, Chern MS et al. Short- and long-term results of catheter balloon percutaneous transvenous mitral commissurotomy. Am. J. Cardiol. 67(9), 854–862 (1991).
- Dev V, Shrivastava S. Time course of changes in pulmonary vascular resistance and the mechanism of regression of pulmonary arterial hypertension after balloon mitral valvuloplasty. Am. J. Cardiol. 67(5), 439–442 (1991).
- Patel J, Vythilingum S, Mitha A. Balloon dilatation of the mitral valve by a single bifoil (2 x 19 mm) or trefoil (3 x 15 mm) catheter. Br. Heart J. 64(5), 342–346 (1990).
- Wunderlich NC, Beigel R, Siegel R. The role of echocardiography during mitral valve percutaneous interventions. Cardiol. Clin. 31(2), 237–270 (2013).
- Gamra H, Ben-Farhat M, Betbout F et al. Long term outcome of balloon mitral commissurotomy during pregnancy: a prospective physical and mental evaluation of babies. EuroIntervention 2(3), 302–309 (2006).
- Esteves CA, Munoz JS, Braga S et al. Immediate and long-term follow-up of percutaneous balloon mitral valvuloplasty in pregnant patients with rheumatic mitral stenosis. Am.J. Cardiol. 98(6), 812–816 (2006).
- Rapaport E. Natural history of aortic and mitral valve disease. Am. J. Cardiol. 35(2), 221–227 (1975).
- Banning AP, Pearson JF, Hall RJ. Role of balloon dilatation of the aortic valve in pregnant patients with severe aortic stenosis. Br. Heart J. 70(6), 544–545 (1993).
- Ormerod O, Newton JD, Westaby S, Wilson N. Percutaneous pulmonary valve replacement during pregnancy. Circulation 129(10), E373–E375 (2014).
- James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation 113(12), 1564–1571 (2006).
- Ladner HE, Danielsen B, Gilbert W. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet. Gynecol. 105(3), 480–484 (2005).
- Roth A, Elkayam U. Acute myocardial infarction associated with pregnancy. J. Am. Coll. Cardiol. 52(3), 171–180 (2008).
- Smith RL, Young SJ, Greer IA. The parturient with coronary heart disease. Int. J. Obstet. Anesth. 17(1), 46–52 (2008).
- Janion-Sadowska A, Sadowski M, Kurzawski J, Zandecki L, Janion M. Pregnancy after acute coronary syndrome: a proposal for patients’ management and a literature review. Biomed. Res. Int. doi:1155/2013/957027 (2013) (Epub ahead of print).
- Tello-Montoliu A, Seecheran NA, Angiolillo DJ. Successful pregnancy and delivery on prasugrel treatment: considerations for the use of dual antiplatelet therapy during pregnancy in clinical practice. J. Thromb. Thrombolysis 36(3), 348–351 (2013).
- Ahearn GS, Hadjiliadis D, Govert JA, Tapson VF. Massive pulmonary embolism during pregnancy successfully treated with recombinant tissue plasminogen activator: a case report and review of treatment options. Arch. Intern. Med. 162(11), 1221–1227 (2002).
- Bates SM, Greer IA, Pabinger I et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(Suppl. 6), S844–S886 (2008).
- Karaye KM, Henein MY. Peripartum cardiomyopathy: a review article. Int. J. Cardiol. 164(1), 33–38 (2013).
- Baughman KL. Peripartum cardiomyopathy. Curr. Treat. Options Cardiovasc. Med. 3(6), 469–480 (2001).
- Pearson GD, Veille JC, Rahimtoola S et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA 283(9), 1183–1188 (2000).
- Melvin KR, Richardson PJ, Olsen EG, Daly K, Jackson G. Peripartum cardiomyopathy due to myocarditis. N. Engl. J. Med. 307(12), 731–734 (1982).
- Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, Sundstrom JB. Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin. Rev. Allergy Immunol. 23(3), 301–324 (2002).
- Sliwa K, Blauwet L, Tibazarwa K et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 121(13), 1465–1473 (2010).
- Fett JD. Peripartum cardiomyopathy: a puzzle closer to solution. World J. Cardiol. 6(3), 87–99 (2014).
- Haghikia A, Podewski E, Libhaber E et al. Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res.Cardiol. 108(4), 366 (2013).
- Heider AL, Kuller JA, Strauss RA, Wells SR. Peripartum cardiomyopathy: a review of the literature. Obstet. Gynecol. Surv. 54(8), 526–531 (1999).
- Keogh A, Macdonald P, Spratt P, Marshman D, Larbalestier R, Kaan A. Outcome in peripartum cardiomyopathy after heart transplantation. J. Heart Lung Transplant. 13(2), 202–207 (1994).
- Schroeter MR, Unsöld B, Holke K, Schillinger W. Pro-thrombotic condition in a woman with peripartum cardiomyopathy treated with bromocriptine and an Impella LP 2.5 heart pump. Clin. Res. Cardiol. 102(2), 155–157 (2013).
- Li Y, Margraf J, Kluck B, Jenny D, Castaldo J. Thrombolytic therapy for ischemic stroke secondary to paradoxical embolism in pregnancy: a case report and literature review. Neurologist 18(1), 44–48 (2012).
- Schrale RG, Ormerod J, Ormerod OJ. Percutaneous device closure of the patent foramen ovale during pregnancy. Catheter Cardiovasc. Interv. 69(4), 579–583 (2007).