Review Article - Interventional Cardiology (2012) Volume 4, Issue 4
Percutaneous circulatory support during percutaneous coronary intervention
- Corresponding Author:
- Robert L Wilensky
Division of Cardiovascular Medicine
Hospital of the University of Pennsylvania & Cardiovascular Institute
University of Pennsylvania
3400 Spruce Street, 9 Gates, Philadelphia, PA 19104, USA
Tel: +1 215 615 3060
Fax: +1 215 615 3073
E-mail: robert.wilensky@uphs.upenn.edu
Abstract
Keywords
acute myocardial infarction, cardiogenic shock, extracorporal membrane oxygenation, Impella®, intra-aortic balloon pump, left ventricular assist device, TandemHeart®
Improvements in catheter, guide wire, balloon and stent technologies as well as improved diagnostic tools, adjunctive antithrombotic and antiplatelet agents and the application of randomized clinical studies have directly led to improvement in outcomes of percutaneous coronary intervention (PCI). As a result, operators are more comfortable with safely performing PCI on higher-risk lesions in patients with comorbidities, which would have been considered foolhardy a decade ago. For example, PCI is now performed on lesions in the last remaining patent saphenous vein graft or coronary artery, multivessel PCI is carried out in cardiogenic shock patients, and PCI is conducted in patients with left main coronary artery disease in the setting of an occluded right coronary artery or in patients with severely decreased left ventricular ejection fraction. However, PCI in these patient subgroups remains associated with an increased risk of death and myocardial infarction and so operators have elected to perform the PCI with circulatory support in the hopes of decreasing the peri-procedural risk. We, hereby, review the pathophysiology of cardiogenic shock, the indications and uses of the available left ventricular support devices and the procedural results of these devices to support the performance of PCI in higher-risk patients.
Cardiogenic shock
Cardiogenic shock is the presence of a systolic blood pressure less than 90 mmHg and target organ hypoperfusion due to cardiac dysfunction [1]. In the absence of treatment, cardiogenic shock carries an in-hospital mortality rate that approaches 80% [2]. The use of thrombolytic therapy reduced the mortality rate of cardiogenic shock to 55%, but shock still accounts for the highest number of deaths from an acute myocardial infarction [3]. The Should We Emergently Revascularize Coronary Arteries for Cardiogenic Shock (SHOCK) trial randomized patients presenting with a myocardial infarction and cardiogenic shock to either early revascularization (either PCI or coronary artery bypass surgery) or no revascularization. Although there was no difference in 30-day mortality, the group randomized to revascularization had reduced 6- and 12-month mortality rates (relative risk: 0.8; 95% CI: 0.65–0.98) [4,5]. However, despite the use of emergent PCI, approximately 10% of acute coronary syndrome events are complicated by cardiogenic shock and the mortality rate of these patients has only minimally improved, from 60% in 1995 to 47% in 2004 [6].
Cardiogenic shock during an acute myocardial infarction is predominately due to left ventricular dysfunction. Indeed, in the SHOCK registry, the cause of shock in 1190 patients with acute myocardial infarction and cardiogenic shock was left ventricular dysfunction in 78.5% of cases, while mitral regurgitation, isolated right ventricular failure, ventricular septal rupture and tamponade comprised the cause of shock in a minority of cases [5]. In the GUSTO-1 trial, only 11% of patients presented with shock initially, while 89% subsequently developed shock after the initial presentation [3]. In a National Registry of Myocardial Infarction (NRMI) review, 21% of patients presented with shock while the remaining 79% developed shock during the hours or days following the initial infarction, despite urgent coronary revascularization [6].
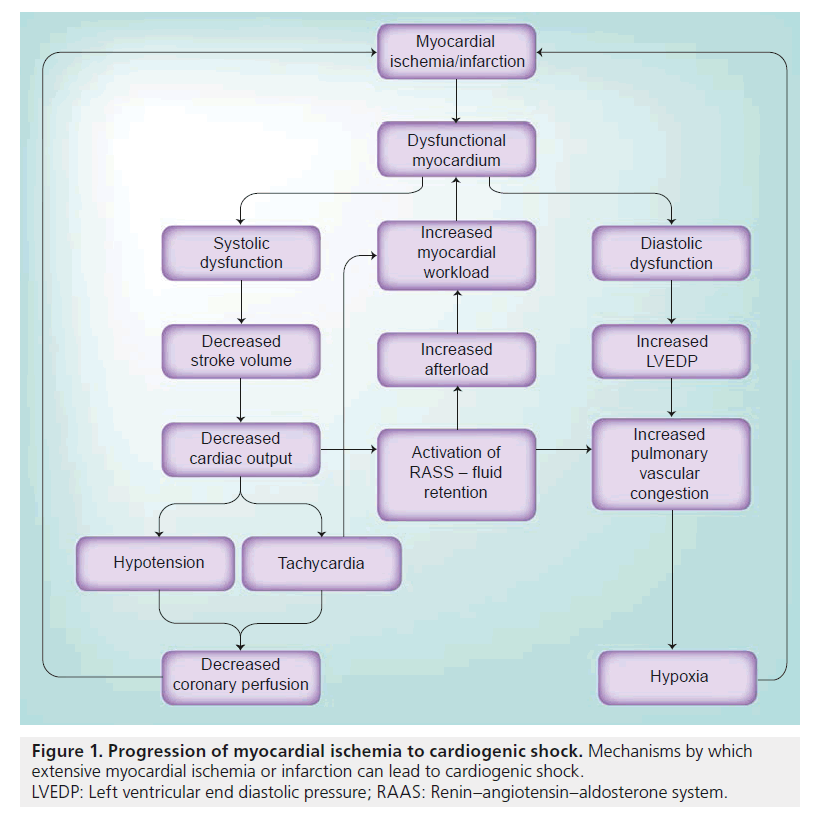
Myocardial ischemia/infarct leads to both systolic and diastolic myocardial dysfunction (Figure 1). Systolic abnormalities result in decreased stroke volume, decreased cardiac output and lower blood pressure. The normal physiologic response is to increase the heart rate and increase peripheral vasoconstriction. However, the resulting hypotension and tachycardia leads to decreased coronary perfusion, which is dependent on diastolic blood pressure and the diastolic filling period. Decreasing coronary perfusion results in further myocardial ischemia and a worsening cycle of myocardial dysfunction ensues. Decreased blood pressure and thus, lower renal blood flow, is a stimulant for the activation of the renin–angiotensin–aldosterone system, which results in peripheral vasoconstriction and increased afterload. Tachycardia and the increased afterload leads to increased myocardial demand and further myocardial dysfunction.
