Review Article - Interventional Cardiology (2013) Volume 5, Issue 3
Primary percutaneous coronary intervention: devices to prevent no-reflow phenomenon
- Corresponding Author:
- David Meerkin
Department of Cardiology, Shaare Zedek Medical Center, PO Box 3235, Jerusalem 91031, Israel
Tel: +972 265 559 75
Fax: +972 265 554 37
E-mail: meerkin@szmc.org.il
Abstract
Keywords
mesh-covered stent;primary PCI;thrombus aspiration.
Introduction
The superiority of primary percutaneous coronary intervention (PPCI) over conventional thrombolytic treatment for ST-elevation myocardial infarction (STEMI) has been demonstrated in randomized controlled trials (RCTs). This has resulted in it becoming the treatment of choice when available [1–3]. PPCI aims to restore normal coronary arterial perfusion and is successful in most cases. However, in up to 40% of patients, normal perfusion is not completely achieved despite successful treatment of the occlusive culprit lesion, as evidenced by reduced thrombolysis in myocardial infarction (TIMI) flow, failure of ST-segment resolution (STR) and poor myocardial blush grade (MBG) [4].
This phenomenon, known as no or slow reflow in its most pronounced manifestations, has been associated with adverse clinical outcomes and has been attributed to a combination of distal embolization of plaque debris, vasoconstriction and reperfusion injury [5,6]. Several STEMI studies demonstrated that percutaneous coronary intervention (PCI) resulted in approximately 15% distal embolization rate [7,8]. Microembolization may lead to the occlusion of arterioles in the microcirculation, thus impairing end myocardial perfusion, whereas embolization of larger atherosclerotic particles can lead to the occlusion of prearterioles and side branches [9,10]. The resulting capillary edema, along with endothelial dysfunction and leukocyte activation, leads to impaired oxygen delivery and eventually to myocardial cell necrosis [11]. It is, therefore, not surprising that in patients with no reflow, infarct sizes are larger, early postinfarction complications are more common and longer-term outcomes, such as left ventricular function and survival, are worse [12–15]. According to the TYPHOON STEMI trial, 7.5–14.3% of patients with acute myocardial infarction (MI) treated with primary PCI experience major adverse cardiac events (MACEs), including cardiac death, heart attack and restenting of the artery [16].
Protection & thrombectomy devices in PPCI
In limited instances, distal embolization may occur with spontaneous reperfusion or in association with diagnostic angiography; protection devices, in these cases, would be of reduced value. However, the clinical need to limit such phenomena and the fact that distal embolization appears to occur predominantly at the time of intervention in association with a large thrombus burden, has led to the development of dedicated devices to prevent this problem [10].
These devices, although aimed at reducing the thrombus burden washed downstream, fall into three categories: protection (distal or proximal), thrombectomy devices and mesh-covered stent. Although distal protection devices have demonstrated efficacy in preventing reduced flow following saphenous vein graft interventions [17], studies, to date, have failed to demonstrate a benefit for these devices in the setting of PPCI [18–20]. Although not proven, it seems likely to be due to the native coronary artery not being a conduit vessel with a tapered vessel caliber and multiple side branches emerging and escaping protection. This, associated with imperfect vessel-wall apposition by the assessed devices, appears to be the principal cause for their lack of benefit in native coronary occlusion treated by PPCI for STEMI. Potential additional explanations include embolization during passing of the device, embolization prior to device crossing and the possibility that the microvasculature is already dysfunctional prior to use of the device. It should, however, be noted that distal protection devices have not been evaluated by RCTs in PPCI in vein grafts. Proximal protection overcomes some of these limitations, including poor wall apposition, side-branch escape close to the occlusion and intervention site, as well as a small distal vessel. The limitations of these systems are that the vessel needs to be occluded and aspirated during the intervention, possibly causing an even greater ischemic area, as well as the possibility of bringing thrombus to more proximal sites and branches.
To date, evidence appears to be more promising for thrombectomy devices, as recognized by recent international guidelines [21].
Thrombectomy devices vary in design and mechanism of action but can be broadly divided into two groups depending on the presence or absence of a motorized system.
Mechanical thrombectomy
Mechanical thrombectomy devices vary in their working mechanism depending on their ability to actively fragment atherosclerotic thrombus material prior to aspiration (Table 1). The Angio- Jet® (MEDRAD, PA, USA), X-Sizer® (eV3 Inc., MN, USA) and Rinspirator™ System (eV3 Inc.) catheters are capable of such active thrombus fragmentation.
| Device | Description |
|---|---|
| (manufacturer) | |
| AngioJet® (MEDRAD, | Rheolytic thrombectomy system consisting of a drive unit, a disposable pump set and a thrombectomy catheter |
| PA, USA) | that tracks over a guidewire (6 F compatible) |
| High-velocity saline jets are directed back into the catheter, creating a low-pressure zone at the distal tip | |
| (Bernoulli principle), which results in suction, break-up and removal of thrombus through the outflow lumen | |
| (Figure 1) | |
| X-Sizer® (eV3 Inc., MN, | Double lumen over-the-wire system (7 F compatible) with a helical shape cutter at its distal tip |
| USA) | The cutter rotates at 2100 rpm driven by a handheld battery motor unit |
| One catheter lumen is connected to a 250-ml vacuum bottle, and aspirated debris is collected in an inline filter | |
| Two or three passages across the lesion are performed (Figure 2) | |
| Rinspirator™ (eV3 Inc.) | Double lumen over-the-wire system (6 F compatible) for simultaneous aspiration and infusion of heparinized |
| saline | |
| Injection of saline through perforations located proximal to the aspiration hole of the catheter generates | |
| turbulent flow that rinses the vessel wall, detaches any adherent thrombus and simultaneously evacuates the | |
| thrombotic material from the vessel | |
| Rescue™ (Boston | 4.5 F aspiration catheter advanced over a guidewire through a 7 F guiding catheter |
| Scientific, MA, USA) | The proximal end of the catheter has an extension tube connected to a vacuum pump (0.8 bar) with a collection |
| bottle | |
| The catheter is slowly advanced and pulled back through the thrombus, while continuous suction is applied | |
| TransVascular Aspiration | Single lumen catheter (7 F compatible) with a beak-shaped distal tip |
| Catheter® (Nipro, | The catheter is attached to an aspiration pump for vacuum formation and removal of thrombotic material |
| Osaka, Japan) |
Table 1. Mechanical thrombectomy devices.
