Mini Review - Interventional Cardiology (2024)
Recent evidence of cardiovascular disease prevention by polypills: A mini review
- Corresponding Author:
- Antonio Coca
Department of Internal Medicine. University of Barcelona, Barcelona, Spain,
E-mail: acoca1492@gmail.com
Received date: 07-Mar-2024, Manuscript No. FMIC-24-129060; Editor assigned: 11-Mar-2024, PreQC No. FMIC-24-129060 (PQ); Reviewed date: 26-Mar-2024, QC No. FMIC-24-129060; Revised date: 03-Apr-2024, Manuscript No. FMIC-24-129060 (R); Published date: 10-Apr-2024, ![]()
Abstract
Despite explicit guideline recommendations for the diagnosis and management of Cardiovascular Risk Factors (CVRF) such as hypertension, dyslipidaemia, obesity, and hyperglycaemia, a large proportion of patients remain undiagnosed, untreated, or treated but uncontrolled. Inadequate control of CVRF is associated with many complex factors including physician’s inertia, health systems disparities, and poor adherence to prescribed antihypertensive drug treatment by patients. This problem has been seen not only in the primary prevention of patients with different CVRF but also in the secondary prevention of recurrent Cardiovascular (CV) events in patients with atherosclerotic CV disease such as Coronary Artery Disease (CAD) or stroke. All guidelines recommend treatment with at least a ReninâAngiotensinâAldosterone System (RAS) blocker (AngiotensinâConverting Enzyme Inhibitor (ACEI) or Angiotensin Receptor Blocker (ARB), statin, and antiagregant. To improve adherence with this multiple drug therapy the preferred use of a single pill combination, the polypill, is recommended in most guidelines. This strategy is supported by robust evidence demonstrating better adherence and long-term persistence on treatment, with the consequent reduction of CV events and CV mortality in primary or secondary CV disease prevention. This review summarizes the latest evidence supporting these recommendations.
Keywords
Polypill; Single pill combination; Adherence; Primary cardiovascular prevention; Secondary cardiovascular prevention
Introduction
One of the main targets of the Sustainable Development Goals program is a onethird reduction in premature mortality related to non-communicable diseases by 2030 [1]. This goal should be achieved by improving their prevention and treatment, particularly Cardiovascular Disease (CVD). However, in the last three decades, CVD has remained the leading cause of death and disability worldwide [2]. The above has led to the proposal of innovative strategies to reduce the burden of CVD. In the early 2000s, it was proposed that a combination of medications with proven CVD benefits (betaâblocker, Angiotensin-Converting Enzyme Inhibitor (ACEI), statin, and aspirin) in a single pill might reduce CVD events in people at high risk [3]. The efficacy of this intervention, named polypill, was confirmed by a meta-analysis from Wald and Law that demonstrated that a Single Pill Combination (SPC) of six components (three antihypertensive agents, statin, aspirin, and folic acid) reduced the risk of myocardial infarction by 88% (95% Confidence Interval (CI): 84-91%) and stroke by 80% (95% CI: 71-87%) [4]. Nowadays, the most accepted definition of polypill is the one proposed by the World Heart Federation, which defines it as an SPC therapy that includes one or two antihypertensive agents and a statin, with or without aspirin [5].
Literature Review
The rationale for the use of a polypill
The use of medications with proven CVD benefits is a mainstay in CV risk treatment and prevention of new events. In secondary prevention, the combination of an antiplatelet agent, a Renin- Angiotensin System (RAS) blocker, a beta-blocker, and a statin reduces the risk of myocardial infarction by 27% (95% CI: 17-36%) and stroke by 21% (95% CI: 9-32%) compared to monotherapy or no therapy [6]. However, evidence-based pharmacotherapy is underused worldwide [7,8]. In the INTERASPIRE survey, it was shown that less than half of the participants were taking the four medications indicated in secondary prevention [9]. The barriers described for these low levels of adherence are related to the low availability, accessibility, and affordability of the medications [10]. In addition, patients at high risk for CVD usually require complex drug regimens with multiple daily intakes. In other words, a patient’s increased risk translates into an increased pill burden that predisposes to lower adherence and persistence in treatment [11]. Therefore, some of these barriers could be addressed with the polypill strategy.