Figure 1. Progression of myocardial ischemia to cardiogenic shock. Mechanisms by which extensive myocardial ischemia or infarction can lead to cardiogenic shock.
LVEDP: Left ventricular end diastolic pressure; RAAS: Renin–angiotensin–aldosterone system.
Diastolic dysfunction leads to an elevated left ventricular end diastolic pressure, which, in combination with a decreased diastolic blood pressure, results in a lower coronary perfusion pressure and lower coronary blood flow. The higher left ventricular end diastolic pressure leads to increased pulmonary vascular congestion and hypoxia, which, in turn, results in further myocardial ischemia.
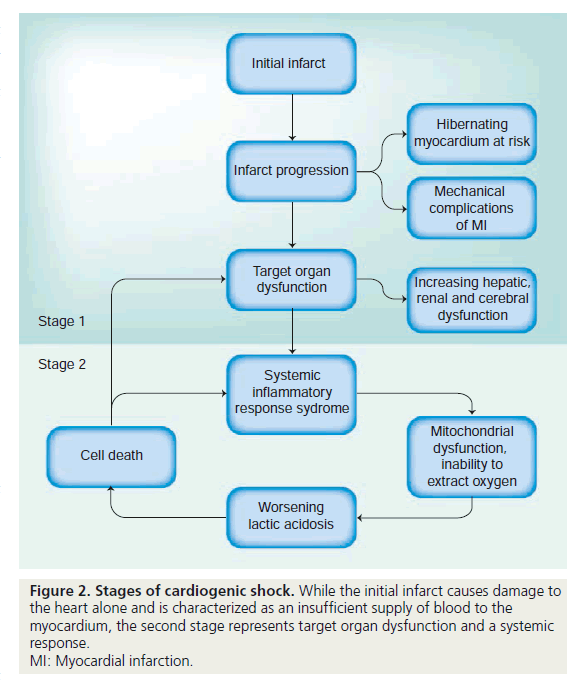
There are two stages of cardiogenic shock (Figure 2): the initial infarct and subsequent cycle of cardiovascular changes that lead to worsening myocardial function; and target organ dysfunction and systemic inflammatory response syndrome (SIRS). If unchecked, cardiogenic shock results in severe lactic acidosis, cell death and, in association with SIRS, severely decreased systemic vascular resistance. Unlike the first stage of cardiogenic shock, which is primarily a problem of insufficient supply, the second stage can best be thought of as an inability to extract and use oxygen due to microvascular dysfunction, mitochondrial disarray and, eventually, cell death. Hence, efforts to treat cardiogenic shock that either address the underlying cause or supplement cardiac output (such as PCI and mechanical support devices) are most efficacious if utilized before the onset of the second stage. Once target organ dysfunction and the SIRS response develop, it is unlikely that simply augmenting cardiac output will result in complete recovery.
Use of circulatory support
In addition to urgent revascularization, the initial management of cardiogenic shock involves administration of vasopressors and inotropes, as well as mechanical circulatory support. Despite these therapeutic strategies, some patients remain hypotensive with concomitant signs and symptoms of poor organ perfusion, and, as such, may benefit from more aggressive means of circulatory support, such as left ventricular assist devices (LVADs). Second- and third-generation surgical LVADs are safe and efficacious alternatives for bridging patients to cardiac transplant, as well as destination therapy for patients with advanced heart failure [7,8]. However, the implantation of these permanent devices in patients who are in severe refractory cardiogenic shock is often associated with a high early mortality rate, likely due to the need for cardiopulmonary bypass during a lengthy surgery. Indeed, as several studies have shown, patients deteriorating most rapidly are the least likely to recover after implantation of a LVAD [9]. Percutaneous ventricular assist devices, such as the Impella® (Abiomed, Danvers, MA, USA) and the TandemHeart® (CardiacAssist, PA, USA), as well circulatory support via extracorporeal membrane oxygenation (ECMO) may provide safer and less invasive alternatives for the most critically ill cardiac patients with potential for future improvement via revascularization or as a bridge to surgical LVAD or heart transplant.
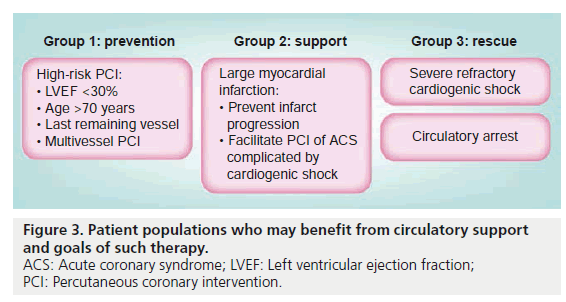
Mechanical circulatory support is a treatment option for three patient populations (Figure 3). The least acute cohort is the planned percutaneous intervention in a high-risk patient. While the definition of a high-risk patient is open to debate we classify a patient as high-risk when there is decreased left ventricular ejection fraction, severe aortic or mitral valvular disease, or deemed to not be a surgical candidate, and vessel-specific factors such as intervention on last remaining vessel or graft, left main or ostial left anterior descending artery or a bifurcation lesion involving large side branches (Figure 4). In the setting of an acute myocardial infarction patient aged >75 years, left ventricular ejection fraction being <40%, presence of hypotension, arrhythmia requiring treatment, in-hospital cerebral vascular accident and the final TIMI grade blood flow were all predictive of increased in-hospital mortality [10]. Other risk stratification systems such as the SYNTAX score that are used to predict major adverse cardiovascular events when comparing PCI with bypass surgery take into account lesion/vessel anatomic characteristics such as the number, location and complexity of the lesions [11]. While this score can lend insight into those anatomic characteristics which may be associated with worse outcomes, it has not been validated for use in the acute setting to differentiate the high risk versus low risk PCI candidate. Insofar that it remains difficult to a priori predict those patients with a high likelihood of peri-procedural events it therefore, it is difficult to predict which patients would or have benefitted by the use of circulatory support. The use of circulatory support in these setting is to ensure support in case the intervention proves more difficult than anticipated or results in a complication which may potentially lead to cardiogenic shock. In these cases, if the procedure goes well the device is removed prior to the patient leaving the catheterization laboratory.