▪ AngioJet
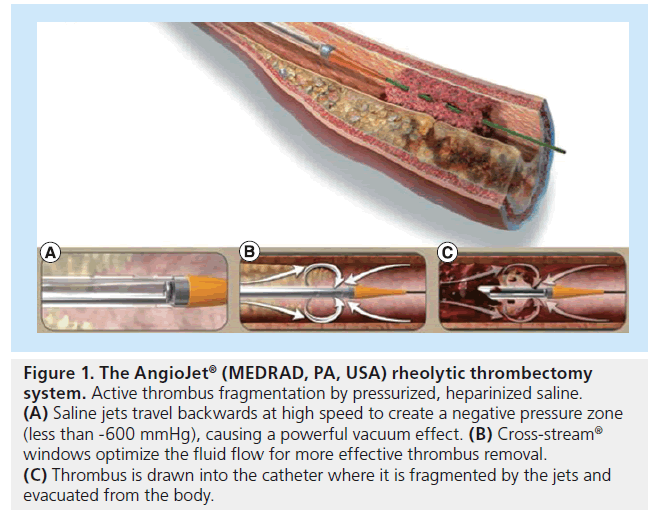
The mechanism in the AngioJet rheolytic thrombectomy (RT) system (Figure 1) involves the delivery of pressurized heparinized saline from the catheter, where saline jets travel backwards creating a low-pressure zone and, thus, a powerful vacuum effect. Thrombus, as a result, is drawn back into the catheter, where it is fragmented by the saline jets prior to being evacuated from the body.
Figure 1: The AngioJet® (MEDR AD, PA, USA) rheolytic thrombectomy system. Active thrombus fragmentation by pressurized, heparinized saline. (A) Saline jets travel backwards at high speed to create a negative pressure zone (less than -600 mmHg), causing a powerful vacuum effect. (B) Cross-stream® windows optimize the fluid flow for more effective thrombus removal. (C) Thrombus is drawn into the catheter where it is fragmented by the jets and evacuated from the body.
The use of the AngioJet system as an adjunct in PPCI has been tested in three randomized trials. An initial single-center study enrolling 100 patients demonstrated a reduction of infarct size as assessed by Tc-99m sestamibi scintigraphy and better STR when compared with standard PPCI [22]. The larger, multicenter, randomized AiMI trial that included 480 patients, however, failed to reproduce these initial findings [23]. Importantly, the MACE rate was higher in the thrombectomy group, driven by an increase in 30-day mortality. The discrepancy between the two studies could be explained to some extent by the complexity of the device and, therefore, the impact of operator experience in its use. This also may explain the higher rate of coronary perforation observed in the AiMI trial. In addition, TIMI 3 flow prior to intervention was encountered significantly more often in the standard PPCI than in the thrombectomy group, potentially biasing the differences in infarct size between the two groups. Finally, angiographic evidence of thrombus was absent in a large percentage of both groups (25%) raising the possibility that the AngioJet device is best suited in cases with significant and visible thrombus burden. Use of the AngioJet device, on the other hand, has been associated with an increased incidence of symptomatic bradycardia, thus requiring positioning of a temporary pacing wire, which, in itself, is not without potential complications. Both of these studies were performed over 7 years ago with suboptimal antiplatelet regimens [22,23].
The recently published JETSTENT trial aimed to answer the questions raised by the previous two conflicting studies [24]. It recruited 501 patients, all of whom were treated with dual antiplatelet therapy (300-mg aspirin and 600- mg clopidogrel) and glycoprotein (GP) IIb/IIIa antagonists. Exclusion criteria included TIMI thrombus grade <3 and infarct artery reference diameter <2.5 mm on visual assessment. The presence of thrombus as a prerequisite for entry into the study was an important difference between the JETSTENT and AiMI studies. No significant difference in STR, angiographic end points or myocardial infarct size between the AngioJet and conventional treatment groups were detected by Tc-99m sestamibi scanning. Interestingly, however, a significant decrease in MACE was noted at 6 and 12 months in the AngioJet group, driven primarily by a lower incidence of death and target-vessel revascularization. This was attributed by the authors to improved myocardial perfusion and better stent length and diameter assessment following RT. The results of the JETSTENT, however, need to be interpreted with caution. In this study, infarct size and STR, both established surrogates of prognosis following MI, showed no benefit with the AngioJet device. It is, therefore, difficult to explain what drives the MACE advantage associated with its use. Owing to this anomaly and the associated costs of the device, it has not gained widespread clinical acceptance in PPCI.
▪ X-Sizer
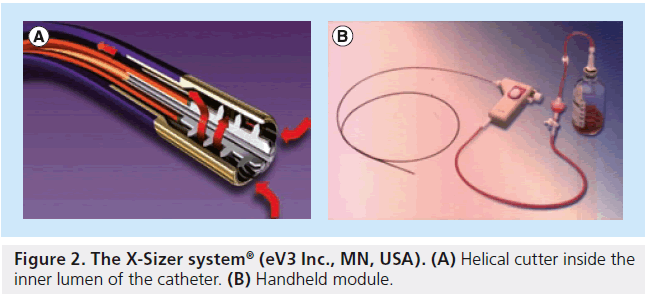
The X-Sizer device consists of a dual-lumen hydrophilic-coated catheter shaft connected to a handheld control module (Figure 2). Once the catheter is engaged, the vacuum captures the thrombus, and the helical cutter present inside the inner lumen and inside the distal tip shears this off. The efficacy of the X-Sizer catheter in STEMI patients has been assessed in three randomized trials, and although it has been shown to improve STR at 60 min post-PCI, achieve better angiographic flow, reduce no reflow and distal embolization of atherosclerotic plaque debris, its use did not provide significant clinical benefit at 1 and 6 months (X-AMINE ST trial) [25–27]. Moreover, the routine use of the X-Sizer catheter in PPCI is limited by its rigidity and, thus, inability to navigate tortuous and heavily calcified vessels, as well as by its bulky size, which limits its use to vessels >2.5 mm and without very tight lesions. In one study, failure of X-Sizer thrombectomy was encountered in 24% of cases, and this was associated with increased incidence of 30-day adverse events [28]. It has also been associated with an increased risk of coronary artery perforation [29].
▪ Rinspirator
The Rinspirator system is a newer non-manual thrombectomy device consisting of three lumens. The first is a standard coronary guidewire lumen, whereas the second allows for distal aspiration. The third lumen allows injection of a rinsing solution (heparinized saline) through perforations located proximal to the aspiration lumen and distributed circumferentially along a short length of the catheter. This generates turbulent flow that rinses the vessel wall, detaches any adherent thrombus and simultaneously evacuates the thrombotic material from the vessel. According to the initial data from an international registry, this device is safe and its use does not seem to be associated with higher complication rates [30]. To date, however, no clinical benefit has been established.
