Perspective - Interventional Cardiology (2012) Volume 4, Issue 4
Pericardium-covered stent: a reality for coronary interventions of the future?
- Corresponding Author:
- Alaide Chieffo
Interventional Cardiology Unit
San Raffaele Scientific Institute
Via Olgettina 60, 20132, Milan, Italy
Tel: +39 022 643 7331
Fax: +39 022 643 7339
E-mail: chieffo.alaide@hsr.it
Abstract
Keywords
coronary perforation, no-reflow, percutaneous coronary intervention, pericardium-covered stent, saphenous vein graft, ST-elevation myocardial infarction
The advent of percutaneous coronary intervention (PCI) and the introduction of the coronary stent have revolutionized the treatment of coronary artery disease. However, despite rapid advances in techniques and technologies, some procedural complications can still occur and these are potentially catastrophic. These include coronary perforation and the ‘no-reflow’ phenomenon. The first is sometimes unpredictable, even though it occurs more frequently in heavily calcified vessels, tortuous vessels and chronic total occlusions [1–4]. In such cases, cardiac tamponade can develop rapidly and this can lead to a life-threatening situation. No-reflow may happen more frequently following intervention for ST-elevation myocardial infarction (STEMI) where there is a high thrombus burden, and in saphenous vein-graft (SVG) PCI due to the friable degenerate material present. In both these situations, distal embolization can contribute to the occurrence of no-reflow, causing hemodynamic deterioration and worse outcomes [5–9]. Indeed, it has been demonstrated that no-reflow is an independent correlate of mortality at 5 years following STEMI [10]. Furthermore, owing to the lack of beneficial effects of pharmacological therapy following the development of no-reflow, it is believed that the aim should be to prevent this phenomenon from occurring at the outset [11,12].
The pericardium-covered stent (PCS) ‘Overand- Under™’ (IGTM Medical, Or Akiva, Israel) was the first in a series of heterologous tissue-covered stents, designed to set a barrier between the stent and the vessel wall. It became commercially available in Europe for clinical use in May 2006. It was thought that this type of stent might reduce complications by preventing distal embolization in a high-risk subset of patients, such as those with STEMI and SVG lesions, by trapping material against the vessel wall. In small clinical studies the PCS has been demonstrated to have some benefit. In addition, this stent, and the subsequent newer generations of this stent (i.e., the Aneugraft™ and the Aneugraft™ Dx [ITGI Medical, Or Akiva, Israel]), have been utilized for the urgent treatment of coronary perforations with some success.
This article aims to review the PCS, the lesion subgroups in which the PCS may be helpful and current clinical experience, and, finally, provide a perspective of what we think the role of the PCS will be in coronary intervention in the future.
Pericardium as a graft material
Pericardium, as a biocompatible graft material, has been utilized for over 30 years, with a demonstrated benefit compared with other grafting materials in specific subsets [13,14].
In view of excellent results with the use of pericardium, including in patients undergoing carotid endarterectomy and incisional hernia repair, it was decided it could be a possible advantageous platform for covered coronary stents. The Over-and-Under stent gained a CE mark in May 2006. Initially, the stent was made utilizing bovine pericardium. A study in dogs comparing glutaraldehyde-treated bovine and equine pericardium reported no differences in results between the two materials and when concerns were raised regarding the spread of bovine spongiform encephalopathy, the stent was changed to that using equine pericardium. In addition, it has been demosntrated that when equine pericardium is cross-linked with collagen, it has higher enzymatic stability.
There remains only one potential concern regarding the use of equine pericardium as a graft material, and that is the possibility of host reactivity to the foreign protein contained in the material in the long term [15]. This has not yet been seen clinically but, nevertheless, it needs to be considered if issues develop in the future.
Current stents available
At the present time, the Over-and-Under and the Aneugraft stents are commercially available in Europe, Israel and Latin America. The current indications are: stenoses and aneurysms of SVG and native coronary artery aneurysms. They are not yet licensed for use in STEMI and in the USA, they only have US FDA approval for humanitarian use in the case of perforations or dissections of SVG and native coronary artery lesions. Both these stents are wet devices that are stored in saline and a 0.2% glutaraldehyde solution.