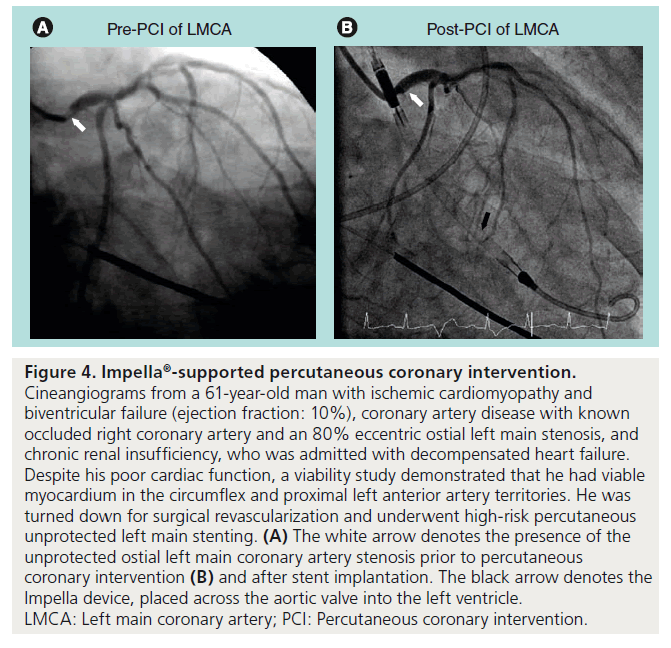
Figure 4. Impella ® -supported percutaneous coronary intervention. Cineangiograms from a 61-year-old man with ischemic cardiomyopathy and biventricular failure (ejection fraction: 10%), coronary artery disease with known occluded right coronary artery and an 80% eccentric ostial left main stenosis, and chronic renal insufficiency, who was admitted with decompensated heart failure. Despite his poor cardiac function, a viability study demonstrated that he had viable myocardium in the circumflex and proximal left anterior artery territories. He was turned down for surgical revascularization and underwent high-risk percutaneous unprotected left main stenting. (A) The white arrow denotes the presence of the unprotected ostial left main coronary artery stenosis prior to percutaneous coronary intervention (B) and after stent implantation. The black arrow denotes the Impella device, placed across the aortic valve into the left ventricle.
LMCA: Left main coronary artery; PCI: Percutaneous coronary intervention.
Patients with an acute myocardial infarction and cardiogenic shock are the second group of patients in whom mechanical circulatory support is used. These patients might be stable on one inotrope or pressor but there is concern that the hemodynamic profile might worsen while the percutaneous intervention is being performed. The use of mechanical circulatory support, thusly, allows for a more complete revascularization in a more stable environment. Following a successful PCI and a favorable hemodynamic profile, the device is removed shortly thereafter.
Severe refractory cardiogenic shock and/or circulatory arrest patients represent the third group in which mechanical circulatory support is used in order to salvage myocardium and prevent death. The use of circulatory assist devices in these scenarios often facilitates subsequent definitive therapy (coronary artery bypass surgery, PCI, ventricular tachycardia ablation, ventricular septal defect repair and so on) and may be a bridge to a permanent LVAD or heart transplantation. Infrequently, the device can be sufficient to allow for myocardial recovery. These patients usually require multiple days or even weeks of circulatory support prior to device removal.
Mechanical circulatory support devices
▪ Intra-aortic balloon pump
The intra-aortic balloon pump (IABP) is a balloon catheter that is inserted via a 7- or 8-French sheath in the femoral artery and positioned in the descending aorta. The balloon can be inflated with an electrocardiographic or aortic pressure trigger. The device inflates during diastole, providing greater coronary blood flow, and deflates during systole, creating a venturi effect, thus decreasing afterload and left ventricular end diastolic pressure [12].
The use of the IABP in cardiogenic shock was initally described by Kantrovitz in 1968 and its use increased in the thrombolytic era after data showed that the combination of IABP and thrombolysis in acute myocardial infarction increased patency of the infarct-related artery and improved thrombolysis in myocardial infarction (TIMI) grade blood flow [13–15].
However, this benefit has not been observed in several trials that examined the use of an IABP during PCI. The Primary Angioplasty in Myocardial Infarction (PAMI) 2 trial randomized 1100 patients with acute myocardial infarction who, in turn, were stratified into a high-risk group (>one of the following criteria: age >70 years, three-vessel disease, left ventricular ejection fraction <45%, vein graft occlusion, malignant ventricular arrhythmias or suboptimal angioplasty result) and low-risk group [16]. The high-risk group was subsequently randomized to either IABP or to standard medical care. Use of the IABP did not reduce the combined end point of death, reinfarction, stroke, heart failure or sustained hypotension. Indeed, the group randomized to IABP had a 2.4% incidence of stroke compared with 0% in control patients. The trial excluded all patients with triple-vessel or left main disease who underwent coronary artery bypass grafting. Furthermore, the use of IABP for cardiogenic shock excluded patients from enrollment. As such, the PAMI 2 trial was a select group of patients who were deemed high risk by study criteria but did not include such high-risk patients that they might have benefited from IABP support (triple-vessel and left main coronary artery disease or cardiogenic shock).
In a recent meta-analysis of seven randomized trials and nine cohort studies of IABP use in ST elevation myocardial infarction complicated by cardiogenic shock, IABP demonstrated no survival benefit or improvement in left ventricular ejection fraction [17]. The aforementioned studies included a wide range of patients (average age: 50–65 years), various treatment modalities for ST elevation myocardial infarction (no therapy, thrombolytic therapy or primary PCI) and a wide range of inclusion criteria (some studies also included non-ST elevation myocardial infarction). The timing from initial infarct to study inclusion ranged from 3 to 24 h. IABP use in the setting of primary PCI was associated with a 6% increase in 30-day mortality.
