Review Article - Interventional Cardiology (2009) Volume 1, Issue 2
Role of filter design in embolic protection during carotid artery stenting
- Corresponding Author:
- Sumaira Macdonald
Consultant Vascular Radiologist & Honorary Clinical Senior Lecturer
Freeman Hospital, Newcastle-Upon-Tyne, NE7 7DN, UK
Tel: +44 191 223 1120
Fax: +44 191 223 1168
E-mail: sumaira.macdonald@ nuth.nhs.uk
Abstract
Keywords
carotid stenting, cerebral protection, filter
Recent randomized trials comparing carotid artery stenting (CAS) with carotid endarterectomy (CEA) in symptomatic patients have had mixed 30‑day (safety) results [1,2]. What is clear, however, is that survival free of ipsilateral stroke for both CAS and CEA within these trials in the intermediate term is comparable, suggesting that if CAS can be performed safely, the results can be durable and competitive with CEA [3,4].
Cerebral protection devices (CPDs) were introduced into clinical practice soon after the development of carotid stents with the express aim of improving the procedural safety of CAS by reducing the embolic complication rate. The first commercially available CPDs were based on the distal balloon occlusion concept of Théron et al. [5]. This system relied on protection by temporary flow arrest. After filters were introduced they rapidly gained in popularity, perhaps because they promised control of the embolic burden associated with CAS whilst allowing procedural cerebral perfusion. They remain the most commonly used CPD, employed in over 90% of protected CAS cases.
Whether CPDs in general have reduced the stroke rate associated with CAS is still open to debate; of the recent randomized trials of CEA versus CAS, the incidence of stroke after CAS was lowest in the trial in which the use of CPD (largely of the filter-type) was also lowest (27% of patients) [2]. Regardless, the literature attests to the fact that these devices trap macroemboli of a size that would otherwise pose a considerable threat to the brain [6–8]. While filters are relatively simple to use, they are quite a feat of complex engineering, and although the outcome of filter-protected CAS is dependent on operator expertise, it is also potentially influenced by the design of the filter (and stent) systems employed.
This article seeks to explore the procedural parameters during CAS that may be influenced by the filter, to explore filter variables that impact on technical outcome and to provide experimental and clinical data where possible.
Filter design
Filters, as a class of CPD, have a number of features in common; they are distal protection devices, are all rapid-exchange systems compatible with 0.014-inch guidewires (a feature common to all other aspects of CAS procedures) and all allow varying degrees of procedural cerebral perfusion.
There are, however, a number of differences between filters. Basic variables include the relationship of the guidewire to the filtration element (i.e., ‘bare-wire’ vs ‘wire-mounted’ systems), position of filtration element in relation to the central guidewire (i.e., eccentric vs concentric), composition of the filtration element (i.e., perforated polyurethane mesh vs nitinol mesh), size of the pores in the filtration element, crossing profile of the filter delivery system, wall apposition of the filter and ‘landing zone’ required (i.e., length of straight portion of internal carotid artery [ICA] necessary for safe placement). A number of these variables are codependent.
Each aspect of filter design can influence the following procedural parameters:
ƒƒThe embolic burden associated with lesion crossing whilst flow in the ICA is antegrade
ƒƒThe penalty associated with filter deployment and retrieval
ƒƒThe penalty associated with filter movements during the procedure
▪ Filter capture efficiency
▪ Filter ‘through-f low’ and ‘perif low’ (i.e., number of particles evading capture by passing through and around the filter)
▪ Filter ‘seeding’ (i.e., microemboli composed of formed blood elements collecting on the outer surface of the membrane with subsequent embolization)
ƒƒCerebral perfusion (i.e., the cerebral flow velocity in the middle cerebral artery ipsilateral to the lesion being treated)
Lesion crossing
The lesion to be crossed is often friable and prone to embolization. In order to cross such lesions safely, minimal device manipulation near or in the lesion is mandatory. Bulky filterdelivery systems, or filters that are cumbersome to advance or manipulate, pose clear disadvantages. Safe lesion crossing is thus dependent on the filter crossing-profile and whether it is a wire-mounted or a bare-wire system. Table 1 gives the crossing profiles of currently available filters. The innovative FiberNet® (Lumen Medical, WA, USA) comprises a matrix of synthetic fibers in a 3-dimensional design resulting in an effective pore size of 40 mm. The FiberNet, the Medtronic Interceptor® (CA, USA) and the Boston Scientific Rubicon™ (MA, USA) have the lowest crossing profiles of current filters, do not require separate delivery systems and are deployed by remote actuation.
Tight, tortuous, complex lesions are perhaps better crossed with a bare-wire system (i.e., one in which a high-quality 0.014-inch guidewire is advanced through the lesion and the filter subsequently advanced) rather than a wiremounted system (i.e., a 0.014 inch wire onto which a filter has been premounted, rendering it less responsive [in terms of one-to-one torque]).