▪ Vacuum thrombectomy
The TransVascular Aspiration Catheter® (Nipro, Osaka, Japan) and Rescue™ (Boston Scientific, MA, USA) devices do not offer active thrombus fragmentation, but as they are connected to motorized vacuum units they can be considered mechanical thrombectomy devices. Only the TransVascular Aspiration Catheter system has shown some promise based on the results of a multicenter randomized trial (VAMPIRE) [31]. This study showed a marginal benefit of thrombectomy on myocardial perfusion as assessed by final TIMI flow and MBG, with the most benefit observed in patients presenting 6 h after symptom onset. MACE rates were similar at 30 days to standard PPCI, but a significant reduction in MACE was seen at 8 months in the thrombectomy group, mainly as a result of lower rates of revascularization in the treatment group. This was attributed to the better TIMI flow following thrombectomy, which may have facilitated better selection of stent diameter and length, as well as to the removal of inflammatory thrombus material. The use of glycoprotein IIb/IIIa inhibitors and drug-eluting stents were not allowed in the VAMPIRE study and it has been conjectured that in the presence of such adjunctive pharmacological intervention, the rate of future revascularization in the control group may have been more comparable to that of the treatment group.
The Rescue system has not been shown to improve infarct size or myocardial salvage as measured by sestamibi SPECT, nor have a beneficial effect on STR, MBG and left ventricular ejection fraction in randomized trials. Equally important, it has been associated with a high rate of procedural failure in the randomized trial by Kaltoft et al. and other study groups due to failure of the catheter to reach the culprit lesion [32–35].
▪ Meta-analyses
In a meta-analysis including multiple devices, Bavry et al. showed that routine use of mechanical thrombectomy in setting of PPCI appears to significantly increase mortality 5.3 versus 2.8% for PCI alone, whereas the use of embolic protection devices has no impact on survival [36]. Negative results of mechanical thrombectomy in this meta-analysis were driven primarily by the largest study in this category – the AiMI trial (AngioJet system) that showed increased infarct size and 30-day mortality rate in RT group. It must be noted that very low mortality rate was observed in patients treated with PCI alone (0.8 vs 4.6% in patients treated with adjunct RT; p = 0.02). Limitations of this work are described above.
Another meta-analysis, the ATTEMPT study, which included pooled analysis on 2686 individual patients’ data from 11 randomized trials, comparing thrombectomy plus PPCI with standard PPCI, showed that allocation to mechanical thrombectomy devices, particularly those which involve thrombus fragmentation prior to aspiration, failed to produce a survival benefit [37]. These data are in agreement with a recent meta-analysis by Costopoulos et al., showing that although mechanical thrombectomy was associated with better STR, it failed to produce any benefit in regarding to MBG, TIMI 3 flow, mortality and MACE [38]. When taken alone, mechanical vacuum aspiration was shown to improve STR and MBG but not TIMI 3 flow. Improvement in clinical outcomes was not seen with mechanical vacuum devices, although this, to some extent, could be the result of low overall patient numbers from the four pooled RCTs.
There are several possible explanations for the disappointing results seen with mechanical thrombectomy. First, they tend to be bulkier and have a longer setup time, resulting in longer procedure times. In fact, all mechanical thrombectomy trials [23,27,33,39] showed longer procedure time compared with conventional PCI, a finding not present in the manual aspiration trials [31,40,41]. Such differences in procedure times may have an impact especially in patients presenting within 3 h of the onset of infarction. Second, mechanical thrombectomy devices are more complex to operate than manual aspiration catheters, with resultant less favorable learning curves. Operator and staff experience regarding the use of these complex devices is, thus, important and perhaps limited if one considers that most PPCIs occur out-of-hours when staffing levels are reduced and the likelihood of complications is higher. They are, therefore, best avoided in routine PPCIs, although they may have a role in patients with large vessels and a particularly heavy thrombus burden as some studies suggest [42].
Manual thrombectomy
Manual thrombectomy devices offer a major benefit over their motorized equivalents, in that they are much simpler to use. Most of these systems comprise a monorail catheter with a central lumen, which communicates with one or more holes located at the tip. The catheter is connected proximally to a syringe for manual aspiration. Manual thrombectomy devices in current clinical use include the Pronto™ (Vascular Solutions, MN, USA), Export® (Medtronic, MN, USA), Diver™ CE (Invatec, Roncadelle, Italy), QuickCat™ (Spectranetics Inc., CO, USA) and Hunter® (IHT Cordynamic, Barcelona, Spain) amongst others (Table 2). All of these devices are based upon similar principles, but differ in terms of catheter material, aspiration lumen size and configuration, with theoretical differences in deliverability and thrombus extraction.
| Device (manufacturer) | Description | |
|---|---|---|
| Diver™ CE (Invatec, Roncadelle, Italy) | Dual-lumen catheter with hydrophilic coating (6 F compatible) It has a central aspiration lumen running through its full length and a soft tip with multiple holes communicating with the lumen A 30-ml Luer lock syringe is connected to proximal end for blood aspiration and clot removal | |
| Pronto™ (Vascular Solutions, MN, USA) | Dual-lumen, monorail design (6 F compatible) catheter The smaller lumen accommodates a standard 0.014-inch guidewire The larger extraction lumen allows the removal of the thrombus, which is aspirated in a 30-ml syringe The catheter has a rounded distal tip designed to maximize thrombus aspiration and to protect the vessel, while advancing and during aspiration | |
| Export® (Medtronic, MN, USA) | 6 F catheter, which crosses the target lesion over a floppy guidewire and aspirates the thrombus into a 20-ml syringe The aspiration rate is >30 ml of fluid per minute The total usable length is 145 cm | |
| QuickCat™ (Spectranetics Inc., CO, USA) | Dual-lumen catheter with hydrophilic coating (6 F compatible) | |
| Hunter® (IHT Cordynamic, Barcelona, Spain) | Dual-lumen catheter (6 F compatible) | |
Table 2. Manual thrombectomy devices.
▪ Trial results
The first randomized PPCI trial that tested a manual thrombectomy device was the REMEDIA study (Diver CE), which randomized a small number of patients (n = 99) to PPCI plus manual thrombectomy or standard PPCI [40]. Results showed that Diver CE device delivery was not only successful and safe, but its use was also associated with significantly better MBG and STR, as well as decreased risk of no reflow, slow reflow and distal embolization. A subgroup analysis showed that patients with an occluded artery or higher thrombus burden benefited most from manual thrombus aspiration. These positive findings, however, did not extrapolate to a clinical advantage, as the study was underpowered to assess this as an end point. In another small (n = 76) randomized study, De Luca et al. found that in patients with anterior STEMI, the use of Diver CE was associated with better postprocedure MBG and more effective STR at 90 min [43]. Echocardiography 6-months post-PPCI showed that thrombectomy use was associated with smaller end-diastolic and endsystolic volumes. These beneficial effects in LV size again did not lead to improved clinical outcomes compared with the standard PCI group, although here too the study was underpowered to detect any clinical benefit. Similar results to the De Luca et al. and REMEDIA studies were observed in the slightly larger (n = 196) multicenter randomized PIHRATE trial, where no difference between the groups in 6-month mortality or re-infarction rate were observed [44].