In an effort to see if the use of IABP could reduce infarct size when used in conjunction with primary PCI for ST elevation myocardial infarction, the Counterpulsation to Reduce Infarct Size Pre-PCI Acute Myocardial Infarction (CRISP AMI) trial randomized patients to pre-PCI IABP placement versus PCI without IABP placement [18]. There were no differences between the two groups in left ventricular mass as measured by cardiac MRI up to 5 days post-intervention. The potential benefit of IABP placement prior to PCI may have been offset by the time it took to place the IABP, although placement added roughly 9 min to the procedure. The death rate was statistically insignificantly lower in the IABP plus PCI group. The observation that 8.5% of patients in the PCI group crossed over to rescue IABP supports the use of IABP as standby strategy.
So, despite the early promising data for the use of the IABP in acute myocardial infarction and the continued American College of Cardiology/American Heart Association 1B recommendation for the use of IABP in acute myocardial infarction complicated by cardiogenic shock, there is a lack of data to support the routine use of IABP during primary PCI [19].
▪ Impella
The HemoPump® (Medtronics Corp, MN, USA) was a surgical LVAD mounted on a catheter and placed retrograde into the left ventricle via the femoral artery [20]. The concept behind this device was eventually used to engineer the Impella percutaneous LVAD. The Impella is an axial flow rotary pump that is placed retrograde into the left ventricle and directly decompresses the left ventricle, with an outflow of blood into the ascending aorta. The Impella 2.5 provides up to 2.5 l/min of flow while the Impella 5.0 can provide up to 5.0 l/min of flow but requires surgical cut-down for femoral arterial placement. The Impella 2.5 has been demonstrated to decrease mean pulmonary capillary wedge pressure, increase coronary blood flow and improve cardiac output [21].
The Prospective Feasibility Trial Investigating the Use of the Impella 2.5 System in Patients Undergoing High-Risk Percutaneous Coronary Intervention (PROTECT 1) was a small multicenter feasibility trial that assessed the use of the Impella 2.5 device in high-risk PCI, defined by low ejection fraction (mean left ventricular ejection fraction [LVEF]: 26 ± 6%) and either left main (n = 14) or last remaining vessel intervention (n = 6). The primary end point was the incidence of major adverse cardiovascular events at 30 days. The Impella 2.5 was implanted successfully in all patients without evidence of aortic valve injury, LV perforation or limb ischemia [22]. The subsequent PROTECT II randomized trial was designed to assess the effect of the Impella 2.5 versus the IABP on mortality in a high-risk PCI cohort. However, it was prematurely terminated at the halfway point (~300 patients) when it was apparent that the study would be unable to meet its prespecified end points and the study is, as yet, unpublished [101]. A critique of the study has been that rotational atherectomy was used in almost twice the number of patients in the Impella cohort, and this might have biased the effect towards the null hypothesis owing to the known increased risk of peri-procedral myocardial infarctions related to rotational atherectomy use. Indeed, rotational atherectomy may have been used more often after randomization to Impella as a result of the operator’s bias toward Impella use in high-risk patients.
Data to support the use of Impella 2.5 in highrisk PCI come from European and American registries. The Europella registry reported on 144 consecutive high-risk patients (elderly, those with lower ejection fraction, and requiring PCI in the left main, multiple vessel and last remaining vessel) in whom the Impella 2.5 was used to facilitate PCI [23]. The 30-day mortality was 5.5%. There were no myocardial infarctions but 6.2% of patients suffered bleeding that required transfusions and 4% of patients had vascular complications. The USpella registry report contained outcomes on 175 patients with Impella 2.5 implantation prior to high-risk PCI [24]. Patients with a ST elevation myocardial infarction and/or cardiogenic shock were excluded from this registry. In the USpella registry, 30-day mortality was 4% and there was an 8% rate of major adverse cardiovascular events (death, myocardial infarction, stroke, emergency cardiac or vascular surgical operation). The rate of groin hematomas was 8.6%, and 9.7% of patients required blood transfusions. An additional 3.4% of patients had vessel dissection and/or arteriovenous fistulas. There were no clinically important cases of hemolysis due to Impella use. Aortic stenosis is considered a contra- indication for the placement of an Impella; however, in a small series of patients, successful use of the Impella was demonstrated [25].
The ISAR-SHOCK trial randomized 25 patients with acute myocardial infarction complicated by cardiogenic shock who were undergoing primary PCI with an IABP or the Impella 2.5 and compared the change in hemodynamics [26]. The average time from the initial myocardial infraction to randomization was 4.5 h (range: 3.3–13 h). The Impella group had an increase in cardiac index of 0.49 l/min/m2 and improved pulmonary capillary wedge pressure but there was no difference in 30-day mortality between the two groups (46%). The group implanted with the Impella 2.5 had no major bleeding, but there was one case of limb ischemia post-device removal. Engstrom et al. evaluated the value of the Impella 5.0 versus the Impella 2.5 in patients presenting with a ST elevation myocardial infarction complicated by cardiogenic shock in a case series of 34 patients at a single center [27]. Although mortality rates in both groups were high, improved survival was observed in the group implanted with the Impella 5 (30-day mortality for Impella 5.0: 66%; Impella 2.5: 76%). Eight of 25 patients initially implanted with the Impella 2.5 were later upgraded to the Impella 5.0 owing to lack of support and persistent hypotension.
Hence, there are no current randomized clinical data that indicate that the use of the Impella 2.5 or the Impella 5.0 carries a mortality benefit in high-risk PCI. However, the use of this device does lead to favorable hemodynamic changes and the outcomes of high-risk patients in nonrandomized case series show a low complication rate and a high success rate with the Impella 2.5 device. The use of this device in shock is also of unproven mortality benefit as no large studies on acute cardiogenic shock have been performed with the Impella 2.5. Furthermore, there is concern that 2.5 l/min of flow may not be sufficient to adequately support a completely failed native circulation. Although the Impella 5.0 device does offer significantly more cardiac support, there is a requirement for a surgical cut-down to implant this device.