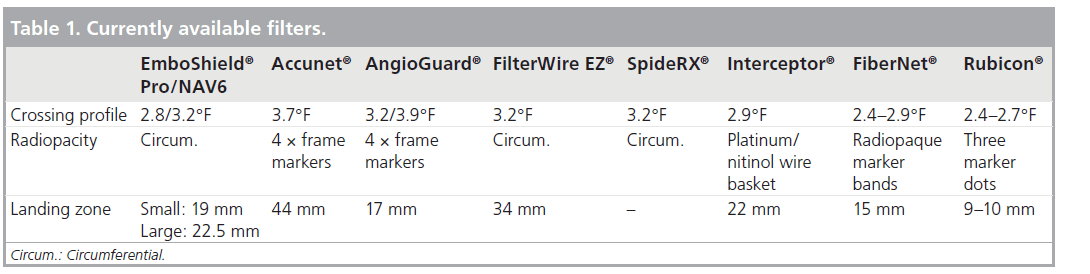
Current bare-wire systems include the EmboShield® (Abbott Vascular, IL, USA – latest iteration the NAV6™) (Figure 1) and the SpideRX™ (ev3, MN, USA). The EmboShield/NAV6 must be used with a dedicated wire. This wire has a 0.019-inch bead mounted 3 cm from the leading platinum tip of the 0.014-inch wire. This bead serves to prevent cephalad migration of the 0.014-inch filter into the brain. The EmboShield/NAV6 is the only system that allows the operator to retrieve the filter whilst leaving the wire across the lesion at the end of the procedure before completion angiography, in case further intervention is required. The SpideRX can be used with a 0.014-inch wire of the operator’s choice, but once the delivery system has been advanced over this wire and beyond the lesion, the original 0.014-inch wire is removed, and the filter is deployed on its own 0.014-inch wire (i.e., it becomes a wire‑mounted system).
Work based on evaluating microembolic signals (MES) on transcranial Doppler (TCD) as a measure of procedural microembolization indicates that lesion crossing with a wiremounted filter (in this study, the FilterWire™ [Boston Scientific]) is associated with significantly more microembolic signals than lesion crossing with a bare wire (as part of a proximal balloon occlusion protection system) [9]. The FilterWire was associated with MES in 95% of cases and the bare wire used during a MoMa protected case was associated with MES in 29% of cases (p < 0.0001).
Embolic penalty associated with filter movements & with filter deployment & retrieval
Ex vivo work was performed in porcine carotid arteries in order to evaluate the effect of distal protection devices (to include filters and distal balloon occlusion) on the delicate intimal lining of the distal ICA where these devices are deployed. This study revealed interesting findings on the embolic penalty associated with filter deployment and retrieval [10].
Four filters (AngioGuard™, Cordis, Johnson & Johnson, FL, USA; FilterWire EX, Boston Scientific; TRAP®, Microvena, MN, USA; NeuroShield™, Abbott Vascular) and the PercuSurge™ distal balloon occlusion device (Medtronic) were compared in a flow rig. ‘Adverse movements’, defined as 1 cm up, 2 cm down and 1 cm up again, not infrequently encountered during CAS, were compared with filter deployment and retrieval stages. The effluent downstream of the deployed CPD was analyzed. The debris released from the porcine vessel wall was correlated with the degree of intimal damage as assessed at light and scanning electron microscopy. The authors concluded that all devices caused histologically visible wall damage, with a direct correlation between degree of intimal denudation and the mass of the debris detected in the effluent. The TRAP caused the most severe intimal and subintimal wall damage; notably, this was the only nitinol mesh filter evaluated (the other filters analyzed were perforated polymer sheets). Interestingly, compared with adverse movements (up and down), which resulted in no significant increase in distal embolization, there was a significant embolic penalty (and vessel-wall injury) associated with device deployment and retrieval stages. A randomized trial comparing unprotected with filter-protected carotid stenting (EmboShield, then NeuroShield) demonstrated that there were significantly more MES on TCD in the protected limb of this trial (to include emboli judged on physical parameters to be particulate) and that the most relevant procedural phases responsible for the difference between protected and unprotected CAS were: lesion crossing, filter deployment and retrieval, which were universally emboligenic [11]. During these phases of the procedure, there were 82.7 particulate emboli released during protected CAS compared with only 9.4 during unprotected CAS (standard error: 16.5; 95% CI: 32.8–113.8; p < 0.01).
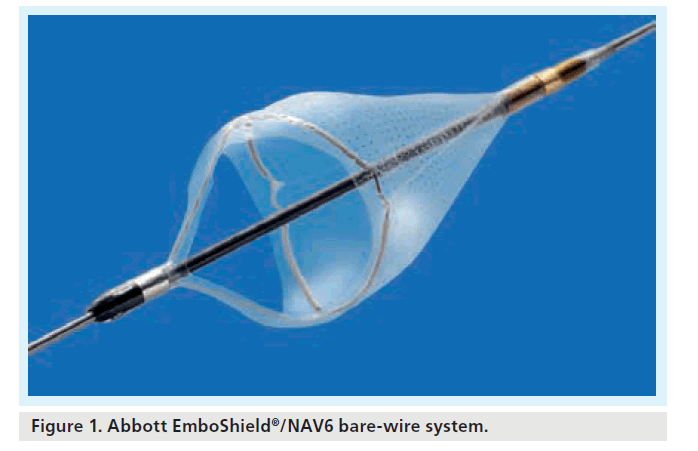
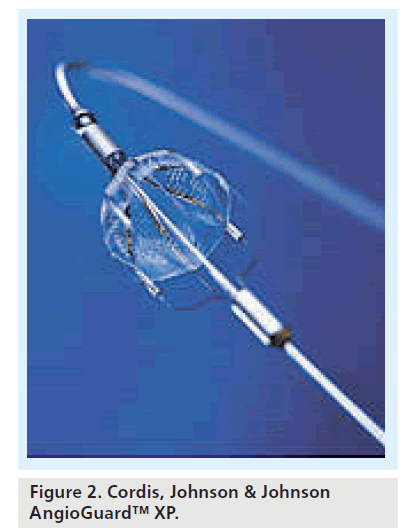
One might argue that the stability of the deployed filter is influential to the integrity of the intima in the landing zone of the distal ICA. Furthermore, the safety of deployment and retrieval phases may be influenced by the opacity of the filter and of the deployment and retrieval catheters. Table 1 gives the radiopacity of currently available filters. Some designs incorporate complete radiopaque hoop markers (i.e., that aspect of the filter that provides continuous wall apposition is radiopaque), whilst some have point markers (e.g., four discrete markers along the wall-opposing edge); in this circumstance, deployment is visually confirmed when four discrete marker dots are seen, and retrieval confirmed when these four dots converge into one dot (e.g., AngioGuard XP) (Figure 2). With filters that have complete radiopaque hoop markers, retrieval is confirmed when the radiopaque marker hoop opposing the vessel wall is collapsed into a slit or streak on fluoroscopy (e.g., FilterWire EZ [Figure 3], SpideRX and EmboShield/NAV6). The visibility of the filter will inform the operator of the condition as well as the position of the filter. Careful review of the radiopaque wall-opposing markers on filtration elements allows differentiation between procedural flow arrest owing to flow-limiting spasm or distal ICA dissection (the markers are drawn together) and a full filter (the markers remain unchanged compared with initial postdeployment images). This is clearly an important distinction as the remedies for these complications depend very much on the underlying problem.