Polypill in primary prevention
The pivotal trial demonstrating that a polypill was effective in reducing major cardiovascular events in primary prevention was the International Polycap Study (TIPS)-3 study. The TIPS-3 [12], was a 2-by-2-by-2 factorial design trial that randomly assessed 5,713 adults with intermediate or high INTERHEART risk scores to the polycap (containing 40 mg of simvastatin, 100 mg of atenolol, 25 mg of hydrochlorothiazide, and 10 mg of ramipril) or placebo daily, aspirin (75 mg) or placebo daily, and vitamin D or placebo monthly. After a 4.6-year follow-up, the polycap plus aspirin reduced the composite primary outcome (CV death, myocardial infarction, stroke, heart failure, cardiac arrest) by 31% compared to the control group (Hazard Ratio (HR): 0.69; CI 95%: 0.50 -0.97). The benefits were independent of the level of reduction of blood cholesterol or Blood Pressure (BP). Likewise, the PolyIran study, a pragmatic, cluster-randomized trial, nested in a prospective cohort that included 6,838 adults between 40-75 years in primary and secondary prevention to receive polypill or usual care. The assessed intervention was a Statistical Process Control (SPC) containing hydrochlorothiazide 12.5 mg, aspirin 81 mg, atorvastatin 20 mg, and enalapril 5 mg (in case of cough, enalapril was replaced by valsartan 40 mg). The primary outcome was a composite of Major Cardiovascular Events (MACE) including hospitalization for acute coronary syndrome, fatal myocardial infarction, sudden death, heart failure, coronary artery revascularisation procedures, and non-fatal and fatal stroke. After a median follow-up of 60 months, was significantly lower in the polypill arm, compared to the control (HR: 0.66; 95% CI: 0.55-0.80) [13]. Interestingly, when the analysis was restricted to participants with high adherence, the reduction in the risk of MACE was even greater compared to the control group (adjusted HR 0.43, 95% CI 0.33-0.55). Furthermore, a meta-analysis of 3 randomized controlled trials (TIPS-3, HOPE-3, and PolyIran) with 18,162 adults at intermediate CVD risk showed that polypill decreased a composite outcome of CV death, myocardial infarction, stroke, or revascularization by 38% (95% CI: 27- 47%, P<0·0001) compared to standard treatment, after a median follow-up of 5 years. The addition of aspirin showed an increased 47% reduction in the main outcome (95%CI: 33-59%) [14]. In conclusion, a SPC therapy of antihypertensive drugs with statin, with or without aspirin, substantially reduces MACE in primary prevention, with greater reductions in those receiving aspirin.
Polypill in secondary prevention
The NEPTUNO observational retrospective study assessed the efficacy of the Centro Nacional de Investigaciones Cardiovasculares (CNIC)-Polypill with data obtained from administrative anonymized electronic health records from 6,456 participants in Spain [15]. After a propensity score matching and two years’ followup, the CNIC-Polypill, containing aspirin 100 mg, atorvastatin 20/40 mg, and ramipril 2.5/5/10 mg, administered once daily, improved BP (12.5% vs 6.3%; p<0.05) and LDL-C (10.3% vs 4.9%; p<0.001) control rates compared to standard treatment. The incidence of recurrent MACE was lower in the polypill cohort (19.8%) compared to the cohort using medications separately (23.3%) (p<0.001). Subsequently, the Secondary Prevention of Cardiovascular Disease in the Elderly (SECURE) trial randomized 2,499 participants with a history of myocardial infarction in the previous six months to a polypill-based strategy with the CNICPolypill or usual care. At three years of follow-up, the polypill arm significantly reduced the risk of MACE (HR: 0.76; 95%CI: 0.60-0.90), including the reduction of CV death (HR: 0.67; 95% CI: 0.47-0.97) [16]. As mentioned before, also the PolyIran trial reported a significant 20% reduction in MACE (HR: 0.80; 95% CI: 0.57-1.12) in the polypill arm after a 5-year follow-up in the secondary prevention groups. However, a meta-analysis of 8 studies with 25,584 patients [17], found no differences in MACE (RR: 0.85; 95% CI: 0.70-1.02) but a 10% reduction in the risk of all-cause mortality (RR: 0.90; 95% CI: 0.81-1.00) with the polypill intervention in secondary prevention of patients with CVD. This meta-analysis had a significant limitation, including a high range of heterogeneity, especially when evaluating adherence and treatment discontinuation. Therefore, evidence suggests that incorporating a once-daily fixed-dose cardiovascular polypill into clinical practice could significantly decrease the risk of recurrent CV events. Using a once-daily fixedâdose cardiovascular polypill should be integrated into clinical practice [18,19].
Safety of the polypill
A major reason to promote the SPC therapy strategy is that combining different classes of medications will improve efficacy while reducing side effects due to lower medication dosing. Participants using a polypill did not experience fewer side effects compared to those assigned to usual care. Therefore, reducing the side effects of individual medications does not represent an important reason for its clinical use [20]. Given the high prevalence of comorbidities in subjects with established CVD, a careful review of each polypill component is essential. For example, a polypill containing aspirin should be avoided in patients who require longterm or permanent chronic anticoagulation. Additionally, polypills should not be prescribed for patients with heart failure when an ARB/neprilysin inhibitor is indicated.
Possible explanations of the polypill benefits
In addition to its efficacy in reducing MACE, polypills increase medication adherence. A higher adherence could be driven by a reduction in pill burden, enhanced patient preferences, less therapeutic inertia, and a synergistic effect of the individual components of the pill. The polypill has been shown to improve quality of life. In the AURORA study, it was reported that 98% of participants would choose a medication regimen that included the polypill, with 92% highlighting the ease of use, and 97% considering the polypill practical and feasible [21]. Similarly, the participants in the polypill arm of the IMPACT study more frequently reported “very easy” use of this strategy compared with usual care (53% vs 46%) [22]. Therefore, regimen simplification is effective in addressing low medication adherence [23,24]. A metaanalysis of 8 randomized clinical trials with 25,584 adults showed improvement in adherence with polypill (HR: 1.31; 95%CI: 1.11- 1.55). In a recent review including real-world data, the polypill improved overall medication adherence by 13% (95% CI, 7.6% to 34.9%) compared with standard medication. For clinicians, prescribing a polypill may reduce therapeutic inertia and improve patient adherence.
Guidelines recommendations on the clinical use of polypills
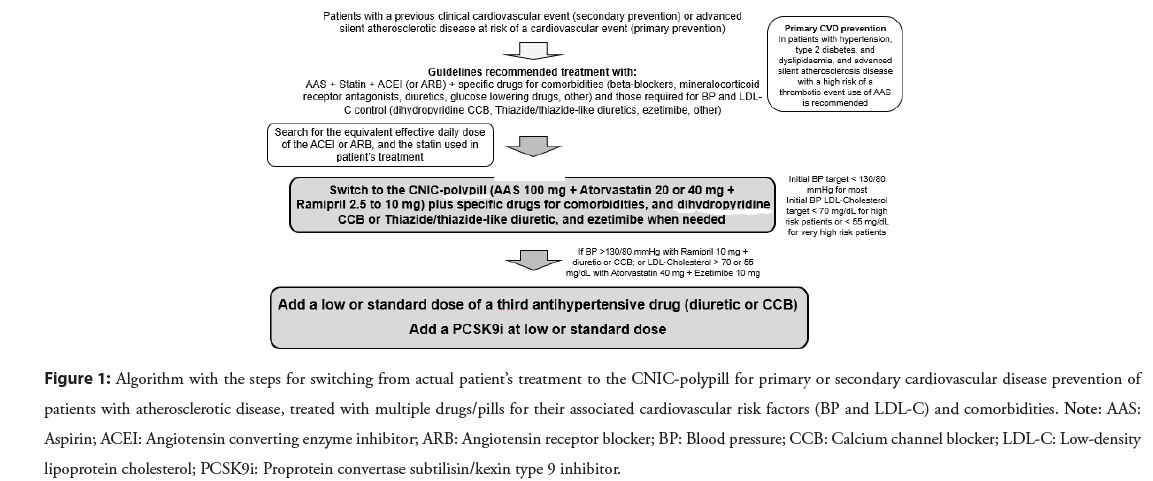
Based on the results of clinical trials demonstrating the potential benefits of CV polypills to increase adherence and reduce MACE, the 2023 European Society of Cardiology (ESC) guidelines for the management of acute coronary syndromes recommend polypills as an option to improve adherence and outcomes in secondary prevention (Class IIa, Level B) [25]. Furthermore, the 2023 European Society of Hypertension (ESH) Guidelines for the management of arterial hypertension recommends polypills in both primary and secondary prevention, clarifying that those with aspirin should be preferred in secondary prevention and polypills without aspirin in primary prevention (Class II, Level A) [26]. However, some barriers to the routine use of a polypill in clinical practice must be addressed and overcome: a) the adoption of multiple combination dosing regimens of antihypertensive medications and statin to achieve BP and cholesterol targets is difficult; b) the inclusion of dual antihypertensive therapy in some polypills to follow the recommendations of current guidelines; c) finding the correct combination of antihypertensive drugs in the polypill may be problematic since the antihypertensive agents recommended for primary prevention (ACEI or ARB plus Calcium Channel Blocker (CCB) or thiazide diuretic) differ from antihypertensive therapies required for secondary prevention (e.g. beta-blocker instead of diuretic); and d) standardized clinical algorithms with polypills for primary and secondary prevention will require more validation before their widespread use. Figure 1 shows an algorithm to help clinicians when switching from current multiple pill combination patient’s actual treatment to a polypill based treatment.
Figure 1: Algorithm with the steps for switching from actual patient’s treatment to the CNIC-polypill for primary or secondary cardiovascular disease prevention of patients with atherosclerotic disease, treated with multiple drugs/pills for their associated cardiovascular risk factors (BP and LDL-C) and comorbidities. Note: AAS: Aspirin; ACEI: Angiotensin converting enzyme inhibitor; ARB: Angiotensin receptor blocker; BP: Blood pressure; CCB: Calcium channel blocker; LDL-C: Low-density lipoprotein cholesterol; PCSK9i: Proprotein convertase subtilisin/kexin type 9 inhibitor.
Cost-effectiveness of polypills
The cost-effectiveness of a strategy should ideally be documented before being implemented, especially in regions with limited resources. Using a probabilistic approach, a study in the United Kingdom reported that a polypill was only cost-effective depending on price and population demographics [27]. Recently, a costeffectiveness study using a Markov model with the data from the NEPTUNO observational study and administrative data from Portugal was conducted [28]. The study showed that independently from the length of the horizon, the polypill improved quality of life (starting at 54.6% when the time horizon selected was two years and reaching 82% at a 20-year time horizon) compared to monotherapy. In addition, the cost-effectiveness and cost-utility ratios were below the generally recommended cost-effectiveness threshold of €10,000/QALY to €100,000/QALY. Therefore, in secondary prevention, the polypill could have a potential public health benefit as a cost-effective intervention compared to treatment with monocomponents in a multiple pill therapy.
Conclusion
Results of clinical trials have demonstrated that CV polypills increase adherence and persistence on treatment compared with the strategy based on free components in multiple pills. In addition, recent randomised clinical trials have also demonstrated that the polypill strategy reduce MACE. For these reasons, the 2023 ESC Guidelines for the Management of Acute Coronary Syndromes has recommended polypills as an option to improve adherence and outcomes in secondary prevention (CoR IIa - LoE B). Moreover, the 2023 ESH Guidelines for the management of arterial hypertension recommend the clinical use of polypills in the primary and secondary CV prevention. Polypills containing aspirin should be used in secondary CV prevention, and may be used in primary prevention of CVD in patients with advanced atherosclerotic process at high risk of CV event (CoR II LoE A).
References
- United nations department of economic and social affairs sustainable development. Goals: 3 ensure healthy lives and promote well-being for all at all ages. (2024).
- Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors,1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol 76(25):2982-3021(2020).
- World health organization. Secondary prevention of non-communicable diseases in low-and middle-income countries through community-based and health service interventions: World health organization-well come trust meeting report, 1-3 August 2001. (2002).
- Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ.326(7404):1419 (2003).
- World Heart Federation. The polypill could avoid millions of premature deaths, heart attacks and strokes every year, say leading cardiology experts. (2023).
- Ma TT, Wong ICK, Man KKC, et al. Effect of evidence-based therapy for secondary prevention of cardiovascular disease: Systematic review and meta-analysis. PLoS One.14(1):e0210988 (2019).
- Murphy A, Palafox B, O'Donnell O, et al. Inequalities in the use of secondary prevention of cardiovascular disease by socioeconomic status: Evidence from the PURE observational study. Lancet Glob Health. 6(3):e292-e301 (2018).
- Avezum A, Oliveira GBF, Lanas F, et al. Secondary cv prevention in South America in a community setting: The PURE study. Glob Heart.12(4):305-313 (2017).
- McEvoy JW, Jennings C, Kotseva K, et al. INTERASPIRE: An international survey of coronary patients; their cardio metabolic, renal and biomarker status; and the quality of preventive care delivered in all who regions: In partnership with the world heart federation, European society of cardiology, Asia pacific society of cardiology, inter American society of cardiology, and Panafrican society of cardiology. Curr Cardiol Rep.23(10):136 (2021).
- Chow CK, Nguyen TN, Marschner S, et al. Availability and affordability of medicines and cardiovascular outcomes in 21 high-income, middle-income and low-income countries. BMJ Glob Health. 5(11) (2020).
- Grigorian-Shamagian L, Coca A, Morais J, et al. The use of the CNIC-Polypill in real-life clinical practice: Opportunities and challenges in patients at very high risk of atherosclerotic cardiovascular disease expert panel meeting report. BMC Proc. 17(Suppl 8):20 (2023).
- Yusuf S, Joseph P, Dans A, et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med. 384(3):216-228 (2021).
- Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): A pragmatic, cluster-randomised trial. Lancet. 394(10199):672-683 (2019).
- Joseph P, Roshandel G, Gao P, et al. Fixed-dose combination therapies with and without aspirin for primary prevention of cardiovascular disease: an individual participant data meta-analysis. Lancet. 398(10306):1133-1146 (2021).
- Gonzalez-Juanatey JR, Cordero A, Castellano JM, et al. The CNIC-Polypill reduces recurrent major cardiovascular events in real-life secondary prevention patients in Spain: The NEPTUNO study. Int J Cardiol. 361:116-123 (2022).
- Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 387(11):967-977 (2022).
- Rao S, Jamal Siddiqi T, Khan MS, et al. Association of polypill therapy with cardiovascular outcomes, mortality, and adherence: A systematic review and meta-analysis of randomized controlled trials. Prog Cardiovasc Dis.73:48-55 (2022).
- Coca A, Kreutz R, Manolis A, et al. A practical approach to switch from a multiple pill therapeutic strategy to a polypill-based strategy for cardiovascular prevention in patients with hypertension. J Hypertens.38(10):1890-1898 (2020).
- Coca A, Castellano JM, Camafort M, et al. Polypill in cardiovascular disease prevention: Recent advances. Pol Arch Intern Med.133(3):16460 (2023).
- Lopez-Lopez JP, Gonzalez AM, Lanza P, et al. Benefits of the polypill on medication adherence in the primary and secondary prevention of cardiovascular disease: A systematic review. Vasc Health Risk Manag. 19:605-615 (2023).
- Cosin-Sales J, Murcia-Zaragoza JM, Pereyra-Rico HO, et al. Evaluating patients' satisfaction and preferences with a secondary prevention cardiovascular polypill: The aurora study. J Comp Eff Res.10(13):975-985 (2021).
- Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: Randomised controlled trial in primary care. BMJ. 348:g3318 (2014).
- Parati G, Kjeldsen S, Coca A, et al. Adherence to single-pill versus free-equivalent combination therapy in hypertension. A systematic review and meta-analysis. Hypertension.77(2):692-705 (2021).
- Tsioufis K, Kreutz R, Sykara G, et al. Impact of single-pill combination therapy on adherence, blood pressure control, and clinical outcomes: A rapid evidence assessment of recent literature. J Hypertens.38(6):1016-1028 (2020).
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 44(38):3720-3826 (2023).
- Mancia G, Kreutz R, Brunström M, et al. 2023 ESH Guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the European society of hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J Hypertens.41(12):1874-2071 (2023).
- Jowett S, Barton P, Roalfe A, et al. Cost-effectiveness analysis of use of a polypill versus usual care or best practice for primary prevention in people at high risk of cardiovascular disease. PloS One.12(9):e0182625 (2017).
- Aguiar C, Araujo F, Rubio-Mercade G, et al. Cost-Effectiveness of the CNIC-Polypill strategy compared with separate monocomponents in secondary prevention of cardiovascular and cerebrovascular disease in Portugal: The MERCURY Study. J Health Econ Outcomes Res.9(2):134-146 (2022).