▪ TandemHeart
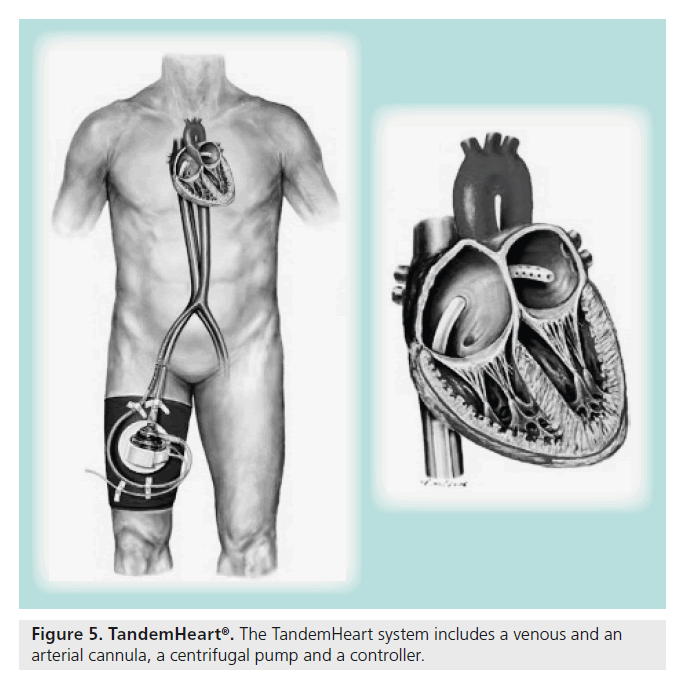
In 1962, Dennis reported the use of percutaneous trans-septal puncture as a means to provide left heart bypass [28]. This straightforward means of decompressing the left of the heart eventually evolved into the TandemHeart (Figure 5). The TandemHeart is a percutaneous mechanical support device comprised of three parts – a 21-French venous sheath (inflow) placed via the femoral vein across the interatrial septum, which then decompresses the left atrium, a 15–17-French arterial sheath (outflow) placed in the femoral artery, and a centrifugal pump that drives the circuit. The device on average takes 30 min to implant and requires the user to be facile with trans-septal puncture. The TandemHeart provides up to 5.0 l/min of flow and allows for direct left heart decompression.
The TandemHeart, like the Impella, can be used to support high-risk patients during planned percutaneous interventions. Recently, an approach utilizing a stepwise increase in the degree of support used (Impella 2.5 vs TandemHeart) based on the patient risk profile has been described [29]. Patients with severely reduced ejection fractions (<10%) or severely decompensated heart failure (pulmonary capillary wedge pressure >25 mmHg) undergoing intervention of the last remaining vessel or a left main intervention might be better supported with a device that can provide complete circulatory support such as the TandemHeart over the other devices that might not be able provide a similar level of support. Patients with aortic stenosis may benefit from the use of TandemHeart rather than an Impella.
There has only been one extensive case series that describes the outcome of patients in whom the TandemHeart was used to support a planned high-risk PCI. Thomas et al. reported a case series of 37 patients who were supported with the TandemHeart during PCI of which 47% of interventions were on the left main coronary artery and 21% of patients had concomitant severe aortic stenosis [30]. Twenty six of the 37 patients survived to hospital discharge but bleeding was a frequent complication, with 30 requiring blood transfusions.
The TandemHeart is more likely to be used in cases of rescue from cardiogenic shock. Thiele et al. randomized 41 patients who presented with acute myocardial infarction and subsequently developed cardiogenic shock to either an IABP or to the TandemHeart, with hemodynamic change as the primary end point [31]. The TandemHeart markedly improved hemodynamic function as compared with the IABP, as evidenced by a higher cardiac power index (cardiac power index = cardiac index × mean arterial pressure × 0.0022) and lower pulmonary artery and pulmonary capillary wedge pressures. The decrease in serum lactate was more pronounced in the group treated with the TandemHeart compared with the group treated with an IABP. Despite the improved hemodynamics, there was no mortality difference between the two groups (45% for IABP and 43% for TandemHeart). In addition, the group randomized to the TandemHeart was more likely to have major bleeding requiring transfusion (19 patients with LVADs vs eight patients with an IABP) and to suffer limb ischemia (seven patients with LVADs versus zero patients with IABP). Bleeding was predominately at the arterial access site for the patients with the TandemHeart. In addition, the authors postulated that the increased blood transfusion requirement in this group was secondary to the large cannulas used and activation of the complement cascade leading to a disseminated intravascular coagulation-like picture; a phenomenon which is also seen in patients placed on extracorporeal membrane oxygenation [32]. The cause for the lack of mortality benefit may have been related to the prolonged time from shock onset to randomization (11 h).
More recently, Kar et al. described a series of 117 patients that were implanted with the TandemHeart at a single center from 2003 to 2008 [33]. The patient population included 80 patients with an ischemic cardiomyopathy, 48 of whom had initially presented with a ST elevation myocardial infarction, although only five had had the LVAD implanted during an acute coronary syndrome. Of the 117 patients, 47.9% underwent cardiopulmonary resuscitation during the LVAD implant and 82.1% were supported with inotropes and an IABP. The only patients not supported with an IABP had been actively undergoing cardiopulmonary resuscitation. The median LVEF was 20% (interquartile range: 5%) and the average preimplant mean pulmonary capillary wedge pressure was 31.5 ± 10.2 mmHg. Despite the high anticipated mortality rate of this cohort, the investigators reported a 30-day survival rate of 59.8% and a 6-month survival rate of 54.7%. In the cardiac arrest cohort, the 30-day survival rate was 43% higher than expected. One reason for the relatively impressive mortality results may have been the relatively rapid implantation of the LVAD after onset of severe refractory shock, with an average time to implant from CPR onset of 65.6 ± 41.3 min. One patient suffered a wire perforation of the left atrium, only 3% suffered limb ischemia and 29% of patients had bleeding around the groin cannula site. The markedly lower limb ischemia rate was likely due to the increased use of an antegrade perfusion catheter.
▪ Extracorporeal membrane oxygenation
A machine designed to provide artificial oxygenation and perfusion was initially developed by Gibbon in 1953 and then further refined by Lillehei and Kirklin into what eventually became the current cardiopulmonary bypass system [34–36]. ECMO has seen widespread use in the last 5 decades in providing mechanical circulatory support in the perioperative setting and in the congenital heart and lung pediatric population. Many adult medical centers have turned to ECMO as the salvage mechanism for refractory cardiogenic shock and cardiac arrest with mixed results. Analysis of ECMO outcomes is complicated by several factors including the wide variety of diagnoses for implant (i.e., rescue for postcardiotomy syndrome, congenital abnormalities, univentricular versus bi-ventricular failure, and cardiac versus cardiac and respiratory failure), different implant strategies (peripheral vs central, size of implant cannulas), multiple different types and lengths of connecting tubing, and the use of different pumps to drive the circuit.
Due to the much larger sheath size and the associated greater risk of vessel injury, bleeding and limb ischemia, only a few cases of ECMO for planned high-risk PCI have been reported [37,38]. In the few case reports in which ECMO was used for cardiac support, the choice to use this support over other previously described support options was usually made owing to the greater comfort level of the performing institution with ECMO compared with the other options. The discussion here will be limited to ECMO that is peripherally inserted for the indication of isolated cardiogenic shock. In this setting, a catheter is placed in the femoral vein (23–29 French) and femoral artery (17–20 French) and connected to a pump and oxygenator. Like the Impella, TandemHeart and the IABP, systemic anticoagulation with heparin is needed for as long as the device is implanted. The flow rates delivered by such a system can reach up to 10 l/min and, in addition to providing perfusion, also provide complete oxygenation.
At our institution, ECMO has become the default salvage mechanism for patients presenting in severe cardiogenic shock or circulatory arrest. Thiagarajan et al. published the largest case series describing outcomes with ECMO as a salvage mechanism for cardiopulmonary resuscitation – the outcomes of the Extracorporeal Life Support Organization (ELSO) registry from 1992 to 2007 [39]. During this timeframe, 295 patients, who presented in cardiac arrest, were placed on ECMO when traditional cardiopulmonary resuscitation was insufficient. The average age of the patients was 52 years (interquartile range: 35–64) and 75% of them had a cardiac cause of cardiac arrest (other causes included pulmonary embolus, accidental injury, other respiratory disease and, infrequently, sepsis). The majority of patients were peripherally cannulated in the femoral artery (81%) and femoral vein (70%). Overall survival to discharge was 27%, comparing favorably to the previously published survival rate of 17% in 14,720 in-hospital cardiac arrests in which only traditional CPR was used [40]. Factors associated with a higher likelihood of survival included cause of cardiac arrest, higher pre-ECMO mean arterial pressure, pre-ECMO pulmonary artery O2 saturation and the use of percutaneous cannulation. Patients with viral myocarditis had the highest survival with ECMO support, although these patients comprised a minority of cases. The need for renal replacement therapy was associated with a twofold increase in mortality. A high percentage (33%) of patients experienced neurologic injury, 19% developed clots in the ECMO circuit, 21% had culture-proven infection and 24% of patients had bleeding requiring surgical intervention.
In an effort to minimize the complications associated with peripheral ECMO, El-Banayosy simplified the ECMO circuit to remove any extraneous tubing (platelet activation due to contact with plastic tubing surface is thought to promote ECMO circuit clot formation and possibly ischemia and stroke) and standardized the pump to a more powerful device (CentriMag; Thoratec, Pleasanton, CA, USA). Recently, this group reported a case series of ten patients (mean age: 45 years; LVEF: 30%) who all presented with cardiogenic shock refractory to conventional therapy (MAP <65 and cardiac index <2.2 on maximal inotropic support) and were then placed on the aforementioned ECMO circuit [41]. The in-hospital survival was 60% with no mechanical errors of the ECMO circuit. In addition, none of the ten patients experienced distal limb ischemia (likely due to the routine use of an anterograde perfusion catheter). However, bleeding continued to be a major complication as all patients required transfusions of one to four units of packed red blood cells daily.
The advantage of ECMO over the TandemHeart and Impella is the ability to support a patient with bi-ventricular failure and combined cardiac and respiratory failure. Furthermore, peripheral ECMO can be conducted at the patient’s bedside and does not require a trans-septal puncture, nor does it even necessitate activation of the cardiac catheterization laboratory. Owing to the nonproprietary nature of the ECMO circuit, the cost associated with carrying out ECMO is a tenth of the cost associated with either the Impella or the TandemHeart. The cost difference between the various devices is especially pertinent because the neurologic status and the ultimate outcome of many cardiac arrest patients is unknown and many health systems are reluctant to use an expensive device in a patient that may, even with optimal support, exhibit irreversible anoxic brain injury.
The disadvantage of the ECMO circuit is twofold: it has an inability to provide left ventricular decompression and a higher incidence of bleeding, limb ischemia and intravascular clot formation. When providing mechanical circulatory support to a young patient, one exit strategy is for myocardial recovery. However, if there is not adequate left ventricular decompression, worsening preload could hamper recovery and lead to further cardiac damage. However, while hypothesized and generally adhered to in the management of surgical LVADs, the theory that left ventricular decompression could promote myocardial recovery has not been conclusively proven. The increased bleeding and limb ischemia rates seen with ECMO are likely due to cannula size, although one could postulate that the increased surface area of the ECMO circuit could promote a DIC-like picture and cause clotting abnormalities.
Conclusion
It is perhaps unfair to subject the indications for the use of circulatory assist devices to the results of randomized clinical trials. Generally, the use of such devices is at the behest of the interventionalist if they believe that, after assessment of the patient’s comorbidities and anatomical characteristics, circulatory support is necessary in order to perform the required procedure safely. As such, the actual risk of the untoward events with such a procedure may be less than 10% so that 90% of patients undergo implantation of such devices without benefit. As such, clinical benefit in a randomized prospective study may be impossible to demonstrate unless a very large number of patients were randomized. However, the piece of mind afforded by such circulatory support devices and the possibility of performing the procedure in the absence of hemodynamic instability may make the risks associated with the placement of circulatory support devices well worthwhile.
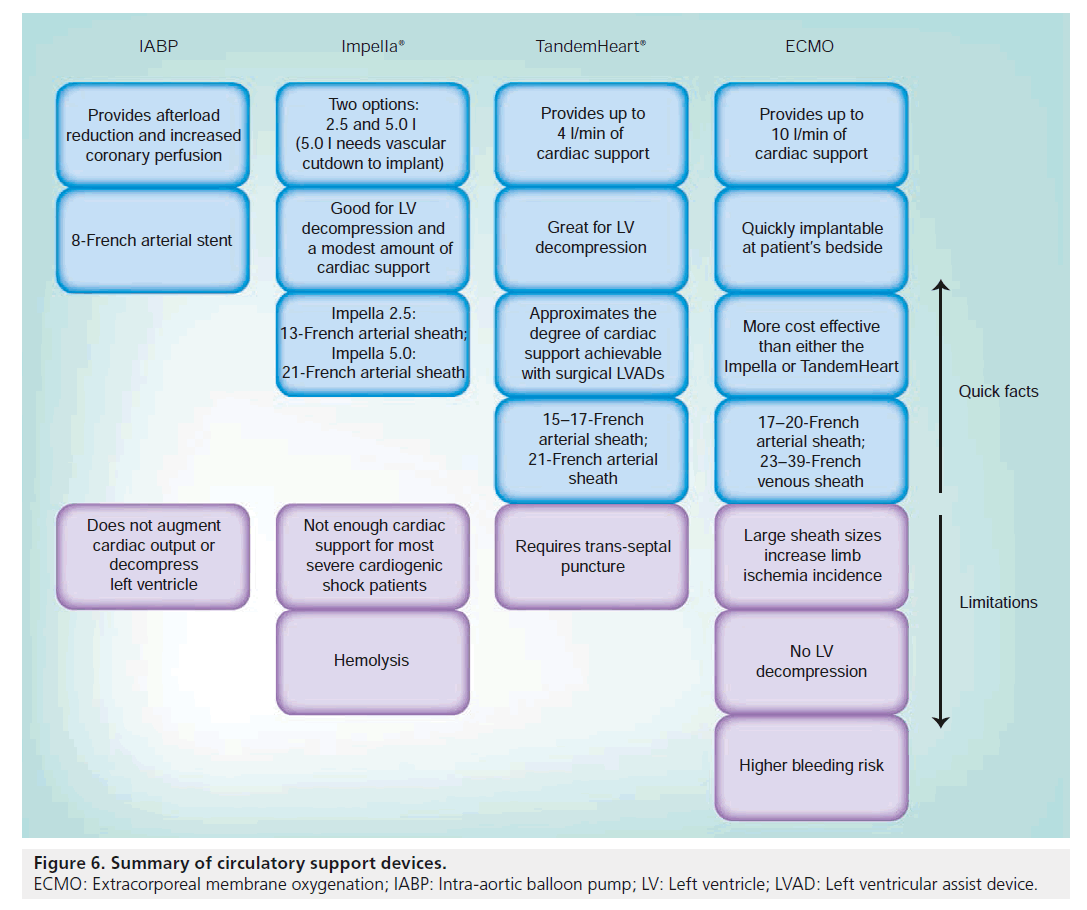
In summary, IABP (Figure 6) requires a baseline residual cardiac function and a stable cardiac rhythm. Its use in a patient who is undergoing cardiac arrest adds little to the stabilization of the patient. It is best used in a high-risk PCI cohort where it might lessen peri-procedural complications, but, even in this group, there is no proven mortality benefit.
The Impella 2.5 provides up to 2.5 l/min of circulatory support, which is frequently enough to temporarily stabilize a patient in extremis. However, the flow rates with this axial pump can change in response to the patient’s systemic vascular resistance such that a patient with a very low cardiac output and a very high systemic vascular resistance can frequently see much lower flow rates than 2.5 l/min. Also, this amount of support is not sufficient for a patient in cardiac arrest.
The TandemHeart can provide up to 4.0 l/min of circulatory support but requires both venous and arterial access and a trans-septal puncture. This amount of circulatory support rivals that seen with ECMO and is frequently enough to provide complete circulatory support.
Finally, if a patient has bi-ventricular failure or combined respiratory and cardiac failure, ECMO has the ability to provide both oxygenation and perfusion and thus provides complete cardiopulmonary support. However, the complication rate with ECMO is higher.
Future perspective
In order to make the PCI procedures safer, there is a need for better, less-invasive mechanical circulatory support options. An easily implantable device that could provide at least 3.5 l/min of flow with minimal bleeding complications would expand the use of these devices to many more patients. Currently, Abiomed has plans for a version of the Impella that will provide up to 3.5 l/min of flow and stay the same size as the current Impella 2.5 model. Other percutaneous heart pumps are being developed and clinically assessed.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
High-risk patients
▪ Patients at high risk are those who are:
– Aged >70 years;
– Have a left ventricular ejection fraction of <30%;
– Have a pulmonary capillary wedge pressure of >20 mmHg;
– Have a cardiac index of <2.2 l/min/m2;
– Require intervention for the last remaining vessel of bypass graft;
– Require intervention of the left main artery.
Two stages of cardiogenic shock
▪ Stage one of cardiogenic shock: initial insult and subsequent hypotension.
▪ Stage two of cardiogenic shock: target organ hypoperfusion and systemic inflammatory response syndrome.
▪ The goal is to recognize the patient as being at risk of advancing from stage one to stage two and to provide mechanical circulatory support before the patient enters stage two.
When to use which device
▪ High-risk percutaneous coronary intervention: Impella® or intra-aortic balloon pump.
▪ ST elevation myocardial infarction plus cardiogenic shock: TandemHeart® or possibly Impella.
▪ Cardiac arrest: TandemHeart if isolated left ventricular failure and patient can be brought to the catheterization laboratory. Extracorporeal membrane oxygenation in the case of biventricular failure or respiratory and cardiac failure, or if the procedure needs to be done at the bedside.
References
▪ of interest
▪▪ of considerable interest
- Hollenberg SM, Kavinsky CJ, Parillo JE. Cardiogenic shock. Ann. Intern. Med. 131, 47–59 (1999).
- Goldberg RJ, Gore JM, Alpert JS et al. Cardiogenic shock after acute myocardial infarction – incidence and mortality from a community wide perspective 1975–1988. N. Engl. J. Med. 325(16), 1117–1122 (1991).
- Holmes DR, Bates ER, Kleiman NS et al. Contemporary reperfusion therapy for cardiogenic shock: the GUSTO-1 trial Experience. J. Am. Coll. Cardiol. 26, 668–674 (1995).
- Hochman JS, Sleeper LA, Webb JG et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N. Engl. J. Med. 341, 625–634 (1999).
- Hochman JS, Buller CE, Sleeper LA et al. Cardiogenic shock complicating acute myocardial infarction: etiologies, management and outcome – a report from the SHOCK Trial Registry. J. Am. Coll. Cardiol. 36, 1063–1070 (2000).
- Babaev A, Frederick PD, Pasta DJ et al. Trends in management and outcome of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 294, 448–454 (2005).
- Rose EA, Gelijns AC, Moskowitz AJ et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 345, 1435–1443 (2001).
- Miller LW, Pagani FD, Russell SD et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 357, 885–896 (2007).
- Boyle AJ, Ascheim DD, Russo MJ et al. Clinical outcomes for continous-flow left ventricular assist device patients stratified by pre-operative INTERMACS classification. J. Heart Lung Transplant. 30(4), 402–407 (2011).
- DeGeare VS, Stone GW, Grines L et al. Angiographic and clinical characteristics associated with increased in-hospital mortality in elderly patients with acute myocardial infarction undergoing intervention (a pooled analysis of the primary angioplasty in myocardial infarction trials). Am. J. Cardiol. 86(1), 30–34 (2000).
- Serruys PW, Morice M, Kappetein P et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360, 961–972 (2009).
- Mullins CB, Sugg WL, Kennelly BM, Jones DC, Mitchell JH. Effect of arterial counterpulsation on left ventricular volume and pressure. Am. J. Physiol. 220, 694–698 (1971).
- Kantrowitz A, Tjonneland S, Freed PS, Phillips SJ, Butner AN, Sherman JL. Initial clinical experience with intraaortic balloon pumping in cardiogenic shock. JAMA 203, 113–118 (1968).
- Kono T, Morita H, Nishina T et al. Aortic counterpulsation may improve late patency of the occluded coronary artery in patients with early failure of thombolytic therapy. J. Am. Coll. Cardiol. 28(4), 876–881 (1996).
- Ohman E, Geroge B, White CJ et al. Use of aortic conterpulsation to improve sustained coronary artery patency during acute myocardial infaction. Results of a randomized trial. The Randomized IABP Study Group. Circulation 90(2), 792–799 (1994).
- Stone GW, Marsalese D, Brodie BR et al. A prospective randomized evaluation of prophylactic intraaortic balloon counterpulsation in high risk patients with acute myocardial infaction treated with primary angioplasty. J. Am. Coll. Cardiol. 29(7), 1459–1467 (1997).
- Sjauw KD, Engstrom AE, Vis MM et al. A systematic review and meta-analysis of intra-aortic balloon pump therapy in ST-elevation myocardial infarction: should we change the guidelines? Eur. Heart J. 30(4), 459–468 (2009).
- Patel MR, Smalling RW, Thiele H et al. Intra-aortic ballon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: the CRISP AMI randomized trial. JAMA 306(12), 1329–1337 (2011).
- Antman EM, Anbe DT, Armstrong PW et al. ACC/AHA guidelines for the management of patients with ST-elevation myuocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). Circulation 110, E82–E292 (2004).
- Wampler RJ, Moise JC, Frazier OH et al. In vivo evaluation of a peripheral vascular access axial flow blood pump. ASAIO Trans. 34(3), 450–455 (1988).
- Remmelink M, Sjauw KD, Henriques JP et al. Effects of left ventricular unloading by Impella Recover LP 2.5 on coronary hemodynamics. Catheter. Cardiovasc. Interv. 70, 532–537 (2007).
- Dixon SR, Heriques J, Mauri L et al. A prospective feasibility trial investigating the use of the Impella 2.4 system in patients undergoing high-risk percutaneous coronary internvetion (the PROTECT 1 trial): initial U.S. experience. J. Am. Coll. Cardiol. Interv. 2, 91–96 (2009).
- Sjauw KD, Konorza T, Erbel R et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device. J. Am. Coll. Cardiol. 54, 2430–2434 (2009).
- Maini B, Naidu SS, Mulukutla S et al. Real-world use of the Impella 2.5 circulatory support system in complex high-risk percutaneous coronary intervention: the USpella Registry. Catheter. Cardiovasc. Interv. doi:10.1002/ccd.23403 (2011) (Epub ahead of print).
- Martinez CA, Singh V, Londoño JC et al. Percutaneous retrograde left ventricular assist support for interventions in patients with aortic stenosis and left ventricular dysfunction. Catheter. Cardiovasc. Interv. doi:10.1002/ ccd.24303 (2012) (Epub ahead of print).
- Seyfarth M, Sibbing D, Bauer I et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intraaortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 52(19), 1584–1588 (2008).
- Engstrom AE, Cocchieri R, Driessen AH et al. The Impella 2.5 and 5.0 devices for ST-elevation myocardial infarction patients presenting with severe and profound cardiogenic shock: the Academic Medical Center intensive care unit experience. Crit. Care Med. 39(9), 2072–2079 (2011).
- Dennis C. Left atrial cannulation without thoracotomy for total left heart bypass. Acta Chir. Scand. 123, 267–279 (1962).
- