Case Series - Interventional Cardiology (2025) Volume 17, Issue 3
Percutaneous Device Closure of Post-Myocardial Infarction Ventricular Septal Rupture (VSR) â Avoiding AV loop formation: Unlooping the mystery. A Case Series
- Corresponding Author:
- Mirza Mohd Kamran
Department of Paediatric Cardiology and Cardiac surgery, Metromed International Cardiac Centre, Kerala, India
E-mail: kamran16paeds@gmail.com
Received date: 01-Apr-2025, Manuscript No. FMIC-25-166801; Editor assigned: 03-Apr-2025, PreQC No. FMIC-25-166801 (PQ); Reviewed date: 17-Apr-2025, QC No. FMIC-25-166801; Revised date: 24-Apr-2025, Manuscript No. FMIC-25-166801 (R); Published date: 01-May-2025, DOI: 10.37532/1755- 5310.2025.17(3).1007
Abstract
Background: Ventricular Septal Rupture (VSR) is a catastrophic complication following acute Myocardial Infarction (MI), with mortality rates exceeding 80% if untreated. Surgical repair, though effective, is associated with high perioperative mortality, particularly in unstable patients. It has a bimodal incidence, with peak occurrences in the first 24 hours and at day’s 3-5 post-acute myocardial infarction. In the post-thrombolytic and percutaneous coronary intervention era, incidence of post myocardial infarction VSR is less than 0.5% with in hospital and 30-day mortality relating to VSR, ranging from 40% to 90% depending on management technique. T his study is a retrospective review from a tertiary level dedicated cardiac referral centre in South India. We are reporting four cases performed in our centre wherein VSR was closed with different devices with special emphasis on avoiding the formation of Arteriovenous (AV) loop and restricted use of contrast agent over a period spanning one year between February 2023 and January 2024. All patients were followed up post procedure at regular intervals with transthoracic echocardiograms and 12-lead electrocardiograms for a minimum period of 12 months.
Results: All these cases mentioned in our study underwent a complete closure of their respective lesions with no evidence of residual shunt. None of these patients had any major complications, prolong stay, or any vascular injuries. All patients completed minimum 12-months follow-up and were doing well without any residual shunts.
Conclusion: As far as conclusion of this paper is concerned, we suggest that device closure of VSR without the use of contrast and avoiding AV loops is a feasible and effective approach, as demonstrated by these successfully completed cases. This technique minimizes procedural risks, reduces contrast-induced nephropathy, and avoids complications associated with AV loops. Careful imaging guidance and meticulous procedural planning are crucial to ensuring success. Our experience highlights the potential of this method as a safer alternative for high-risk patients, paving the way for broader application in structural heart interventions.
Keywords
Transcatheter. No contrast. Patients. Arteriovenous loops.
Introduction
VSR is a catastrophic complication following acute MI, with mortality rates exceeding 80% if untreated. Surgical repair, though effective, is associated with high perioperative mortality, particularly in unstable patients. It has a bimodal incidence, with peak occurrences in the first 24 hours and at day 3-5 post-acute myocardial infarction. In the post-thrombolytic and percutaneous coronary intervention era, incidence of postmyocardial infarction VSR is less than 0.5% with in-hospital and 30-day mortality relating to VSR, ranging from 40% to 90% depending on management technique [1,2]. Median time to diagnosis of VSR is typically within 24 hours of presentation for acute myocardial infarction [3]. Percutaneous device closure has emerged as a promising alternative, particularly in non-surgical candidates. However, the procedure remains technically demanding due to the fragile nature of the infarcted septum, which is highly prone to further tearing. One of the key technical considerations in optimizing outcomes is avoiding the creation of an AV loop, as this can lead to excessive traction on the septum, increasing the risk of septal tear, conduction disturbances, and device-related complications. Additionally, minimizing the use of contrast, especially by avoiding Left Ventricular (LV) angiography, is crucial in critically ill patients with renal dysfunction or hemodynamic instability. Age, anterior infarction, female gender, and current smoking are the most significant risk factors for development of VSR following AMI. However, forming an AV loop though not studied as an independent risk factor, as far as outcome is concerned but in our case series, we have observed good results when this was avoided. Currently treatment can be classified as: 1) Medical management, 2) Surgical repair, and 3) Transcatheter closure. Medical management carries a grim prognosis with a 94% mortality rate at 30 days. Although open heart surgery is considered standard treatment for VSD, catheter-based intervention has emerged as a promising alternative. The exact timing for closure remains a matter of debate. Current guidelines recommend urgent VSD closure, irrespective of haemodynamic status [4-6]. Despite the first reported percutaneous post-AMI VSR closure occurring in 1988, data regarding this have been limited.