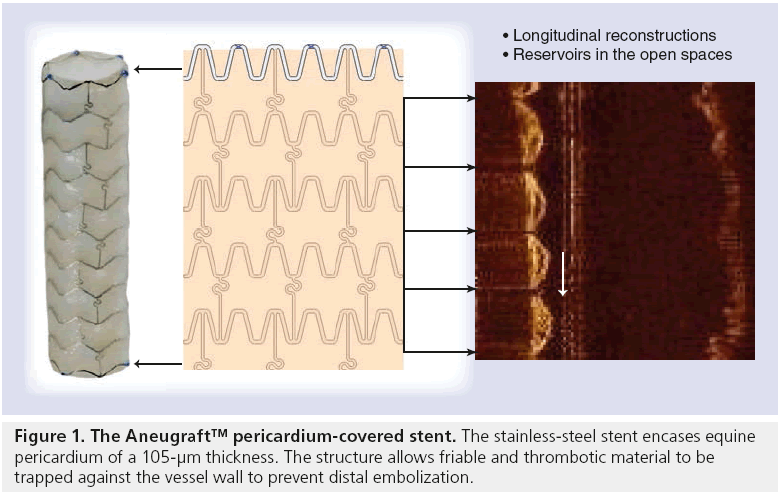
The Aneugraft consists of a highly flexible, laser cut, 316 L stainless-steel balloon-expandable stent, which is covered with a single layer of equine pericardium (Figure 1). This layer of pericardium is approximately 105 μm in thickness and chosen with minimal tissue blemishes. The pericardial tissue is treated with glutaraldehyde in order to cross-link the collagen fibres and hence reduce antigenicity. This passes over the main body of the stent, thereby achieving minimum contact between the steel and the intima of the vessel. The single stent and single layer of pericardium configuration avoids folding or double layers that could increase the profile and compromise the trackability and flexibility of the device. The pericardium tissue is attached to the stent with a polypropylene suture and is subsequently sterilized with liquid chemical and ethylene oxide.
The device, when ready, is packed and stored in sterile glutaraldehyde. In preparation for use, the stent has to be rinsed in sterile physiological saline for at least 2 min and kept wet until usage. It can then be implanted conventionally utilizing a standard rapid-exchange balloon system via a 0.014” coronary guidewire. It is recommended that ‘push-button’ haemostatic valves are not used in order to avoid damaging the device prior to implantation.
The stent is 6-French compatible for sizes 2.5, 3.0 and 3.5 mm and 7-French compatible for 4.0 mm. It is available in lengths of 13, 18, 23 and 27 mm. The balloon is a semi-compliant balloon that deploys in such a way that that the stent edges deploy before the middle of the stent, thereby effectively trapping the friable atheroma behind the pericardium. Post-dilatation can be performed with a new balloon if deemed necessary; however, it must be noted that an important difference between this device and conventional stents is that tearing and disruption of the pericardial covering may occur if the maximal recommended diameter is exceeded. It is recommended that pressures above 8–10 atmospheres should be avoided.
More recently, the Aneugraft Dx has become available, which is similar to the Aneugraft; however, it utilizes a dry tissue technology that does not require any preparation and can therefore be used more quickly in an emergency situation.
With regards to dual antiplatelet therapy following any PCS implantation, the current manufacturer recommendation is to administer antiplatelet therapy for at least 3 months.
Lesion subsets
▪ Saphenous vein grafts
Patients who have previously undergone coronary artery bypass grafting frequently require further revascularization; indeed, within 10 years of coronary artery bypass grafting, 50% of SVGs have been demonstrated to be occluded [16–18]. The percutaneous revascularization of degenerate SVG lesions can be particularly problematic. Typically, the disease is heavily fibrotic with large amounts of friable plaque, which can easily go downstream upon deployment of a stent. Despite the use of distal protection devices to reduce this, plaque material may protrude through the stent struts and prolapse into the lumen causing micro-embolization, which can contribute to the no-reflow phenomenon in 10–15% of cases [19]. There is an associated increase in peri-procedural myocardial infarction (MI) [20–22], which subsequently leads to higher rates of morbidity and mortality [9].