Safe filter retrieval is an important determining factor for good outcomes during CAS. When there has been massive distal embolization, one of the drawbacks of filters is the risk of losing the captured material during retrieval, by extruding it through the pores or around the edges of the filter, thereby causing an in toto cerebral embolic event that may be devastating.
Filter capture efficiency: filter through-flow & periflow
Early work focused on a prototype of the EmboShield filter (then NeuroShield) [12]. Eight carotid bifurcation plaques obtained from patients who had undergone carotid endarterectomy for high-grade atherosclerotic stenosis were encased in polytetrafluoroethylene to simulate adventitia and attached to a perfusion circuit. Self-expanding stents were placed across the lesion with a filter deployed distally. The effluent passing through and beyond the filter was analyzed. The mean number and the maximum size of the particles that were released during initial filter passage, missed and captured by the filter were 3.1 and 500 mm, 2.8 and 360 mm, and 20.1 and 1100 mm, respectively. The filter captured 88% of the liberated load. However, more contemporary work on the size and nature of embolic material released during carotid angioplasty (which may be different from the embolic profile of stent placement) suggests that only a small fraction of the liberated load is captured by filters with pore sizes ranging from 60 to 120 mm (reflecting the pore sizes of currently available devices). A substantial number of particles smaller than 60 mm (i.e., microemboli by definition) are clearly released and may pass unhindered to the brain [13–15].
Ex vivo work was performed in order to compare AngioGuard XP (100 mm pores), FilterWire EZ (110 mm pores) and RX Accunet™ (115 mm) in a flow-rig under simulated systolic pressures [16]. The filters were placed at the apex of angles fashioned in silicone tubing measuring 5, 5.5 and 6 mm, and subjected to injections of particles ranging from 297 to 1000 mm in size. The percentage of embolized particles that evaded capture for the 5-, 5.5- and 6-mm tubing for AngioGuard were 7.53, 10.88 and 14.24%, respectively. This was significantly higher than the filter through-flow and periflow occurring with the other filters tested. The RX Accunet had the best overall wall opposition and the FilterWire EZ had the best overall filtration rate, failing to capture only 0.8% of plaque particles.
The degree of filter through-flow is proportional to the pore size and this will vary from filter to filter. Currently available filters have pore sizes between 80 and 297 mm (not including the FiberNet with pores of 40 mm that rely on filtration plus a degree of flow stagnation to exert its protective effect, thereby temporarily compromising cerebral perfusion). It is apparent that beyond a certain size, further reductions in pore size may be counterproductive, owing to the chances of filter-occlusion, ‘slow-flow’ phenomena and/or the potential for causing redcell shear and platelet deposition (see below). Filter through-flow also relates to the degree of wall apposition, to ‘guidewire bias’ and the landing zone required for optimal filter function.
Guidewire bias & landing zones
In most filters (FilterWire EZ, SpideRX, AngioGuard and EmboShield/NAV6), the function of the frame is to ensure apposition of the filter membrane to the vessel wall. This apposition can be adversely affected in some CPDs if the guidewire is placed under tension or compression, or if it is biased to one side of the vessel. FilterWire EX (which was an eccentric design) was prone to this problem. FilterWire EZ, also of an eccentric design, performs better because the frame loop is not as directly connected to the guidewire, and there is a ‘suspension arm’ joining the hoop base and its free end to the guidewire. EmboShield/NAV6 have significant advantages in that they are not connected to the guidewire, meaning that they are less affected by tension or compression of the guidewire. However, there is an important consideration to take into account when using this device: because the filter floats completely freely on the wire, traction on the wire will cause the distal 0.019-inch bead to pull the 0.014-inch filter caudally (the worstcase scenario being to pull the fully deployed filter into an untreated friable lesion). Pushing the wire will then cause the wire only to advance, most often leaving the filter opposed to the vessel walls and immobile. This situation can be remedied by advancing either a balloon or a stent on the 0.014-inch guidewire without actually holding the guidewire. It is accepted that this maneuver is counterintuitive but it has worked for the author on a number of occasions when proctoring less experienced operators. Perhaps the vibration along the 0.014-inch wire promotes gentle cephalad ‘walking’ of the filter.
The radial force of the frame arms is intended to overcome the stiffness of the guidewire and remain concentric and apposed to the vessel even when the guidewire is biased towards one wall of the vessel. Tension on the guidewires of some types of eccentric filters and wiremounted systems is sufficient to pull the filtration element away from the vessel wall in some aspect and thus produce a ‘protection blind zone’ whereby emboli can reach the brain by evading capture. This situation may be exacerbated in situations where the landing zone is short and angulated and the filter has a relatively long landing zone requirement. Table 1 provides the landing zones for currently available filters.
Ex vivo work was also carried out on variations in carotid anatomy. Three different flow models, each simulating degrees of tortuosity of the distal ICA were used and the degree of anatomic complexity was correlated with the capture of polyvinyl alcohol particles of three size ranges (small, medium and large). The authors stated that none of the tested devices (AngioGuard, FilterWire EX, TRAP and NeuroShield) prevented embolization completely. However, the only filter that showed no significant reduction in efficacy in the tortuous models was the FilterWire EX, with its eccentric filtration element [17]. The differences in outcome between this experiment and the issues of guidewire bias highlighted above are likely to relate to two factors. First, older iterations of the EmboShield (then NeuroShield) and AngioGuard were used in this study with improved versions of each filter available currently. Second, the ex vivo study perhaps did not reflect the issues pertinent to a live CAS procedure whereby any undue tension on the guidewire (on which the filter is mounted), exerted as countertraction in order to facilitate tracking of the stent and balloons may lift the filtration element clear of the vessel wall causing a ‘blind zone’.
For all filters, but perhaps more so for those with concentric hoop markers rather than discrete marker dots (see above), wall apposition is best evaluated by angiography in at least two orthogonal plains to ensure that the hoop is not constrained in any particular orientation and has expanded fully, or that the marker dots are spread evenly about the circumference of the filtration element, depending on the device used.
Filter seeding
Blood flow is known to suffer high shear stress during passage through filter pores and so platelets are activated. Platelet activation is known to occur at lower shear stress in the presence of foreign surfaces than in the presence of fully biocompatible surfaces. However, platelets will adhere even to biocompatible surfaces when they are activated, and the critical shear stress for platelet activation is surface characteristicdependent. Prototype filters of the uncoated biocompatible polyurethane type were subject to high shear stress and to platelet activation with formed blood elements clumping to the outer, cephalad surface of the filter, risking uncontrolled cerebral embolization. This is less of a problem with newer filters; for example, the NAV6 has a hydrophilic coating (which is intended to inhibit fibrin formation). The small version of NAV6 (which has 1000 pores of 120 mm) causes a shear stress of 89 t compared with 214 t for the generation III EmboShield (3 mm). The shear stress is greater across smaller filtration elements, and it remains true that reductions in filter pore size and in number of pores will generally be associated with increased blood shear stress and platelet activation.
Cerebral perfusion
The vast majority of currently available filters comprise a perforated polyurethane membrane, although some are comprised of nitinol mesh, such as the SpideRX and the Interceptor.
Quite apart from particle capture efficiency, it is imperative to be sure that use of a filter does not incur a penalty with respect to procedural cerebral perfusion. Despite reassurances from industry that there is no significant flow reduction caused by contemporary filter devices, there is some experimental work that suggests otherwise. Pressure gradients were compared across three membrane filters; AngioGuard, FilterWire EZ and Accunet RX, and the results were compared with findings for the SpideRX nitinol mesh filter [18]. In this ex vivo analysis, the pressure gradient caused by the filtration element was correlated with the degree of flow reduction. It was concluded that all evaluated filters caused a pressure gradient and flow obstruction and this may amount to a temporary 40% reduction in mean middle cerebral artery perfusion. This effect seemed most marked with perforated membrane filters and almost absent for the nitinol mesh filter evaluated. Indeed, industry studies for the Interceptor showed the best results of all for the SpideRX; after around 9 mg of entrapped emboli, the cerebral perfusion rate through filters of the perforated polyurethane mesh type fell to less than 100 ml/min compared with the flow rate in the nitinol mesh filters, which consistently provided more than 150 ml/ min perfusion despite entrapped loads of up to 30 mg. This might be an important consideration when a filter-type CPD is considered mandatory on the basis of precarious cerebral perfusion (i.e., in a patient with contralateral carotid occlusion, isolated hemisphere and/or insufficiency of the circle of Willis).
The following paragraphs will focus on filters as a group with a number of factors in common.
Experimental data
▪ Outcomes based on surrogate markers of stroke: microemboli measured as MES on TCD
If surrogate markers of neurological injury occurring more frequently than stroke are utilized as a primary outcome, small, randomized trials may be appropriate to compare protected and unprotected carotid stenting. MES measured on TCD are one such surrogate.
A small, randomized trial comparing protected and unprotected carotid stenting indicated significantly more MES when a filter (EmboShield, then NeuroShield) was used compared with unprotected stenting [11]. This work was corroborated in a larger, nonrandomized clinical evaluation [19]. A total of 509 patients were divided into three groups in this subsequent study; 161 patients treated before filter devices became available, 151 patients treated with filters (including FilterWire EX, FilterWire EZ, AngioGuard and AngioGuard XP, Accunet RX, TRAP, SpideRX, EmboShield and NeuroShield) and 197 patients undergoing unprotected carotid stenting after these devices had become available. The authors concluded that carotid stenting with filter-type cerebral protection yielded significantly more microemboli when filters were employed compared with unprotected stenting. The infrequent occurrence of neurological sequelae did not allow comprehensive statistical comparison between groups.
The clinical relevance of the findings of both these studies is unclear and warrants further study. What is clear, however, is that the results are unlikely to be due to choice of filter. The use of filters with various pore sizes was also associated with significantly more MES than in unprotected patients [19].
▪ Outcomes based on surrogate markers of stroke: new white lesions on diffusion-weighted imaging of the brain
Nonrandomized comparison of unprotected and protected populations suggests a reduction in diffusion-weighted imaging (DWI) lesions when protection (usually of the filtertype) is used. However, these series compare protected series with historical controls that encompass considerable variation in presenting symptoms and age, and overlook important technical advances such as fine guidewire technology, dedicated carotid stents, improvements in periprocedural pharmacological support and, of course, operator learning curve [20,21]. Regarding differences in patient demographics, Kastrup et al. demonstrated that age and symptom status influenced the incidence of lesions on DWI following CAS [22].
A recent publication by Barbato et al. constitutes the only published randomized trial to date comparing unprotected CAS with filterprotection (utilizing the Accunet filter, then Guidant, CA, USA [now Abbott Vascular]) [23]. This work confirms the earlier trial published as a PhD thesis [11]. It has the same findings, namely a nonsignificant increase in lesions on DWI in the filter-protected group. With respect to DWI findings, both trials (the latter trial being terminated short of recruitment target) showed an increase in lesions with filter-type protection that did not reach significance, probably because there were insufficient numbers. Kastrup et al. later stated that approximately 120 to 140 patients would be needed for a randomized trial based on DWI lesions to be adequately powered; however, this analysis postdates the earlier randomized trial [11,24]. Furthermore, it is important to note that Kastrup’s estimated numbers are based on a “retrospective analysis of nonrandomized data with all its inherent limitations” [25].
The results are unlikely to be due to choice of filter in general and filter-pore size specifically. Recent work suggests no relationship between filter pore size and rate of DWI lesions during filter-protected CAS [26]. A substudy of the International Carotid Stenting Study (ICSS) (the main study comparing CAS with CEA in lowrisk symptomatic patients) focused on new DWI lesions. It again demonstrated more lesions when a filter was used than when the CAS procedure was unprotected [27].
Caution must be exercised when interpreting these results. Many DWI lesions reverse within months and the extent of permanent injury may be overestimated [28].
▪ Outcomes based on surrogate markers of stroke: macroemboli
The capture of visible debris constitutes perhaps another surrogate marker of neurological injury. It is a logical assumption that the debris collected and retrieved from filters used during carotid stenting would, without that filter being in place, embolize to the brain, causing some degree of neurological damage. Analysis of 270 registry patients revealed the clinical factors that were predictive for the presence or absence of visible debris collected in filters to include FilterWire EX and EZ, SpideRX, AngioGuard and “four others” [29]. Visible debris was present in 169 filters (60.3%). There was an increased risk of visible debris found with several variables: hypertension (odds ratio [OR]: 2.9; 95% CI: 1.7–5.2), hyper-cholesterolemia (OR: 2.3; 95% CI: 1.4–3.9), stent diameter of more than 9 mm (OR: 16.6; 95% CI: 9.0–30.0) and any neurological event (OR: 4.2; 95% CI: 1.5–9.9). The negative predictive value failed to exceed 0.80 (80%) for any variable. It was concluded that the study failed to identify any variables capable of consistently predicting the absence of visible debris. It was suggested, therefore, that the findings supported the routine rather than the selective use of filters.
Clinical data: outcomes based on stroke & death
There are a number of single-center or collaborative works evaluating a particular filter, but no level I evidence supporting the routine use of filters and only one small trial to date comparing the relative merits of any one filter over any other based on clinical outcomes.
▪ Comparative outcomes
A small, randomized trial comprising 162 consecutive patients randomly assigned to EmboShield (n = 46), FilterWire EZ (n = 57) or the SpideRX (n = 59) was presented at Transcatheter Cardiovascular Therapeutics in 2005 but to date has not been published [30]. The primary end point was ‘filter success’ (defined as effective lesion crossing, filter positioning and retrieval without complications). Secondary end points included procedural success, and the incidence of all-stroke, death and myocardial infarction at 30 days. EmboShield was significantly poorer than the other filters evaluated with respect to ‘filter success’. It was more frequently associated with spasm and with transient ischemic attack. Procedural time was significantly lower with the FilterWire EZ. Caution must be exercised in the interpretation of these findings as the populations treated were a mixture of symptomatic patients and asymptomatic patients with variable procedural risk. It is not clear, therefore, whether there were more symptomatic patients in the group protected by means of the EmboShield device, which could help to explain the higher rate of transient ischemic attack in this group. Furthermore, the operators’ prior experience with the devices is not given and it is possible that they were more familiar with one device than the other. In addition, it is not clear which generation of the EmboShield device was used – the latest generation has a much shorter filtration element than the earlier iterations and differences in radial force at the base of the filter. Lastly, the trial is seriously underpowered and thus meaningful conclusions on the basis of infrequently occurring adverse clinical events cannot be drawn.
A nonrandomized analysis of 3160 CAS procedures sought to evaluate the efficacy of CPDs and to compare clinical outcomes with specific devices and types of CPDs [31]. A total of nine CPDs were included. The risk of a procedural adverse event was 0.9% in protected and 2.3% in (an historical) unprotected cohort (p = 0.12). Compared with the most frequently used device (FilterWire), there was no significant difference in the risk of procedural adverse events for any of the other CPDs. There was, however, an increased risk of 30‑day adverse events with the Accunet (Abbott Vascular) filter compared with the FilterWire (relative risk [RR]: 2.67; 95% CI: 1.41–5.04; p = 0.005). Pairwise comparison of proximal occlusion balloons to filters, distal occlusion balloons to filters and proximal-to-distal occlusion balloons revealed no significant difference in the risk of procedural or 30‑day adverse events. There was no significant difference in the risk of procedural events between eccentric and concentric filters; however, the relative risk of eccentric compared with concentric filters at 30 days was 0.59 (unadjusted 95% CI: 0.38–0.92; p = 0.04). This difference was still apparent after adjustment for risk factors (RR: 0.61; 95% CI: 0.39–0.95; p = 0.06), but not after adjustment for risk factors and stent-type ([open-cell vs closed-cell] RR: 0.76; 95% CI: 0.47–1.22; p = 0.51). The authors concluded that the use of CPDs was associated with a low risk of procedural adverse events. They were unable to detect significant differences in the risk of procedural adverse events between different devices or types of device and speculated that the observed differences at 30 days were largely attributable to differences in stent-type rather than CPD used.
▪ Group outcomes:
filter-associated complications
A series of 442 consecutive patients was evaluated for the clinical advantages and complications incurred by use of available protection devices [32]. A mixture of CPDs were employed to include distal occlusion, proximal occlusion and flow reversal in addition to AngioGuard (37.8%), FilterWire EX (25.1%), TRAP (18.8%) and NeuroShield filters (9.3%). Of those filter-related complications defined as major, there was one instance of failure to retrieve a loaded filter (AngioGuard), necessitating surgical intervention. It must be highlighted that this must have been fairly early on in the center’s experience, as the stent used in this case was a Palmaz-Schatz that has long since fallen from favor in the carotid territory as a result of its susceptibility to compression deformation. Of those complications deemed to be side-effects of a filter, 58 cases (13.1%) were owing to flow impairment resistant to nitroglycerin, although all patients involved remained asymptomatic. After filter retrieval, flow was restored in all patients. A large amount of macroscopic visible debris was visible inside these filters and was considered to have been responsible for the temporary but significant flow impairment. The study was not designed either to compare various protection systems or to detect differences between balloon occlusive devices, filter devices and flow reversal devices.
Corroborative work on significant reductions in antegrade ICA flow with a filter in situ, designated the ‘slow-flow’ phenomenon, focused on 414 patients undergoing 453 filter-protected carotid stenting procedures [33]. The filters used were AngioGuard (64%), FilterWire EX and EZ, Accunet and NeuroShield. Multivariate logistic regression analysis identified the following predictors of slow-flow: recently symptomatic patients (i.e., patients treated within 6 months of index event), increased stent diameter and increased patient age. Among those with slow-flow, the 30‑day incidence of stroke or death was 9.5% compared with 2.9% in patients with normal flow (p = 0.03). It was concluded that embolization of vulnerable plaque elements played a pathogenic role.
Results from large registries & randomized trials in which filters are the most commonly used protection device
The German Cardiology Carotid Stenting Registry described 1734 patients treated between 1996 and 2003, 729 of whom were treated with a protection device: 553 (75.9%) with a variety of filters and 176 (24.1%) with distal balloon occlusion [34]. There was no significant difference in clinical outcome between these two populations, despite the confounding variable of more symptomatic patients protected by means of distal balloon occlusion. There was a significant difference in all-stroke/death for protected and unprotected patients (4.9% unprotected and 2.1% protected; p = 0.004). However, the unprotected patients were those treated early and the protected patients were those treated later chronologically. Furthermore, there was a significant increase in asymptotic patients treated latterly and these patients are known to be associated with lower procedural risk regardless of technical advances. During the time of data accrual for this registry, technical and pharmacological advances occurred and both these and learning curve issues may each have had a profound influence on outcome quite separate from the influence of a CPD, and this should not be overlooked.
Interestingly, another German carotid stenting registry did not corroborate these results [35]. This was a prospective concurrent registry of 2532 protected and unprotected patients, 923 without protection and 1609 with protection. Distal balloon occlusive systems were used in 12% of these cases, proximal balloon occlusive systems in 9% and filters in 76%. There was no significant difference in permanent neurological deficits and death in these nonrandomized groups (2.2% unprotected and 2.1% protected) but interestingly, there were more transient symptoms when a protection device was used (4.6% unprotected and 7.6% protected). Updated analysis of this registry, comprising 5341 patients, revealed that use of a CPD was not an independent predictor of stroke or death [36].
Regarding available evidence from recent randomized trials of CAS versus CEA, the use of CPDs – of the filter-type in the vast majority of cases – proved to be no panacea against stroke and death. Unfortunately, the procedural or 24‑h results from within these trials are somewhat opaque to scrutiny, and the 30‑day results are not necessarily a reflection of the efficacy of a CPD employed during the procedural time-frame. Regardless, in the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) trial, the 30‑day rate of ipsilateral stroke and death was 7.3% in those patients in whom protection had been employed, and 6.7% in unprotected patients (p = nonsignificant) [2]. In the Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA‑3S) trial, the 30‑day stroke and death rate was 7.9% in patients in whom cerebral protection had been employed, although it is notable that the majority of those who suffered stroke in the carotid stenting limb of the trial (17 out of 24) did so on the day of the procedure, implying that stroke was a direct complication of the procedure [1]. After routine cerebral protection had been mandated by the safety committee, almost half of all cases were protected by means of a membrane filter, a third by distal balloon occlusion and a fifth by nitinol mesh filters.
Conclusion
Despite absence of level I evidence of clinical efficacy (reduction in incidence of procedural stroke), it is widely believed that CPDs are beneficial and most operators (including the author of this article) performing CAS advocate routine use of these devices.
To date, filter protection has proved to be the most popular form of cerebral protection, perhaps because the concept of embolic capture whilst permitting cerebral perfusion is attractive. However, it should be noted that there is inevitably a compromise between capture efficiency and cerebral perfusion. Small, randomized trials have demonstrated more microemboli (in the form of MES on TCD and new white lesions on DWI) when filters are used compared with unprotected CAS, but the prognostic relevance and clinical impact of these surrogate-marker findings remain unknown, and under these circumstances many operators will accept a tradeoff between capture of macroemboli despite microembolic burden. It should be noted, however, that much of the published work pertains to symptomatic patients or to mixtures of symptomatic and asymptomatic patients. It is not at all clear whether the same conclusions can be made for patients with asymptomatic carotid stenoses undergoing filter‑protected CAS.
Further research is required to more clearly define the relative advantages and disadvantages of each type of filter as well as to establish the relative merits of filters with proximal balloon occlusion/flow reversal.
Executive summary
Filter design
▪ Filters as a group of cerebral protection devices have certain features in common (i.e., they allow cerebral perfusion, they are distal devices and all are 0.014 inch rapid-exchange compatible).
▪ There are a number of subtle differences in design between filters and these differences can influence filter performance.
Clinical data: registries & randomized trials
▪ Use of filter-type protection devices is intuitive but there is no supporting level I evidence.
▪ Clinical outcome data are hampered by comparisons against historical controls of unprotected CAS with many confounding variables that may influence results.
Experimental data: capture of macroemboli
▪ Substantial in vivo analyses demonstrate that filters trap macroemboli thought to have been liberated by endovascular manipulation of plaques.
Experimental data: outcomes based on surrogate markers of stroke: microembolic signals on transcranial Doppler
▪ Filters may generate more microemboli than are demonstrated in unprotected carotid artery stenting as shown on procedural transcranial Doppler; the clinical relevance of these microemboli is unknown.
▪ Filters may be associated with more new white lesions on diffusion-weighted imaging although the clinical relevance of this finding also requires elucidation.
Conclusion
▪ It has been said that filters provide protection by allowing a ‘controlled embolization’ and are thus very different from proximal balloon occlusion devices and flow reversal systems that may offer more complete control of microembolization but do so at the expense of cerebral perfusion.
Future perspective
Filter-protection and filter-type CPDs need to improve in order to address the microembolic burden associated with them in particular. The first steps to be taken are towards a better understanding of the nature of this microembolic load; for example, do they comprise platelet aggregates, microthrombi or atheroma fragments? The available literature suggest that lesion crossing, filter deployment and retrieval are emboligenic stages of the CAS procedure and so these stages need to be better understood and refined. Lesion crossing should be rendered safer by further reductions in crossing profile of the filter delivery system. The embolic penalty of deployment may relate to air and, thus, improved methods of eliminating air from the delivery systems should be developed. Analysis of where microemboli arise at device retrieval should be carried out – are these emboli impacting around the filter edges (i.e., not trapped within) and then released on retrieval or are they squeezed through the pores on retrieval? Understanding this should allow refinement in technique. Finally, a careful review of the filter materials may reveal that intelligent coatings are the way forwards.
Financial & competing interests disclosure
Sumaira Macdonald has received research grants and/or consultancy fees from the following companies: Abbott Vascular; CR Bard; Cordis, Johnson & Johnson; ev3; WL Gore; Invatec; and Medtronic. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
- Mas JL, Chatellier G, Beyssen B et al.; EVA‑3S Investigators: Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 355(16), 1660–1671 (2006).
- SPACE Collaborative Group; Ringleb PA, Allenberg J, Bruckmann H et al.: 30 day results from the SPACE trial of stentprotected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 368, 1239–1247 (2006).
- Mas JL, Trinquart L, Leys D et al.; EVA‑3S Investigators: Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA‑3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 7(10), 885–892 (2008).
- Eckstein HH, Ringleb P, Allenberg JR et al.: Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective randomised trial. Lancet Neurol. 7(10), 893–890 (2008).
- Theron J, Payelle GG, Coskun O, Huet HF, Guimaraens L: Carotid artery stenosis: treatment with protected balloon angioplasty and stent placement. Radiology 201, 627–636 (1996).
- Angelini A, Reimers B, Della Barbara M et al.: Cerebral protection during carotid artery stenting: collection and histopathologic analysis of embolized debris. Stroke 33, 456–461 (2002).
- Hayashi K, Kitagawa N, Morikawa M: Observing the carotid debris aspirated during carotid stenting: technical note. Neurol. Res. 27, 22–26 (2005).
- Maleux G, Demaerel P, Verbeken E et al.: Cerebral ischaemia after filter-protected carotid artery stenting is common and cannot be predicted by the presence of substantial amount of debris captured by the filter device. Am. J. Neuroradiol 27, 1830–1833 (2006).
- Schmidt A, Diederich KW, Scheinert S et al.: Effect of two different neuroprotection systems on microembolization during carotid artery stenting. J. Am. Coll. Cardiol. 44, 1966–1969 (2004).
- Muller-Hulsbeck S, Stolzmann P, Liess C et al.: Vessel wall damage caused by cerebral protection devices: ex vivo evaluation in porcine carotid arteries. Radiology 235, 454–460 (2005).
- Macdonald S: Thesis for the degree of doctor of philosophy: neuroprotection and flow dynamics in carotid stenting. University of Sheffield Main Library Volumes I & II [MO115661SH]. Main Library Thesis 12638 (2004).
- Ohki T, Roubin GS, Veith FJ, Iyer SS, Brady E: Efficacy of a filter device in the prevention of embolic events during carotid angioplasty: an ex vivo analysis. J. Vasc. Surg. 30, 1034–1044 (1999).
- Ohki T, Marin ML, Lyon RT et al.: Ex vivo human carotid artery bifurcation stenting: correlation of lesion characteristics with embolic potential. J. Vasc. Surg. 27, 463–471 (1998).
- Coggia M, Goeau-Brissonniere O, Duvall JL, Leschi JP, Letort M, Nagel MD: Embolic risk of the different stages of carotid bifurcation balloon angioplasty: an experimental study. J. Vasc. Surg. 31, 550–557 (2000).
- Rapp JH, Pan XM, Sharp FR et al.: Atheroemboli to the brain: size threshold for causing acute neuronal cell death. J. Vasc. Surg. 32, 68–76 (2000).