This study is a retrospective review from a tertiary level dedicated cardiac referral center in South India. We are reporting four cases performed in our center wherein VSR was closed with different devices with special emphasis on avoiding the formation of AV loop and restricted use of contrast agent over a period spanning one year between February 2023 and January 2024. Pre-procedure, all patients underwent detailed clinical and diagnostic evaluation comprising of transthoracic echocardiography, transoesophageal echocardiography, 12-lead Electrocardiogram, and Chest X-ray. All patients were symptomatic with haemodynamically significant lesion on Echocardiography. Transcatheter management was preferred after detailed discussion with surgical team in view of either high risk surgery or refusal from patients for surgical options. All patients were followed up post procedure at regular intervals with transthoracic echocardiograms and 12-lead electrocardiograms for a minimum period of 12 months (Table 1).
| S .no | Age | Sex | Detail of lesion treated | Approach | Device used | Issues/Complication | Contrast |
|---|---|---|---|---|---|---|---|
| Case 1 | 50y | M | 22mm VSR (Apical) | Antegrade (RIJV) | 24mm Post MI VSD Device | None | Nil |
| Case 2 | 70y | F | 12 mm VSR (Mid-muscular) | Retrograde (RFA) | Konar-MFO 14X12 | None | Minimal |
| Case 3 | 48y | M | 21 mm VSR (Apical) | Antegrade (RFV) | 24mm Post MI VSD Device | None | Nil |
| Case 4 | 78y | F | 12 mm Apical VSR | Antegrade (RIJV) | 16X14 mm Cocoon PDA device | None | Nil |
Table 1: Details of cases.
Case 1: Device closure of post MI VSR in 50 years old gentlemen after getting a primary PTCA to LAD with severe left ventricular dysfunction.
A 50-year-old male, known chronic smoker with positive family history of CAD, presented with exertional chest pain; ECG showed sinus rhythm with left axis deviation with ST elevation in v1-v6 and biphasic T-waves in aVF (Figure 1).
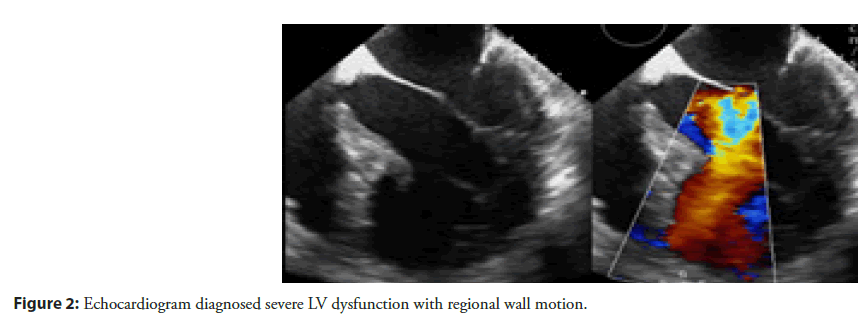
Echocardiography done which revealed showed akinetic mid and distal interventricular septum and apex, severe left ventricular dysfunction (Figure 2).
Patient immediately shifted to Cath lab after primary investigations and informed consent. Coronary angiogram done, which revealed total thrombotic occlusion of proximal LAD segment (Figure 3).
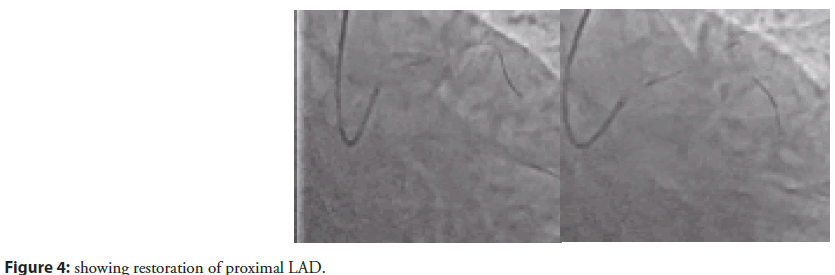
Immediately after CAG, primary PTCA was done to proximal LAD with predilation of lesion with 2.00X9 mm Mozec SC balloon. Target lesion was stented with 3.0X12mm Resolute Integrity stent. Lastly it was dilated with 3.25mmX12mm PIPIT NC balloon (Figure 4).
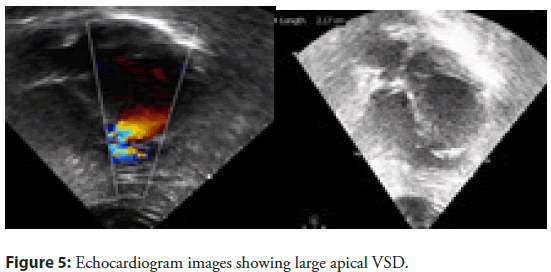
After ensuring good flow across LAD, patient was shifted to CCU in stable condition with excellent results. He was improving till 6 days of PTCA when he developed breathlessness on walking a few steps. He also had history of PND. On examination, his BP was 90/60 mmHg, heart rate 92/min (Figure 5).
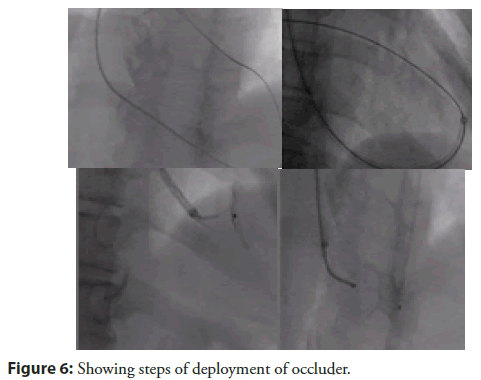
His JVP was raised. Chest showed bilateral basal crept. His heart sounds S1 and S2 were normal. There was left ventricular S3 at apex. Pansystolic murmur was heard at apex, radiating to parasternal area. With no time he developed cardiogenic shock requiring Inotropes. Echocardiogram done at this stage which showed 22 mm large VSR at apex with left to right shunt, severe LV systolic dysfunction (LVEF-35%). After initial stabilization in view of pulmonary oedema and cardiogenic shock, for another 4 days. VSR device closure was planned after detailed discussion with surgical team and family members. Patient shifted to Cath lab with a pretended strategy from the very beginning to avoid formation of AV loop (conventional VSD closure technique) in order to prevent tearing of fragile VSD rims while creating AV loop for device. In addition, other strategies used here were no contrast and utilisation of intra procedure TEE for device size assessment and deployment. Cannulation of the right femoral artery and right internal jugular vein was performed using the Seldinger technique. A guidewire (03500 Terumo guidewire) was introduced from the RIJV and advanced through the VSR into the left ventricle and eventually into right upper pulmonary vein making a course RIJV to RA to RV to VSR to LV to LA and finally guide wire was parked in RUPV (Figure 6).
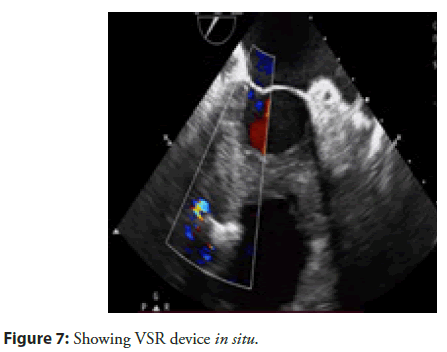
After this the Terumo guide wire was exchanged with Amplatzer super stiff wire and delivery sheath was advanced from the venous side over the wire through the VSR into the left ventricle. Correct positioning of the delivery sheath is confirmed in Fluoroscopy/TEE. The guidewire is then retracted leaving the delivery sheath in position. After the echocardiographic confirmation 24mm Post MI occluder was deployed across VSR using the delivery sheath. Correct positioning of the device and closure was confirmed by TEE and/or Fluoroscopy. After satisfactory placement the occluder was released. Patient tolerated the procedure well and discharged on dual antiplatelet as per unit policy after 4 days, after his heart failure was well controlled. His echocardiography showed well placed device (Figure 7). He is on regular follow up for more than 12 months and is presently in NYHA class 1.
Case 2: Transcatheter closure of postinfarction ventricular septal defect in a critically sick elderly patient.
A 70-year-old lady with a history of hypertension and dyslipidaemia was admitted in our hospital for acute S-T segment elevation anterior wall Myocardial Infarction. Primary coronary angioplasty was planned owing to hemodynamic deterioration. Abnormality with akinesia of anterior, septal and all apical segments (corresponding to Left anterior descending artery drainage territory) with severe LV dysfunction. Mild to moderate ischemic Mitral Regurgitation was also noted. An intra-aortic balloon pump was inserted for hemodynamic support and coronary angiography was performed which showed severe proximal to mid LAD lesion that was treated percutaneously using coronary stent from proximal to mid LAD. After the procedure, she was stable for two days, however, she subsequently developed progressive dyspnoea, tachycardia and hypotension. Cardiovascular examination revealed a new soft early systolic murmur heard maximally at the left lower sternal border. Her blood pressure was borderline low averaging 90/50 mm Hg and fine basal crepitations were noted. Transthoracic echocardiogram revealed a lower muscular, moderate size VSD measuring LV side: 12 mm, RV side: 9 mm with bidirectional shunting (Figure 8).
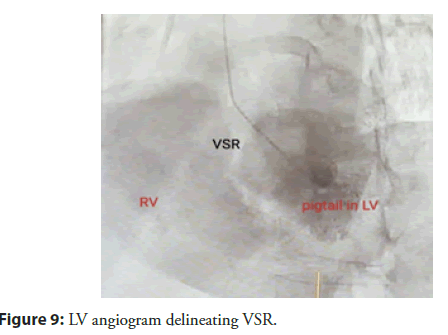
RV systolic pressure was estimated as 64 mm Hg from a tricuspid regurgitant jet velocity of 4 m/s. In view of the patientâs background, clinical condition, and her refusal for cardiac surgery, we decided to proceed with transcatheter closure of VSD under fluoroscopy and real time Transoesophageal Echocardiography (TEE) guidance. Patient was taken up in catheterisation lab. Procedure was performed under general anaesthesia. Both right femoral arterial and venous accesses were taken. LV angiogram (only one) was done with 6F pigtail catheter to delineate the lesion better (Figure 9).
Figure 9: LV angiogram delineating VSR.
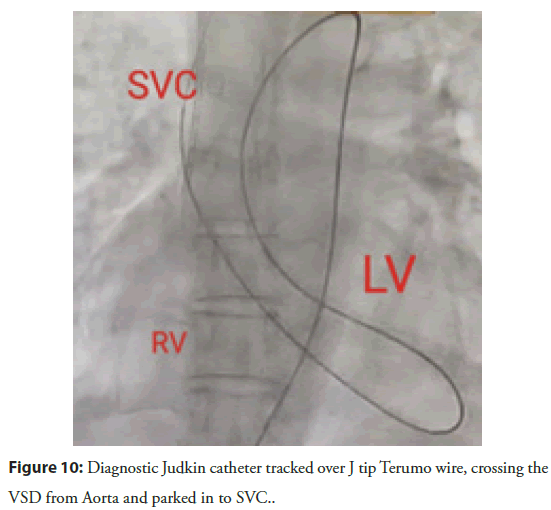
VSD was crossed retrogradely with 5F diagnostic Judkins right catheter guided over 0.032â J tip exchange length wire and parked in superior vena cava. The diagnostic Judkins catheter was then advanced over the wire till SVC (Figure 10).
The 0.032â Terumo wire was exchanged with 0.035â exchange length Amplatzer super stiff guidewire for better support. 9F guiding Judkins catheter was then advanced from Aorta to the RV cavity, through the VSD. Lastly wire was carefully removed and 14X12 mm Konar -MFO (Lifetech) was used. The device was extruded from the catheter until the RV disc was opened under echocardiographic and fluoroscopic guidance (Figure 11). It was then withdrawn toward the interventricular septum. After further satisfactory echocardiographic evaluation of septal alignment, the LV disc was also deployed and the device was released from the delivery cable.
Both TEE and fluoroscopy confirmed good placement of the device with no detectable residual shunt, demonstrating optimal device selection. The patient showed immediate significant symptomatic improvement. She was discharged after 5 days of procedure with mild symptoms (NYHA I). Patient is on regular follow up of more than 12 months and doing well (Figure 12).
Case 3: Device closure of VSR in a moderately symptomatic young adult.
Our third case of series was a 48-year-old gentleman without previous history of hypertension, diabetes mellitus and smoking, sustained an acute anterior wall myocardial infarction, and was thrombolysed with streptokinase within a window period of six hours at a local hospital. He was shifted to our hospital on detection of a ventricular septal defect, ongoing angina and breathlessness at rest next day. On admission, he was in congestive heart failure with a pulse rate of 110/minute, blood pressure of 100/70 mm Hg and a grade IV pan systolic murmur at left lower parasternal area. His 12-lead surface Electrocardiogram (ECG) was consistent with evolved acute anterior wall myocardial infarction. Two-dimensional showed akinetic mid and distal interventricular septum and apex, moderate left ventricular dysfunction and a lower muscular ventricular septal defect of 21 mm size in the anterior-inferior part of the septum (Figure 13).
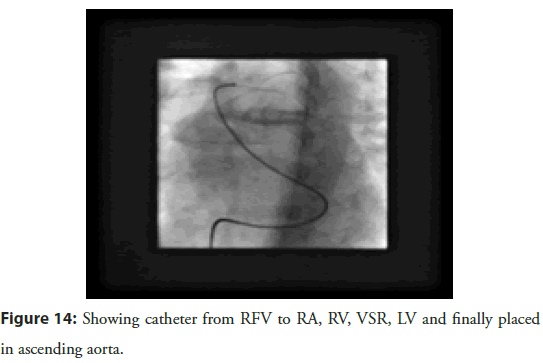
Cardiac catheterization done same day revealed a single muscular ventricular septal defect of size 24mm with a significant step up of oxygen saturation at right ventricular level by 18 % and the ratio of pulmonary blood flow (QP) to the systemic blood flow (Qs) depicted as QP/QS equal to 1.7:1. Pulmonary artery pressure (40/12/15 mm Hg) and pulmonary wedge pressure (mean-17 mmHg) was mildly elevated. Left Anterior Descending artery was the only affected vessel with proximal 90% stenosis. Coronary angioplasty using a drug eluting (Resolute 3 mm à 15 mm) stent was done with good angiographic result. He was pain free and asymptomatic since then. The device closure was planned after 4 weeks. Right femoral approach was taken. VSD was crossed from Right Ventricular (RV) side with right Judkinâs catheter and Amplatzer super stiff wire was passed through it into the Ascending aorta (Figure 14).
Patient tolerated the procedure well and was discharged after 4 days, after his heart failure was well controlled. His echocardiography showed well placed device. He is also on regular follow up for more than 12 months and doing well.
Case 4: Device closure of post MI VSR in 80 years old lady after getting a primary PTCA to LAD with severe left ventricular dysfunction.
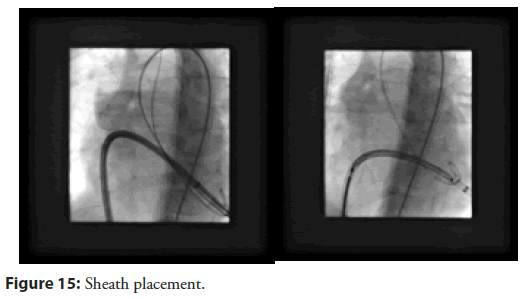
Our last case of series was an elderly female aged 78 years admitted for delayed presentation of anterior ST-elevation MI on a background of treated elsewhere with coronary angioplasty with drug-eluting stent to her mid-left anterior descending artery and to the left anterior descending and left circumflex. Initially treated medically without intervention because of significant anaemia and delayed presentation. His TTE showed an impaired LV ejection fraction of 30% and a 12 mm VSR in the apical muscular part of the septum. Her extreme age and co-morbidities precluded her from surgery. The percutaneous VSR closure procedure was performed under local anaesthesia with TTE guidance. Right Internal Jugular and femoral venous access was obtained, and the right heart study showed Qp/Qs 2.2, mean pulmonary artery pressure of 44 mmHg and the calculated pulmonary vascular resistance was 1.8 Wood units. As the morphology of the defect was simple and not serpiginous, the decision was made to attempt to cross the VSR from the RV side with a Glide wire (Terumo) and diagnostic JR catheter. This was successful and the glide wire exchanged for a support wire. An 8 Fr Amplatzer 45° torque delivery catheter (Abbott) was then introduced into the LV over the support wire. A Cocoon PDA device 16 à 14 mm was successfully deployed with a good seal of the defect. The patient was discharged home well without complications. Notably this procedure done avoiding any AV loop formation, with no contrast used and under local anaesthesia with TTE guidance (Figure 15).
Results
All these four cases mentioned in our study underwent a complete closure of their respective lesions with no evidence of residual shunt. None of these patients had any major complications, prolong stay, or any vascular injuries. All patients completed minimum 12-months follow-up and were doing well without any residual shunts.
Discussion
VSR has incidence of 0.2% among myocardial infarction cases which is commonly seen either as early as 24 hours or as late as 5-6 days with a range of 1-14 days postinfarction period [7]. Mortality is high, exceeding 90% in patients who go untreated.
Acute VSR requires emergency surgery regardless of the patientâs clinical status. However, the incidence rate of residual shunts following surgery is about 20% with a mortality rate close to 50% [8-11].
Device closure of VSR has shown better survival rates being the virtue of less invasive and traumatic in nature [12]. It is not yet clear which occluder device is the best option for the treatment of a VSR. In Bialkowski, et al., study, interventional closure of PMIVSD cases was carried out mainly using atrial septal occluders. Procedure failure occurred in all patients with acute VSR [13].
Traditional transcatheter VSR closure often involves establishing an AV loop, where a wire is passed from the left ventricle to the right ventricle and then externalized through the venous system. While this approach may facilitate device deployment, it poses significant risks:
1. Increased septal tear and defect expansion: The infarcted myocardium is fragile, and excessive wire tension from an AV loop can exacerbate septal rupture, leading to hemodynamic deterioration. Studies have shown that excessive manipulation in the infarcted zone can increase the risk of procedural failure and residual shunting [14]
2. Higher risk of complete heart block: The conduction system is often in close proximity to the VSR, and excessive manipulation from an AV loop can stretch or damage the conduction pathways [15]. Reported a higher incidence of complete heart block in cases where excessive traction was applied during wire externalization.
3. Device malposition and embolization: The AV loop technique can lead to suboptimal device placement, increasing the risk of embolization, particularly in larger defects with friable tissue [16]. Emphasized the importance of avoiding excessive wire tension to improve device stability and procedural success.
We have tried an alternative Strategy without AV Loop Formation in order to mitigate these risks, a direct antegrade approach without an AV loop is preferred.
Key steps in this technique include
Use of a soft or stiff wire without externalization: A guidewire is passed from the RV to the LV and positioned in the Ascending Aorta or RUPV without being externalized. This reduces traction forces on the septum, minimizing the risk of tear and conduction disturbances. Another way to achieve this is to cross from LV to RV and placing the wire to PA in selective cases where device like Konar-MFO can be deployed through guiding JR from LV side avoiding larger sheath and obviously avoiding the AV loop formation as we have done in our second case. Later strategy is possible only in smaller cases as available device which can be used through this manner are size limited and available only up to 14 mm.
• Controlled device deployment: Instead of a conventional arteriovenous rail, a sheath is advanced over the wire in a controlled manner, allowing precise positioning of the closure device.
• Selection of an appropriate device: Using an Amplatzer post-infarct muscular VSD occluder, Konar MFO provides adequate closure while minimizing radial stress on the weak septum.
• Minimizing contrast use: A Strategy without LV Angiography. Sick patients undergoing VSR closure often have multiple comorbidities, including acute kidney injury or cardiogenic shock, making contrast load a major concern. Avoiding LV angiography is a key step in reducing contrast exposure while still ensuring precise defect localization and device placement.
• Alternative imaging and guidance techniques: Transoesophageal Echocardiography (TEE) provide real-time visualization of the defect and help guide wire passage and device deployment without the need for contrast injections.
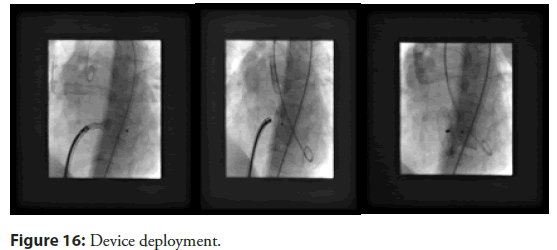
• Right heart catheterization with small contrast injections: If needed, limited contrast injections via the pulmonary artery can provide information about shunt size while minimizing overall dye exposure (Figure 16).
The idea behind deployment of the device from retrograde route to avoid making AV loop which can make this procedure less cumbersome. Since this device can be deployed through a guiding Judkins right catheter, tracking of stiff long sheaths can also be avoided in an already necrosed area as precautionary step to avoid any dislodgement of any embolus from necrosed tissue to lungs.
Another point of concern is possible entrapment of the device in the mitral valve apparatus or damage to the tricuspid leaflets. Continuous TEE guidance during the procedure is strongly recommended to prevent such complication (Figure 17).
Conclusion
As far as conclusion of this paper is concerned, we suggest that device closure of VSR without the use of contrast and avoiding AV loops is a feasible and effective approach, as demonstrated by our three successfully completed cases. This technique minimizes procedural risks, reduces contrast-induced nephropathy, and avoids complications associated with AV loops. Careful imaging guidance and meticulous procedural planning are crucial to ensuring success. Our experience highlights the potential of this method as a safer alternative for high-risk patients, paving the way for broader application in structural heart interventions.
References
- Crenshaw BS, Granger CB, Bimbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-1 (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) trial investigators. Circulation. 101(1):27-32 (2000).
- Yip HK, Fang CY, Tsai KT, et al. The potential impact of primary percutaneous coronary intervention on ventricular septal rupture complicating acute myocardial infarction. Chest. 125(5):1622-1628 (2004).
- Menon Y, Webb JG, Hillis LD, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: A report from the SHOCK trial registry. Should we emergently revascularize occluded coronaries in cardiogenic shock? J Am Coll Cardio. 36(3 Suppl. A):1110-1116 (2000).
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 127:e362-e425 (2013).
- Richter DJ, Schauerte P, Sousa Uva M, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. EuroIntervention. 10(9):1024-1094 (2015).
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 33(20):2569-2619 (2012).
- Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 72(12):1253-1379 (2014).
- Sathananthan J, Ruygrok P. Evolution in the management of postinfarct ventricular septal defects from surgical to percutaneous approach: A single-center experience. J Invasive Cardiol. 25(7):339-343 (2013).
- Dawson AG, Williams SG, Cole D. Does the placement of an Amplatzer septal occluder device confer benefit in patients with a post-infarction ventricular septal defect? Interact Cardiovasc Thorac Surg. 19(6):1040-1047 (2014).
- Thiele H, Kaulfersch C, Daehnert I, et al. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 30:81-88 (2009).
- Calvert PA, Cockburn J, Wynne D, et al. Percutaneous closure of postinfarction ventricular septal defect: In-hospital outcomes and long-term follow-up of UK experience. Circulation. 129:2395-2402 (2014).
- Szkutnik M, Bialkowski J, Kusa J, et al. Postinfarction ventricular septal defect closure with Amplatzer occluders. Eur J Cardiothorac Surg. 23(3):323-327 (2003).
- Demkow M, Ruzyllo W, Kepka C, et al. Primary transcatheter closure of postinfarction ventricular septal defects with the Amplatzer septal occluder- immediate results and up-to 5 years follow-up. Euro Intervention. 1(1):43-47 (2005).
- Bialkowski J, Szkutnik M, Kusa J, et al. Transcatheter closure of postinfarction ventricular septal defects using Amplatzer devices. Rev Esp Cardiol. 60(5):548-551 (2007).
- Matyal R, Wang A, Mahmood F. Percutaneous ventricular septal defect closure with Amplatzer devices resulting in severe tricuspid regurgitation. Catheter Cardiovasc Interv. 82:E817-E820.
- Perez-David E, Garcia Fernandez MA, García E, et al. Successful transcatheter closure of a postmyocardial infarction ventricular septal rupture in a patient rejected for cardiac surgery: Usefulness of transesophageal echocardiography. J Am Soc Echocardiogr 20:1417.e9-e12 (2007).