Membrane-covered stents provide a mechanical barrier to locally trap friable plaque against the graft wall, and prevent this material from distal embolization; therefore, they could be of benefit in such a lesion subset. It was initially thought that stents covered with a polytetrafluroethylene membrane might be associated with favorable clinical outcomes in SVG lesions. However, they were limited by poor flexibility and deliverability as well as the occurrence of in-stent restenosis [23]. In addition, a number of trials failed to show any benefit compared with conventional stents [24–26]. It has been hypothesized that the PCS may be more beneficial in SVG lesions by entrapping the thrombus and preventing distal embolization.
There are a number of coronary clinical studies that have utilized the PCS. The first to be performed was SLEEVE-I, which was designed to evaluate the safety and feasibility of the PCS for patients undergoing PCI of a degenerate SVG lesion. This was an open-label, nonrandomized registry study of 15 patients (in total, 22 SVG lesions) who underwent SVG PCI with the first-generation Over-and-Under PCS. Of note, bovine pericardium was used for the PCS in this study. The primary safety objective was the frequency of clinical and angiographic complications. There was a reported procedural success rate of 100% and no in-hospital major adverse cardiac events (MACE; defined as cardiac mortality, MI and target vessel revascularization). The clinical success (defined as successful delivery of the study device, <50% residual stenosis and no MACE) at 30 days was 93.3%. At 30 days of follow-up, there was one cardiac death adjudicated after 10 days, corresponding to a MACE rate of 6.7%. The technical success (defined as the ability to reach the lesion, accurately position the device and successfully deploy the device) was 100% [27].
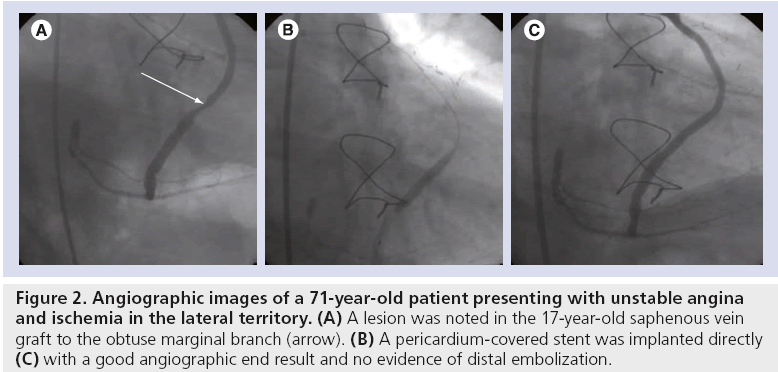
A second similar study was SLEEVE-II. This was a multinational, multicenter registry study that enrolled 47 patients (in total, 58 lesions) requiring SVG PCI. The primary end point of the study was 30-day MACE. The procedural success rate was 89.1% and technical success 85.9%. At 30 days, MACE was 10.6%, mainly as a consequence of MI (8.5%). Of note, there were no reports of stent thrombosis (ST). The study concluded that the bovine PCS was safe to be used in patients with de novo or restenosed SVG lesions [27]. Figure 2 shows the angiographic images of a patient undergoing PCI and implantation of a PCS for a degenerate SVG lesion, recruited into the SLEEVE-II study.
Figure 2: Angiographic images of a 71-year-old patient presenting with unstable angina and ischemia in the lateral territory. (A) A lesion was noted in the 17-year-old saphenous vein graft to the obtuse marginal branch (arrow). (B) A pericardium-covered stent was implanted directly (C) with a good angiographic end result and no evidence of distal embolization.
In addition to the SLEEVE series of studies, there have been a number of case reports describing the use of the PCS in SVG lesions. One such report describes the concurrent use of a paclitaxel drug-eluting balloon with deployment of an Over-and-Under stent afterwards [28]. This group had previously had experience with the drug-eluting balloon and the polytetrafluroethylene-covered stent [29].
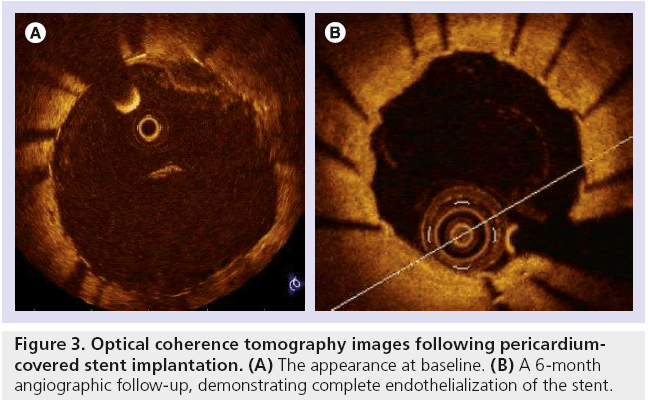
Another study of six cases of SVG PCI utilized pre- and post-intervention optical coherence tomography. This novel means of intravascular imaging detected a characteristic pattern with bulging of the pericardium between the struts, possibly due to the trapping of soft intraluminal plaque behind the pericardial layer [30]. Figure 3 demonstrates optical coherence tomography imaging following PCI in which a PCS was chosen by the operator, with complete endothelialization of the PCS observed at 6 months follow-up.
▪ STEMI
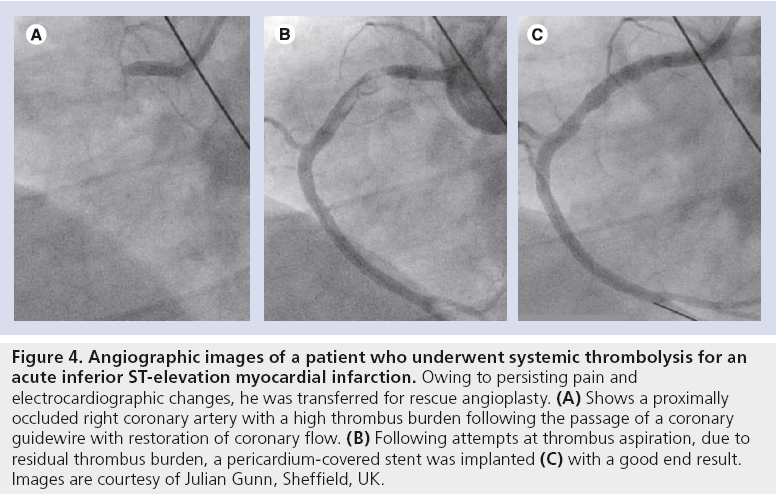
Primary PCI has largely been shown to be the gold-standard treatment for patients presenting with acute STEMI [31]. In such situations, there is often a high thrombus burden that may result in distal embolization and play a contributor role in the development of no-reflow following stent implantation [10–12]. The PCS stent may therefore be well suited for trapping the thrombus behind the graft and, hence, could theoretically reduce complications when conventional percutaneous and pharmacological methods have not been successful. Figure 4 shows the angiographic images of a patient who presented with an acute inferior STEMI and were treated successfully with Aneugraft implantation, which was chosen by the operator.
Figure 4: Angiographic images of a patient who underwent systemic thrombolysis for an acute inferior ST-elevation myocardial infarction. Owing to persisting pain and electrocardiographic changes, he was transferred for rescue angioplasty. (A) Shows a proximally occluded right coronary artery with a high thrombus burden following the passage of a coronary guidewire with restoration of coronary flow. (B) Following attempts at thrombus aspiration, due to residual thrombus burden, a pericardium-covered stent was implanted (C) with a good end result. Images are courtesy of Julian Gunn, Sheffield, UK.
A study by Gunn et al. evaluated the feasibility and safety of the PCS graft in treating massive thrombus burden in native coronary arteries [32]. Patients presenting with STEMI or acute coronary syndrome and undergoing PCI initially underwent thrombus aspiration and administration of glycoprotein IIb/IIIa inhibitors, if there was evidence of significant thrombus. If patients still had persistent massive, localized thrombus (Thrombolysis In MI [TIMI] grades 3 or 4) [33] in proximal lesions of vessels >3 mm in diameter or of a significant (>2mm) branch, they were considered for Aneugraft implantation. In total, nine patients (a total of ten lesions) were included; eight were STEMI, one non-STEMI and the right coronary artery was the culprit in nine lesions. A small balloon was used for predilatation in nine (90%) of the cases with successful device positioning in nine (90%) lesions. Technical success (defined as TIMI grade 0 residual thrombus, TIMI grade 3 flow and <20% residual stenosis) was achieved in nine (90%) lesions. There was one case of ST 1 h after device implantation, who had notably only had aspirin therapy administered previously, owing to a cerebral embolic stroke. This patient subsequently died of complications of the stroke at day 6 following repeat PCI. There were no other inhospital adverse events or any events at 30 days.
▪ Coronary perforation
Coronary perforations are rare (0.1–1.3% of PCI procedures); however, mortality has been reported to be as high as 20% [1,34]. In order to prevent the rapid deterioration of the patient, this complication needs to be promptly solved. Prior to delivery of the covered stent, cardiac surgery was required in approximately half of the cases [35]. The PCS is an option for the treatment of such a complication, when mechanisms such as anticoagulant reversal and prolonged balloon inflation have not worked. The nature of the pericardium graft material that covers the stent enables the perforation to be sealed upon deployment of the stent. The introduction of the Aneugraft Dx dry stent, which requires no preparation time, may greatly help in the urgent treatment of this situation. The use of the PCS has been reported to be successful in case reports in patients with coronary perforation [36]. However, it must be emphasized that the PCS may not be useful in all cases of distal perforations. In these cases, techniques such as embolization may be required.
▪ Other Indications for the PCS
Further clinical scenarios in which the PCS has been used include the treatment of coronary artery aneurysms and hypertrophic obstructive cardiomyopathy.
The presence of coronary artery aneurysms is relatively rare, with a reported incidence in the literature of 1.5–5% [37,38]. The decision to treat these is usually based on the associated complications that can ensue, including angina, although no data are currently available to indicate the relative merits of any approach. Surgery has historically been the preferred treatment modality; however, the PCS may be suitable for the percutaneous treatment of such aneurysms by the neck and may allow the aneurysm to thrombose. Both the Aneugraft and the Over-and-Under PCSs have been reported in the successful treatment of this disease, the latter in the case of a contained ruptured coronary aneurysm [39–41].
In addition to selective septal alcohol ablation for symptomatic hypertrophic obstructive cardiomyopathy, another theoretical approach for effectively ablating the septal myocardium is through occlusion of the septal branches with deployment of a covered stent in the left anterior descending coronary artery. One case has been reported by Gaspar et al. of a 47-year-old woman with three septal perforators distal to the first diagonal artery, in addition to a muscular bridge [42]. Supple Peri-Guard® (Bio-Vascular, MN, USA) bovine pericardium was used to cover stents and deployed with complete reduction of the resting left ventricular outflow-tract gradient through a therapeutic septal MI. Of course, in this situation, there would be some concern regarding the consequences if restenosis or ST were to occur and, therefore, if the PCS is to be used in the future for this indication, the stent implantation should be optimized with the use of adjunctive imaging technology. In addition, as the specific septal branches may not be able to be subselected under echocardiographic guidance as in conventional techniques, there may potentially be a resulting larger therapeutic MI. Unless further investigation is performed in this area, the role of the PCS is purely hypothetical.
Ongoing PCS studies
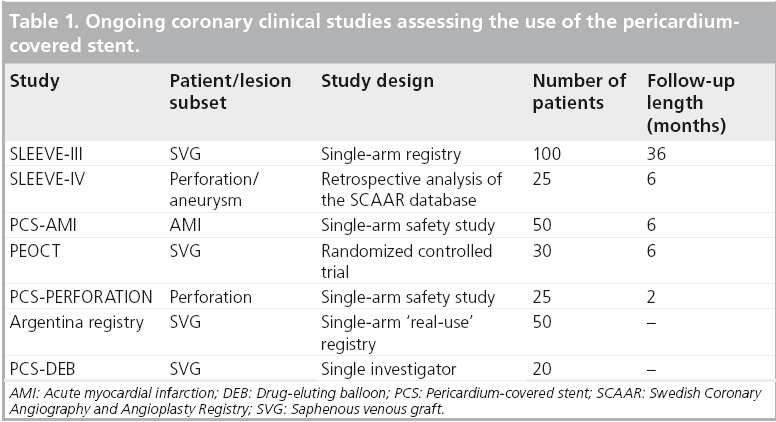
A number of studies are currently underway or are planned to assess the use of the PCS for varying indications. The Over-and-Under/Aneugraft covered stent long-term follow-up registry, SLEEVE-III (NCT01307553), is currently recruiting 75 patients with SVG disease or coronary aneurysms who are undergoing intervention with the PCS. The primary end point is MACE at 6 months clinical follow-up. In addition, SLEEVE-IV, a retrospective analysis of 25 patients from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) database with perforation or aneurysm is currently being analyzed. Other studies assessing the use of the PCS are illustrated in Table 1.
Conclusion
Despite minimal clinical data available, the use of a PCS may be justified in specific situations in current clinical practice. However, we need to await further clinical reports and larger studies, including randomized trials, within these areas in order to endorse widespread clinical usage. There is an unmet need to improve the design of covered stents; a design that incorporates antiproliferative drugs could help improve outcomes when these stents are used for coronary indications.
Future perspective
Contemporary interventional cardiology has been rapidly progressing, but despite the numerous advances in recent years leading to improved outcomes, certain complications of PCI are still present and can result in significant morbidity and mortality.
Coronary perforation, despite being a rare occurrence, needs to be treated promptly and effectively. The PCS appears to be acceptable for the treatment of coronary perforations and as the stent is now available with no preparation required, it can be immediately available to the operator and used to try and salvage the emergency situation. As further improvements are made to increase trackability, the speed at which this life-threatening complication can be treated will increase. The PCS should likely be available to the interventionalist on the shelf for this indication.
No-reflow remains a frightening phenomenon for the interventionalist. However, the PCS has still not been demonstrated to be effective with regard to a reduction in infarct size or, indeed, improved clinical outcomes in a large number of patients or in randomized trials. In addition, results with current drug-eluting stents show such good results with regard to target- vessel revascularization in native coronary arteries that it is unlikely that the use of such a new device would ever be able to be extended beyond these niche applications. However, with newer generations of the PCS having improved trackability, in addition to randomized data, there may be scope for an increase in their usage in the future.
Competing stents are also becoming available with a similar mode of action as the PCS, such as the MGuard™ (Inspire MD Ltd., Tel Aviv, Israel) mesh-covered stent, which, in addition to trapping thrombus, allows side branches to be perfused. The MASTER trial is currently randomizing patients with STEMI to MGuard or commercially available bare-metal or drugeluting stents, with a primary outcome of incidence of complete ST segment resolution. This should give us further information regarding the use of this stent in the acute situation with a high thrombus burden. Although the PCS could be useful in STEMI, this would only be in patients with large vessels with no significant side branches, potentially eliminating a lot of patients requiring intervention for STEMI who are at high risk for distal embolization.
However, the idea of utilizing a PCS in combination with a drug-eluting balloon is an exciting concept, which may play some role in counteracting the levels of restenosis seen in the previous SLEEVE studies. Again, this needs to be evaluated further in larger numbers of patients.
Hence, we still have a lot of work to do in the field of interventional cardiology to continue to improve outcomes, both in reducing complications and treating them as they occur. Although the PCS is potentially a promising option in certain situations, we cannot endorse its general clinical usage until more data become available in the future.
Acknowledgements
The authors would like to thank Julian Gunn (University of Sheffield, Sheffield, UK) for his kind contribution of images for this manuscript.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Background
▪ Complications of percutaneous coronary intervention still exist, including coronary perforation and no-reflow.
Current stents available
▪ The ‘Over-and-Under™’ and Aneugraft™/Aneugraft™ Dx pericardium-covered stents work to trap thrombus and friable atheroma against the vessel wall and, hence, prevent embolization and possibly subsequent no-reflow.
Lesion subsets
▪ These stents are currently licensed for stenoses and aneurysms of saphenous vein graft lesions and native coronary artery aneurysms.
Ongoing studies
▪ Further studies are currently underway; however, a lot of work needs to be done within the field of interventional cardiology to minimize complications in the future.
References
- Ellis SG, Ajluni S, Arnold AZ et al. Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 90, 2725–2730 (1994).
- Gunning MG, Williams IL, Jewitt DE et al. Coronary artery perforation during percutaneous intervention: incidence and outcome. Heart 88, 495–498 (2002).
- Javaid A, Buch AN, Satler LF et al. Management and outcomes of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 98, 911–914 (2006).
- Stankovic G, Orlic D, Corvaja N et al. Incidence, predictors, in-hospital, and late outcomes of coronary artery perforations. Am. J. Cardiol. 93, 213–216 (2004).
- Wu KC, Zerhouni EA, Judd RM et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation 97, 765–772 (1998).
- Resnic FS, Wainstein M, Lee MK et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am. Heart. J. 145, 42–46 (2003).
- Brosh D, Assali AR, Mager A et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am. J. Cardiol. 99, 442–445 (2007).
- Valgimigli M, Campo G, Malagutti P et al. Persistent coronary no flow after wire insertion is an early and readily available mortality risk factor despite successful mechanical intervention in acute myocardial infarction: a pooled analysis from the STRATEGY (Single High-Dose Bolus Tirofiban and Sirolimus-Eluting Stent Versus Abciximab and Bare-Metal Stent in Acute Myocardial Infarction) and MULTISTRATEGY (Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction Study) trials. JACC Cardiovasc. Interv. 4, 51–62 (2011).
- Abbo KM, Dooris M, Glazier S et al. Features and outcome of no-reflow after percutaneous coronary intervention. Am. J. Cardiol. 75, 778–782 (1995).
- Ndrepepa G, Tiroch K, Fusaro M et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J. Am. Coll. Cardiol. 55, 2383–2389 (2010).
- Niccoli G, Kharbanda RK, Crea F, Banning AP. No-reflow: again prevention is better than treatment. Eur. Heart J. 31, 2449–2455 (2010).
- Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstructionrelated myocardial injury and coronary no-reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc. Interv. 3, 695–704 (2010).
- Marien BJ, Raffetto JD, Seidman CS et al. Bovine pericardium vs dacron for patch angioplasty after carotid endarterectomy: a prospective randomized study. Arch. Surg. 137, 785–788 (2002).
- Kapan S, Kapan M, Goksoy E et al. Comparison of PTFE, pericardium bovine and fascia lata for repair of incisional hernia in rat model, experimental study. Hernia 7, 39–43 (2003).
- Butany J, Leask R. The failure modes of biological prosthetic heart valves. J. Long Term Eff. Med. Implants 11, 115–135 (2001).
- Nwasokwa ON. Coronary artery bypass graft disease. Ann. Intern. Med. 123, 528–545 (1995).
- de Feyter PJ. Percutaneous treatment of saphenous vein bypass graft obstructions: a continuing obstinate problem. Circulation 107, 2284–2286 (2003).
- Fitzgibbon GM, Kafka HP, Leach AJ et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 28, 616–626 (1996).
- Sdringola S, Assali AR, Ghani M et al. Risk assessment of slow or no-reflow phenomenon in aortocoronary vein graft percutaneous intervention. Catheter. Cardiovasc. Interv. 54, 318–324 (2001).
- Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331, 489–495 (1994).
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331, 496–501 (1994).
- Bhargava B, Kornowski R, Mehran R et al. Procedural results and intermediate clinical outcomes after multiple saphenous vein graft stenting. J. Am. Coll. Cardiol. 35, 389–397 (2000).
- Schachinger V, Hamm CW, Munzel T et al. A randomized trial of polytetrafluoroethylenemembrane- covered stents compared with conventional stents in aortocoronary saphenous vein grafts. J. Am. Coll. Cardiol. 42, 1360–1369 (2003).
- Turco MA, Buchbinder M, Popma JJ et al. Pivotal, randomized U.S. study of the Symbiottrade mark covered stent system in patients with saphenous vein graft disease: eight-month angiographic and clinical results from the Symbiot III trial. Catheter. Cardiovasc. Interv. 68, 379–388 (2006).
- Stankovic G, Colombo A, Presbitero P et al. Randomized evaluation of polytetrafluoroethylene-covered stent in saphenous vein grafts: the Randomized Evaluation of polytetrafluoroethylene COVERed stent in Saphenous vein grafts (RECOVERS) trial. Circulation 108, 37–42 (2003).
- Stone GW, Goldberg S, O’Shaughnessy C et al. 5-year follow-up of polytetrafluoroethylene-covered stents compared with bare-metal stents in aortocoronary saphenous vein grafts the randomized BARRICADE (Barrier Approach to Restenosis: Restrict Intima to Curtail Adverse Events) trial. JACC Cardiovasc. Interv. 4, 300–309 (2011).
- Colombo A, Almagor Y, Gaspar J, Vonderwalde C. The pericardium covered stent (PCS). EuroIntervention 5, 394–399 (2009).
- Wykrzykowska JJ, Gutierrez-Chico JL, van Geuns RJ. “Over-and-under” pericardial covered stent with paclitaxel balloon in a saphenous vein graft. Catheter. Cardiovasc. Interv. 75, 964–966 (2010).
- Papafaklis MI, Sianos G, Cost B et al. Clinical and angiographic follow-up after overlapping implantation of polytetrafluoroethylene covered stents with drug eluting stents. EuroIntervention 2, 218–223 (2006).
- Tyczynski P, Kukreja N, van Geuns RJ et al. Optical coherence tomography for the assessment of pericardium covered stents for the treatment of degenerated saphenous vein grafts. EuroIntervention 6, 78–85 (2010).
- Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet 361, 13–20 (2003).
- Gunn J, Siotia A, Malkin CJ et al. Novel use of a pericardium-covered stent graft to treat bulky coronary artery thrombus. Catheter. Cardiovasc. Interv. 80(1), 59–64 (2011).
- Early effects of tissue-type plasminogen activator added to conventional therapy on the culprit coronary lesion in patients presenting with ischemic cardiac pain at rest. Results of the Thrombolysis in Myocardial Ischemia (TIMI IIIA) Trial. Circulation 87, 38–52 (1993).
- Gruberg L, Pinnow E, Flood R et al. Incidence, management, and outcome of coronary artery perforation during percutaneous coronary intervention. Am. J. Cardiol. 86, 680–682, A8 (2000).
- Romaguera R, Waksman R. Covered stents for coronary perforations: is there enough evidence? Catheter. Cardiovasc. Interv. 78, 246–253 (2011).
- Jokhi PP, McKenzie DB, O’Kane P. Use of a novel pericardial covered stent to seal an iatrogenic coronary perforation. J. Invasive Cardiol. 21, E187–E190 (2009).
- Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br. Heart J. 54, 392–395 (1985).
- Swaye PS, Fisher LD, Litwin P et al. Aneurysmal coronary artery disease. Circulation 67, 134–138 (1983).
- Ferlini M, Russo F, Marinoni B et al. Images in cardiology. Percutaneous coronary aneurysm obliteration using a novel pericardium-covered stent. J. Am. Coll. Cardiol. 56, 2139 (2010).
- Hayat SA, Ghani S, More RS. Treatment of ruptured coronary aneurysm with a novel covered stent. Catheter. Cardiovasc. Interv. 74, 367–370 (2009).
- Gaspar J, Vonderwalde C, Eid-Lidt G. Treatment of coronary artery aneurysms by percutaneous sealing with bovinepericardium- covered stents. Int. J. Cardiovasc. Interv. 2, 241–246 (1999).
- Gaspar J, Martinez-Rios MA, Vonderwalde C et al. Pericardium-covered stent for septal myocardial ablation in hypertrophic obstructive cardiomyopathy. Catheter. Cardiovasc. Interv. 47, 73–79 (1999).