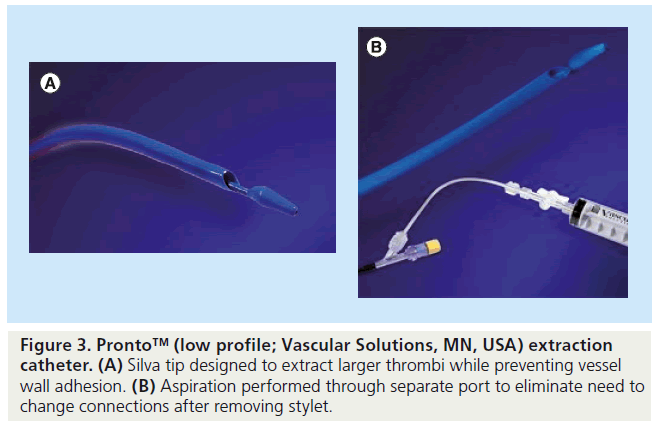
The efficacy of the Pronto catheter (Figure 3) in acute MI was examined in the DEAR-MI trial where 148 patients were randomized to standard PCI or PCI plus manual thrombectomy. The thrombectomy group showed improved STR as well as reduced incidence of distal embolization and no reflow [41]. Pronto catheters used in PPCI include the V3 and low-profile versions with the latter being able to tackle vessels as small as 1.5 mm.
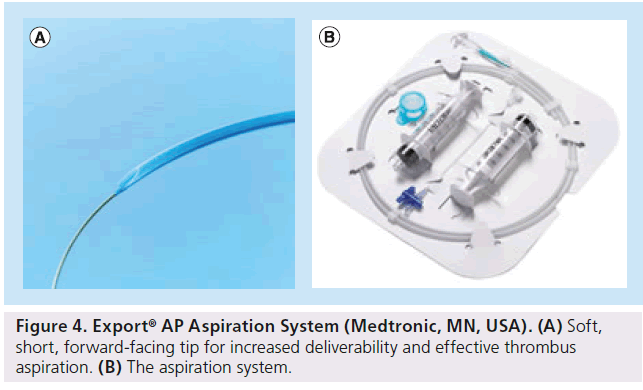
The Export catheter (Figure 4) has been tested in a number of trials. In the EXPIRA trial 175 patients were recruited with STR and MBG being set as primary end points. The secondary end point was MACE at 9 months [45]. GP IIb/IIIa inhibitors were used in all patients and no restrictions were set regarding stent type (i.e., bare-metal stent vs drug-eluting stent). In fact, stents were used in the majority of the patients (58%) and their use was similar in both groups. Results showed that manual thrombectomy was associated with improved STR at 90 min, better MBG, as well as reduced microvascular damage and infarct size at 3 months as assessed by cardiac MRI (only anterior STEMI patients; n = 75). A lower incidence of cardiac death in the thrombectomy group 0 versus 4.6% (p = 0.02) was observed at 9 months. Recently, published data from the same trial at 24 months confirm a clinical benefit associated with manual thrombectomy in the terms of MACE and cardiac death [45].
The Export catheter was also used in the larger TAPAS trial [46]. This was a single-center randomized trial that recruited 1071 patients. All the patients received standard therapy, including GP IIb/IIIa inhibitors, unless contraindicated. MBG and STR were significantly improved in the thrombus aspiration group. In addition, manual thrombectomy was associated with a lower incidence of cardiac death and nonfatal re-infarction at 1 year. Importantly, target-vessel revascularization did not differ significantly between the two groups, indicating that the clinical benefit observed with manual thrombectomy was not owing to a lower incidence of further intervention. Although the TAPAS trial did not assess infarct size or left ventricular function, results suggest that manual thrombectomy in STEMI patients improves myocardial perfusion, and this seems to confer a survival benefit at 1-year follow-up. Surprisingly, however, the survival benefit seems to be more pronounced and not in keeping with the degree of MBG and STR benefit.
In contrast to single-center studies, multicenter aspiration trials have largely been negative. Conflicting results regarding infarct size reduction or improved clinical outcomes are possibly due, in part, to differences in patient selection, devices and study methodology. Moreover, many patients enrolled in these trials had a small amount of myocardium at risk (e.g., nonanterior MI); presented up to 12 h after infarct onset, well beyond the time window for effective myocardial salvage; or both. Interestingly, the most recent multicenter prospective trial, in which 452 patients presenting early with large anterior STEMI undergoing primary PCI were randomized in a 2 × 2 factorial design to bolus intracoronary abciximab versus no abciximab and to manual aspiration thrombectomy versus no aspiration (Infuse-AMI trial [47]), failed to show infarct size reduction assessed by cardiac MRI or 30-day MACE reduction in the thrombus aspiration group. This study was specifically designed to maximize the likelihood that a reduction in infarct size could be demonstrated with intracoronary abciximab, aspiration thrombectomy, or both, if indeed such a reduction truly exists.
It should be noted that the majority of randomized trials have shown that manual thrombectomy is associated with improved MBG, STR and TIMI flow, all of which are well-known predictors of future clinical events. However, until the TAPAS trial, most studies were statistically underpowered to demonstrate clinical benefit.
▪ Meta-analyses
In the absence of large, multicenter trials adequately powered to assess long-term outcome, meta-analyses can contribute to our understanding.
In a Bayesian meta-analysis, including 21 trials (16 that used a simple aspiration thrombectomy device), Mongeon et al. found that thrombectomy yielded substantially less no reflow, more STR and more TIMI 3 perfusion, but there was no evidence of reductions in death, recurrent MI or stroke 30-day post-MI. Restriction of the analysis to trials that used simple aspiration thrombectomy devices did not yield substantially different results [48]. The fact that clinical end points were not reduced likely reflects low statistical power to detect such differences. The overall number of end points were relatively low, and was based on enrollment of relatively lowrisk patients (mortality only 3.2% in the largest trial, for example), and the very short follow-up period [49].
A pooled analysis of 2686 individual patients from 11 randomized trials, comparing thrombectomy plus PPCI to standard PPCI, the ATTEMPT study, showed that allocation to thrombectomy was associated with significantly lower MACE and death plus MI rates [37]. Importantly, the survival benefit of thrombectomy was not only present at 1-year follow-up, but continued in the subgroup of patients that was followed beyond this.
Moreover, ATTEMPT showed that the survival advantage of thrombectomy was confined only to patients treated by manual thrombectomy with an estimated 34 patients needed to be treated to prevent one death at 1 year. In addition, subgroup analysis showed that the greatest benefit from thrombectomy was seen in patients treated with GP IIb/IIIa inhibitors, suggesting that these should be used routinely with thrombectomy.
The recent meta-analysis of ten randomized trials assessing manual thrombectomy (most of them single-center trials including STEMI patients with time from symptoms onset to intervention less than 12 h) performed by Costopoulos et al., not only showed that manual thrombectomy was associated with better STR, MBG and TIMI 3 flow rate, but also a 43% mortality reduction (p = 0.04) and strong trend towards benefit when the composite of death, stroke and MI was examined (relative risk: 37%; p = 0.05) [38]. These findings are in agreement with the meta-analysis performed by Bavry et al. [36] and the recent abstract by Kikkert et al., who examined 5851 patients concluding that aspiration thrombectomy is associated with improved survival [50].
Current evidence supports the routine use of manual thrombectomy in PPCI, as this has been shown to improve angiographic, electrophysiological, as well as clinical outcomes, in PPCI patients. Its effect is likely to be the result of reduced distal embolization and, thus, improved myocardial reperfusion, as well as more precise assessment of stent parameters. It also favors direct stenting over lesion preparation with predilatation possibly due to distal embolization.
Although vessel tortuosity, calcification and poor guide catheter support may not allow the use of thrombectomy devices in all cases, their benefits are becoming more widely recognized. It is, therefore, not surprising that manual thrombectomy has received a class IIa level of evidence B indication in PPCI in the recent American College of Cardiology/American Heart Association and the European Society of Cardiology guidelines [51,21].
In order to provide a conclusive answer to the thrombectomy dilemma, three large multicenter, prospective RCTs are currently being conducted. First, the world’s largest study of thrombus aspiration in MI, ongoing in Scandinavia, comparing PPCI and prior manual thrombectomy with PPCI. It is planned to include 5000 patients over 29 centers in three countries. Primary end points are time to all-cause death at 30 days. The results should be available in the near future (TASTE trial) [101]. The second trial is ongoing in 27 centers of Korea, comparing PPCI with thrombectomy using Export catheter to PPCI. It is planned to include 1400 patients. Primary composite end points of cardiac death, Q-wave MI and triple vessel disease will be assessed 12 months after index intervention. The results should be available in 2014 (ETAMI trial) [102]. Finally, the TOTAL trial, a Canadian-based study aimed at including 4000 patients, is currently randomizing STEMI patients to manual aspiration followed by PCI with PCI alone [103]. Primary outcomes are cardiovascular death, MI, cardiogenic shock and class 4 CHF up to 180 days.
Mesh-covered stent
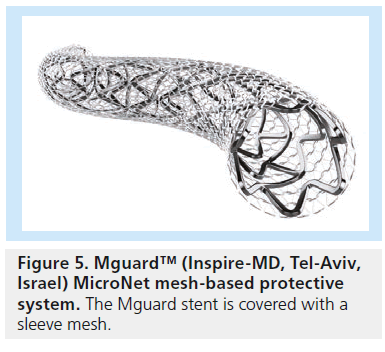
Neither mechanical nor pharmacological strategies have successfully prevented the thrombotic complications coupled with PCI in the context of high thrombotic burden. Although not strictly within the definition of thrombectomy devices, a new strategy has been developed in recent years to reduce the risk of distal embolization during PCI in STEMI patients. The MGuard™ system (Inspire-MD, Tel-Aviv, Israel) was designed using a conventional stainless steel bare-metal stent covered with an ultrathin flexible polyethylene theraphthalate (or Dacron) mesh sleeve that is anchored to the external surface of the struts (Figure 5). During stent deployment, the net stretches and slides over the expanding stent struts, trapping the thromboembolic debris underneath the fiber net and isolating the prothrombotic intimal components from the blood stream. These characteristics make this device very attractive for any intervention where thromboembolic complications might affect procedural and clinical success, such as PCI in a STEMI setting. However, at present, major randomized trials are still lacking to characterize the clinical and/or prognostic utility of the MGuard stent device.
Several feasibility registries and trials have been completed using this approach.
▪ Trial results
The first two studies were conducted in Germany and Brazil and included 71 patients with either saphenous vein graft coronary interventions or native coronary lesions treatable by a stenting procedure [52,53].
The first study in STEMI patients was conducted in Poland and included 60 patients. The purpose was to confirm the clinical performance of MGuard stents in primary PCI. Predilatation was performed in 62% of cases and thrombus aspiration in 18%. TIMI 3 blood flow in the infarctrelated artery was achieved in 90% of patients with myocardial blush grade 3 in 73% of patients and complete (>70%) STR was achieved in 61% of patients. No case of no-reflow phenomenon was observed intraprocedurally, nor MACE during the 6-month follow-up [54].
In a UK registry of 51 patients with STEMI, procedural success was achieved in all patients with final TIMI 3 flow, STR (>50%) in 96% of patients. In-hospital MACE (cardiac death) was in 4% of patients, up to 12-month followup MACE was 6% of patients (all target-vessel revascularization) [55].
An Italian multicenter registry of 100 STEMI patients, including 16 patents with cardiogenic shock, reported postprocedural mean TIMI flow grade 2.85, post-PCI MBG3 was achieved in 90% of patients, STR (>70%) in 90% of patients. During in-hospital follow-up, seven deaths and two cases of stent thrombosis occurred. After hospital discharge, no MACE was reported up to 30-day follow-up [56].
The largest real world, prospective, nonrandomized, multicenter registry of patients receiving the MGuard coronary stent (iMOS registry) is ongoing. A total of 372 patients in 20 centers have been enrolled and presented, 203 of them with STEMI. Thrombus aspiration was performed in 41% of patients and predilatation in 43%. Final TIMI 3 flow was achieved in 93.5% patients, MBG3 80%, STR (>50%) 85% of patients. At 1-year follow-up it was 2.8% ischemic death, MI 3.2%, TLR 4% and MACE 6.8% [57].
To date, PCI with MGuard stent represents an efficient and safe approach in treating STEMI patients, with low rates of MACE and high rates of procedure and angiographic success.
A meta-analysis of data from the completed worldwide registries of STEMI patients treated with MGuard stent in comparison to data contained in published reports of standard PPCI in comparable patients was performed. It revealed that final TIMI 3 flow was reached in 95% of MGuard patients, compared with 87% in patients who underwent PCI with normal bare-metal stents plus thrombus aspiration and compared with 81% in patients without thrombus aspiration. More patients who received MGuard stent experienced restoration of normal electrocardiogram reading (79 vs 57 and 44% accordingly) and MBG3 (83 vs 52 and 32% accordingly) with MGuard than with bare-metal stents plus aspiration or bare-metal stents alone.
In addition, the occurrence of MACEs at 1-year postdeployment was 5.4%, compared with 9.5% in patients treated with drug-eluting stents and 17.8% with bare-metal stents.
In the 2010 European Guidelines on Myocardial Revascularization, mesh-based protection is now recommended for use: “Mesh-based protection may be considered for PCI of highly thrombotic or saphenous vein graft lesions” (class IIb/C recommendation) [58].
Taking into account all these encouraging data generated from MGuard STEMI registries, an international multicenter RCT (MASTER) was designed to compare PPCI with MGuard stent versus BMS or drug-eluting stents in more than 400 STEMI patients with clinical followup for up to 1 year. Enrollment was recently completed and initial results were presented at the Transcatheter Cardiovascular Theraputics (October 2012). These showed improved postprocedural STR in the MGuard stent group, as well as better TIMI flow at procedure completion. A trend to improved survival at 30 days with this novel stent was also apparent (zero out of 217 vs four out of 214; p = 0.06) [59]. For definitive conclusions regarding infarct size or clinical events, longer-term follow-up is necessary.
Conclusion
PPCI has overcome thrombolysis in the management of STEMI as clinical outcomes have been shown to be superior with PPCI. However, in the face of high thrombus burden in a significant proportion of patients, myocardial perfusion is not fully achieved despite TIMI 3 flow in the epicardial vessel. This phenomenon has been attributed mostly to the distal embolization of thrombi particles and atherosclerotic plaque debris, and has been associated with poorer short- and long-term outcomes including heart failure and death. Pharmacological measures, such as adequate antiplatelet therapy, GP IIb/ IIIa antagonists and coronary vasodilators, aim to reduce this phenomenon, but fail to abolish it. This has led to the development of devices dedicated to evacuate or trap thrombus prior to stenting to reduce the risk of distal embolization during PCI. A number of randomized trials have been performed that have tested the efficacy of these devices in improving MBG, TIMI flow, STR, infarct size and clinical outcomes. The majority of these trials (especially with manual thrombectomy devices) have shown that thrombectomy is associated with improved surrogate end points such as MBG, TIMI flow and STR, all of which are well-known predictors of future clinical events. However, until the TAPAS trial, most studies were statistically underpowered to demonstrate clinical benefit with thrombectomy.In agreement with the improved clinical outcome that was shown in the TAPAS trial, a few meta-analyses comparing thrombus aspiration and standard PPCI have confirmed lower death and MACE rates, particularly in patients allocated to manual thrombectomy. This effect is likely to result from reduced distal embolization and, thus, better myocardial reperfusion, as well as better and more accurate assessment of stent parameters. It also favors direct stenting, possibly precluding predilatation that itself can cause distal embolization. It should be noted that mechanical thrombectomy devices have failed to produce such benefits.
Despite the encouraging results of the TAPAS trial and a few meta-analyses, a skepticism regarding the benefit of thrombectomy in acute MI still exists. The results of the INFUSE-AMI trial, which was specifically designed to maximize the likelihood that a MACE reduction could be demonstrated with aspiration thrombectomy, if indeed such a reduction truly exists, were disappointing. The most recent ACC and AHA guidelines upgraded thrombus aspiration to a class IIa recommendation – it is reasonable to perform the procedure – with a level of evidence B (i.e., limited populations evaluated). However, it is widely acknowledged that large outcomes trials are needed to establish whether this procedure should be firmly adopted or discarded.
In recent years, a new strategy, using a meshcovered stent in PPCI aimed at minimizing distal embolization by blocking debris at its source, has been tested. International registries using this mesh stent in PPCI have demonstrated promising results, improved TIMI flow, STR, MBG and MACE 1-year postdeployment. Longer-term follow-up in an ongoing large RCT designed specifically to assess short- and long-term clinical outcome will hopefully resolve any remaining uncertainties regarding use of this device for distal embolization protection during PPCI.
Future perspective
Attempts to adequately cope with the thrombus burden and its incumbent complications in the STEMI patient population has led to the development of pharmacological and mechanical innovations aimed at improved outcomes. Although, benefits have been achieved with thrombectomy devices, and the positive feedback that is achieved by the visible evidence of thrombus extraction, results are unpredictable due to variable vessel and thrombus anatomies. The development of more effective and user-friendly devices associated with novel approaches, such as the mesh-covered stent, as well as an optimal pharmacological regime, may well lead to more reliable and effective results. The challenge of thrombus burden remains an appropriate and desirable avenue of investigation owing to the potential impact on outcomes in the relatively high-risk STEMI population. However, this challenge is complicated by the increasing difficulty in demonstrating true clinical benefit in light of the overall improved outcomes of interventions in this population, resulting in everincreasing trial sizes and, as such, budgets. Our current attempts have placed us on the verge of answers to some of the large questions and will hopefully edge us to further optimization of our device strategy.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Limitations of reperfusion therapy in ST-elevation myocardial infarction patients
▪ For ST-elevation myocardial infarction, primary percutaneous coronary intervention (PPCI) is superior to thrombolytic therapy and, when available, is the treatment of choice. In up to 40% of patients, normal coronary arterial perfusion is not completely achieved, despite successful treatment of the culprit lesion as evidenced by reduced TIMI flow, failure of ST-segment resolution (STR) and poor myocardial blush grade (MBG).
▪ This phenomenon, known as no or slow reflow in its most pronounced manifestations, has been associated with adverse clinical outcomes, such as worse left ventricular function and survival. The clinical need to prevent such phenomena has led to the development of special devices, that can be divided into protection (distal or proximal) and thrombus aspiration devices.
Protection devices in setting of PPCI
▪ Studies using protection devices in the setting of PPCI have failed to demonstrate a benefit. It should, however, be noted that distal protection devices have not been evaluated by randomized controlled trials in PPCI in vein grafts.
Mechanical thrombectomy devices
▪ Thrombectomy devices can be broadly divided into two groups depending on the presence or absence of a motorized system.
▪ Multicenter large randomized trials using mechanical thrombus aspiration devices did not succeed in demonstrating significant difference in STR, angiographic end points or myocardial infarct size between groups and showed conflicting results regarding clinical outcomes. Another meta-analysis including multiple devices showed that routine use of mechanical thrombectomy in the setting of PPCI appears to increase mortality while having neutral effect on angiographic and clinical end points.
Manual thrombectomy devices
▪ Most of the randomized controlled trials that tested manual thrombectomy devices in the setting of PPCI have shown that thrombectomy improves surrogate end points like STR, MBG and TIMI flow, all of which are well-known predictors of future clinical events. However, most of these trials were statistically underpowered to demonstrate clinical benefit with thrombectomy. The largest, although single-center, trial to examine manual thrombectomy was the TAPAS trial. MBG and STR were significantly improved in thrombus aspiration group, with a survival benefit observed at 1-year follow-up. However, the survival benefit seems to be more pronounced and not in keeping with the degree of MBG and STR benefit. In contrast to single-center studies, multicenter aspiration trials have largely been negative.
▪ Manual thrombus aspiration has received a class IIa level of evidence B indication in PPCI in the recent American College of Cardiology/American Heart Association and the European Society of Cardiology guidelines.
Mesh-covered stent in primary PCI
▪ In recent years, a new strategy using a mesh-covered stent in PPCI aiming to minimize distal embolization by blocking debris at its source has been tested. At present, major randomized trials are still lacking to characterize the clinical and/or prognostic utility of the MGuard™ stent device (Inspire-MD, Tel-Aviv, Israel). Data from the completed worldwide registries of ST-elevation myocardial infarction patients treated with MGuard stent give us encouraging results. The first multicenter randomized controlled trial has completed enrollment.
References
Papers of special note have been highlighted as:
▪ of interest
- Zijlstra F, Hoorntje JC, de Boer MJ et al. Long-term benefit of primary angioplasty as compared with thrombolytic therapy for acute myocardial infarction. N. Engl. J. Med. 341(19), 1413–1419 (1999).
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials.Lancet 361(9351), 13–20 (2003).
- Silber S, Albertsson P, Aviles FF et al. Guidelines for percutaneous coronary interventions: the Task Force for Percutaneous Coronary Interventions of the European Society of Cardiology. Eur. Heart J. 26(8), 804–847 (2005).
- Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter. Cardiovasc. Interv. 72(7),950–957 (2008).
- Niccoli G, Burzotta F, Galiuto L et al. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 54(4), 281–292 (2009).
- Henriques JP, Zijlstra F, Ottervanger JP et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 23(14), 1112–1117 (2002).
- Napodano M, Ramondo A, Tarantini G et al. Predictors and time-related impact of distal embolization during primary angioplasty. Eur. Heart J. 30(3), 305–313 (2009).
- Henriques JP, Zijlstra F, Ottervanger JP et al. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart 23(14), 1112–1117 (2002).
- Skyschally A, Leineweber K, Gres P et al. Coronary microembolization. Basic Res. Cardiol. 101, 373–382 (2006).
- Iwakura K, Ito H, Kawanoet S et al. Assessing myocardial perfusion with the transthoracic Doppler technique in patients with reperfused anterior myocardial infarction: comparison with angiographic, enzymatic and electrocardiographic indices. Eur. Heart J. 25, 1526–1533 (2004).
- Prasad A, Stone GW, Holmes DR et al. Reperfusion injury, microvascular dysfunction, and cardioprotection: the ‘dark side’ of reperfusion. Circulation 120(21), 2105–2112 (2009).
- Ito H, Maruyama A, Iwakura K et al. Clinical implications of the ‘no reflow’ phenomenon. predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation 93, 223–228 (1996).
- Claeys MJ, Bosmans J, Veenstra L et al. Determinants and prognostic implications of persistent ST-segment elevation after primary angioplasty for acute myocardial infarction: importance of microvascular reperfusion injury on clinical outcome. Circulation 99, 1972–1977 (1999).
- van’t Hof AW, Liem A, Suryapranata H et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 97(23), 2302–2316 (1998).
- Stone GW, Peterson MA, Lansky AJ et al. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction J. Am. Coll. Cardiol. 39, 591–597 (2002).
- Spaulding C, Henry P, Teiger E et al. Sirolimus-eluting versus uncoated stents in acute myocardial infarction. N. Engl. J. Med. 355, 1093–1104 (2006).
- Baim DS, Wahr D, George B et al. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 105(11), 1285–1290 (2002).
- Stone GW, Webb J, Cox DA et al.Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. J. Am. Med. Assoc. 293(9), 1063–1072 (2005).
- Gick M, Jander N, Bestehorn HP et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 112(10) (2005).
- Kelbaek H, Terkelsen CJ, Helqvist S et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: the drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J. Am. Coll. Cardiol. 51(9), 899–905 (2008).
- Van de Werf F, Bax J, Betriu A et al. ESC guidelines on management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. Eur. Heart J. 29, 2909–2945 (2008).
- Antoniucci D, Valenti R, Migliorini A et al. Comparison of rheolyticthrombectomy before direct infarct artery stenting versus direct stenting alone in patients undergoing percutaneous coronary intervention for acute myocardial infarction. Am. J. Cardiol. 15(93), 1033–1035 (2004).
- Ali A, Cox D, Dib N et al. Rheolyticthrombectomy with percutaneous coronary intervention for infarct size reduction in acute myocardial infarction: 30-day results from a multicenter randomized study. J. Am. Coll. Cardiol. 48, 244–252 (2006).
- Migliorini A, Stabile A, Rodriguez AE et al. Comparison of AngioJet rheolyticthrombectomy before direct infarct artery stenting with direct stenting alone in patients with acute myocardial infarction. The JETSTENT trial. J. Am. Coll. Cardiol.12 (56), 298–1306 (2010).
- Beran G, Lang I, Schreiber W et al. Intracoronary thrombectomy with the X-sizer catheter system improves epicardial flow and accelerates ST-segment resolution in patients with acute coronary syndrome: a prospective, randomized, controlled study. Circulation 105, 2355–2360 (2002).
- Napodano M, Pasquetto G, Saccà S et al. Intracoronary thrombectomy improves myocardial reperfusion in patients undergoing direct angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 42, 1395–1402 (2013).
- Lefèvre T, Garcia E, Reimers B et al. X-sizer for thrombectomy in acute myocardial infarction improves ST-segment resolution: results of the X-sizer in AMI for negligible embolization and optimal ST resolution (X AMINE ST) trial. J. Am. Coll. Cardiol.46, 246–252 (2005).
- Lee CH, Tan HC, Wong HB et al. Incidence, predictors, and outcomes of device failure of X-sizer thrombectomy: real-world experience of 200 cases in 5 years. Am. Heart J. 153(14), e13–e19 (2007).
- Sanmartín M, Goicolea J, Ruiz-Salmerón R et al. Coronary perforation as a potentialcomplication derived from coronary thrombectomy with the X-Sizer device. Catheter. Cardiovasc. Interv. 56, 378–382(2002).
- De Carlo M, Wood DA, Webb JG et al. Adjunctive use of the Rinspiration system for fluidic thrombectomy during primary angioplasty: the Rinspiration international registry. Catheter. Cardiovasc. Interv. 72, 196–203 (2008).
- Ikari Y, Sakurada M, Kozuma K et al. Upfront thrombus aspiration in primary coronary intervention for patients with ST-segment elevation acute myocardial infarction: report of the VAMPIRE (VAcuuMasPIration thrombus REmoval) trial. JACC Cardiovasc. Interv. 1, 424–431 (2008).
- Dudek D, Mielecki W, Legutko J et al. Percutaneous thrombectomy with the RESCUE system in acute myocardial infarction. Kardiol. Pol. 61, 523–533 (2004).
- Kaltoft A, Bøttcher M, Nielsen SS et al. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation 114, 40–47 (2006).
- Andersen NH, Karlsen FM, Gerdes JC et al. No beneficial effects of coronary thrombectomy on left ventricular systolic and diastolic function in patients with acute S-T elevation myocardial infarction: a randomized clinical trial. J. Am. Soc. Echocardiogr. 20, 724–730 (2007).
- Kunii H, Kijima M, Araki T et al. Lack of efficacy of intracoronary thrombus aspiration before coronary stenting in patients with acute myocardial infarction: a multicenter randomized trial. J. Am. Coll. Cardiol. 43, 245A Abstract (2004).
- Bavry AA, Kumbhani DJ, Bhatt DL. Role of adjunctive thrombectomy and embolic protection devices in acute myocardial infarction: a comprehensive meta-analysis of randomized trials. Eur. Heart J. 29, 2989–3001 (2008).
- Burzotta F, De Vita M, Gu YL et al. Clinical impact of thrombectomy in acute ST-elevation myocardial infarction: an individual patient-data pooled analysis of 11 trials. Eur. Heart J. 30, 2193–2203 (2009).
- Costopoulos C, Gorog DA, Di Mario C, Kukreja N. Use of thrombectomy devices in primary percutaneous coronary intervention: a systematic review and meta-analysis. Int. J. Cardiol. 163(3), 229–241 (2013).
- Sardella G, Mancone M, Bucciarelli-Ducci C et al. Thrombus aspiration during primarypercutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J. Am. Coll. Cardiol. 53, 309 (2009).
- Burzotta F, Trani C, Romagnoli E et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J. Am. Coll. Cardiol. 46, 371–376 (2005).
- Silva-Orrego P, Colombo P, Bigi R et al. Thrombus aspiration before primary angioplasty improves myocardial reperfusion in acute myocardial infarction: the DEAR-MI (Dethrombosis to Enhance Acute Reperfusion in Myocardial Infarction) study. J. Am. Coll. Cardiol. 48, 1552–1559 (2006).
- Sharma SK, Tamburrino F, Mares AM et al. Improved outcome with AngioJet thrombectomy during primary stenting in acute myocardial infarction patients with high-grade thrombus. J. Invasive Cardiol. 18(Suppl. C), C8–C11 (2006).
- De Luca L, Sardella G, Davidson CJ et al. Impact of intracoronary aspiration thrombectomy during primary angioplasty on left ventricular remodelling in patients with anterior ST elevation myocardial infarction. Heart 92, 951–957 (2006).
- Dudek D, Mielecki W, Burzotta F et al. Thrombus aspiration followed by direct stenting: a novel strategy of primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. Results of the Polish–Italian–Hungarian RAndomized ThrombEctomy Trial (PIHRATE Trial). Am. Heart J. 160, 966–972 (2010).
- Sardella G, Mancone M, Canali E et al. Impact of thrombectomy with EXPort Catheter in Infarct-Related Artery during Primary Percutaneous Coronary Intervention (EXPIRA trial) on cardiac death. Am. J. Cardiol. 106, 624–629 (2010).
- Vlaar PJ, Svilaas T, van der Horst IC et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371, 1915–1920 (2008).
- Stone GW, Maehara A, Witzenbichler B et al. Intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction: The INFUSE-AMI randomized trial. JAMA 307(17), 1817–1826.
- Mongeon FP, Bélisle P, Joseph L et al. Adjunctive thrombectomy for acute myocardial infarction: a Bayesian meta-analysis. Circ. Cardiovasc. Interv. 3, 6–16 (2010).
- Charanjit S, Rihal. Adjunctive thrombectomy for primary percutaneous coronary intervention: what would Dr Bayes do? Circ. Cardiovasc. Interv. 3, 1–2 (2010).
- Kikkert WJ, van Geloven N, Claessen BEP et al. Increased 1-year survival after adjunctivethrombus aspiration for ST-elevation myocardial infarction patients. J. Am. Coll. Cardiol. 56, B19–B20 (2010).
- Kushner FG, Hand M, Smith SC Jr et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 120, 2271–2306 (2009).
- Grube E, Hauptmann KE, Müller R et al. Coronary stenting with MGuard: extended follow-up of first human trial. Cardiovasc. Revasc. Med. 12(3), 138–146 (2011).
- Costa JR Jr, Abizaid A, Feres F et al. One-year results of the INSPIRE trial with the novel MGuard stent: serial analysis with QCA and IVUS. Catheter. Cardiovasc. Interv. 78(7), 1095–1100 (2011).
- Dudek D, Dziewierz A, Rzeszutko Ł et al. Mesh covered stent in ST-segment elevation myocardial infarction. EuroIntervention 6(5), 582–589 (2010).
- Weerackody R, Jain M, Archbold A et al. A mesh covered stent effectively reduces risk of distal embolization during primary percutaneous intervention for ST-segment elevation myocardial infarction.EuroIntervention 6, H106 (2010).
- Piscione F, Danzi GB, Cassese S et al. Multicentre experience with MGuardTM net protective stent in ST-elevation myocardial infarction: safety, feasibility, and impact on myocardial reperfusion. Catheter. Cardiovasc. Interv. 75, 715–721 (2010).
- Danzi GB. iMOS Interim analysis – on behalf of international MGuard Observational Study (iMOS) registry study investigators. Presented at: EuroPCR Congress. Paris, France, 25–28 May 2010.
- Wijns W, Kolh P, Dnachin N et al. Guidelines on myocardial revascularization. Eur. Heart J. 31, 2501–2555 (2010).
- Stone GW, Abizaid A, Silber S et al. Prospective, randomized, multicenter evaluation of a polyethylene terephthalate micronet mesh–covered stent (MGuard) in ST-segment elevation myocardial infarction (MASTER Trial). J. Am. Coll. Cardiol. 60, 1975–1984 (2012).
- Thrombus Aspiration in Myocardial Infarction (TASTE). www.clinicaltrials.gov/ct2/show/ NCT01093404
- Efficacy of Thrombosuction in Primary Percutaneous Coronary Intervention of Acute Myocardial Infarction (ETAMI). www.clinicaltrials.gov/ct2/show/ NCT01156662
- A Trial of Routine Aspiration Thrombectomy With Percutaneous Coronary Intervention (PCI) Versus PCI Alone in Patients With ST-Segment Elevation Myocardial Infarction (STEMI) Undergoing Primary PCI (TOTAL).www.clinicaltrials.gov/ct2/show/NCT01149044
▪ Comprehensive pooled meta-analysis of randomized controlled trials that confirmed the survival benefit of manual versus mechanical thrombectomy in the setting of primary percutaneous coronary intervention for the long term.
▪ Currently, the largest randomized controlled trial to evaluate clinical outcome of thrombus aspiration in primary percutaneous coronary intervention.
▪ Websites