- Order BM, Glass C, Liess C et al.: Wall apposition assessment and performance comparison of distal protection filters. J. Endovasc. Ther. 15(2), 177–185 (2008).
- Order BM, Glass C, Liess C, Heller M, Muller-Hulsbeck S: Comparison of 4 cerebral protection filters for carotid angioplasty: an in vitro experiment focusing on carotid anatomy. J. Endovasc. Ther. 11, 211–218 (2004).
- Hendriks JM, Zindler JD, van der Lugt A et al.: Embolic protection filters for carotid stenting: differences in flow obstruction depending on filter construction. J. Endovasc. Ther. 13, 47–50 (2006).
- Vos JA, van den Berg JC, Ernst SMPG et al.: Carotid angioplasty and stent placement: comparison of transcranial doppler US data and clinical outcome with and without filtering cerebral protection devices in 509 patients. Radiology 234, 493–499 (2005).
- Jaeger H, Mathias K, Elke H et al.: Cerebral ischaemia detected with diffusion-weighted MR imaging after stent implantation in the carotid artery. Am. J. Neuroradiol. 23, 200–207 (2002).
- Jaeger H, Mathias K, Drescher R et al.: Clinical results of cerebral protection with a filter device during stent implantation of the carotid artery. Cardiovasc. Intervent. Radiol. 24, 249–256 (2001).
- Kastrup A, Groschel K, Nagele T et al.: Effects of age and symptom status on silent ischemic lesions after carotid stenting with and without the use of distal filter devices. Am. J. Neuroradiol. 29(3), 608–612 (2008).
- Barbato JE, Dillavou E, Horowitz MB: A randomized trial of carotid artery stenting with and without cerebral protection. J. Vasc. Surg. 47(4), 760–765 (2008).
- Kastrup A, Nagele T, Groschel K et al.: Incidence of new brain lesions after carotid stenting with and without cerebral protection. Stroke 37(9), 2312–2316 (2006).
- Kastrup A, Schnaudigel S, Groshel K: Regarding ‘A randomized trial of carotid artery stenting with and without cerebral protection’. J. Vasc. Surg. 48(2), 505 (Author reply, 505) (2008).
- Blasel S, Hattingen E, Berkefeld J et al.: Evaluation of angiographic and technical aspects of carotid stenting with diffusion-weighted magnetic resonance imaging. Cardiovasc. Intervent. Radiol. 32, 666–671 (2009).
- Brown M: International Carotid Stenting Study and DWI substudy. Large Clinical Trials Section. Abstract 3. Presented at: European Stroke Conference 2009. Stockholm, Sweden, 26–29 May 2009.
- Palombo G, Faraglia V, Stella N, Giugni E, Bozzao A, Taurino M: Late evaluation of silent cerebral ischemia detected by diffusion-weighted MR imaging after filter-protected carotid artery stenting. Am. J. Neuroradiol. 29, 1340–1343 (2008).
- Sprouse LR 2nd, Peeters P, Bosiers M: The capture of visible debris by distal cerebral protection filters during carotid artery stenting: is it predictable? J. Vasc. Surg. 41, 950–955 (2005).
- Montorsi P, Galli S, Ravagnani P, Trabattoni D, Fabbiocchi F, Lualdi A: Comparison of three filter device performance in carotid stenting: a randomized, single center study. Am. J. Cardiol. 96, 6H (2005).
- Iyer V, de Donato G, Deloose K: The type of embolic protection does not influence the outcome in carotid artery stenting. J. Vasc. Surg. 46(2), 251–256 (2007).
- Cremonesi A, Manetti R, Setacci F, Setacci C, Castriot F: Protected carotid stenting: clinical advantages and complications of embolic protection devices in 442 consecutive patients. Stroke 35, 1936–1941 (2003).
- Casserly IP, Abou-Chebl A, Fathi RB: Slow-flow phenomenon during carotid artery intervention with embolic protection devices: predictors and clinical outcome. J. Am. Coll. Cardiol. 46, 1466–1472 (2005).
- Zahn R, Ischinger T, Mark B et al.; Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK): Embolic protection decvices for carotid artery stenting: is there a difference between filter and distal occlusive devices? J. Am. Coll. Cardiol. 45, 1769–1774 (2005).
- Theiss W, Hermanek P, Mathias K et al.; for the German Societies of Angiology and Radiology: Pro-CAS; a prospective registry of carotid angioplasty and stenting. Stroke 35, 2134–2139 (2004).
- Theiss W, Hermanek P, Mathias K; German Society of Angiology/Vascular Medicine: Predictors of death and stroke after carotid angioplasty and stenting: a subgroup analysis of the Pro-CAS data. Stroke 39, 2325–2330 (2008).
▪ Sizeable randomized trial comparing carotid endarterectomy (CEA) and carotid artery stenting (CAS) demonstrating that if CAS can be performed safely, then it may be as effective at stroke prevention as CEA in the intermediate term.
▪ Provides a good example of the capture of macroemboli by filters and the pathological analysis of the debris.
▪ Provides the first randomized trial of unprotected versus filter-protected carotid stenting using as end points surrogate markers of stroke to include microembolic signals (MES) on transcranial Doppler (TCD) and new white lesions on diffusion-weighted imaging (DWI) of brain.
▪ Sizeable prospective analysis compares unprotected and filter-protected carotid stenting based on the surrogate marker MES on TCD.
▪ Second randomized trial comparing unprotected and filter-protected carotid stenting using the surrogate marker of new white lesions on DWI.
▪ Large, randomized trial of CEA versus CAS incorporating a substudy that evaluated new white lesions on DWI. Most of the CAS procedures were filter-protected but some were unprotected.
▪ Sizeable series evaluating the clinical impact of different protection devices on outcomes for CAS.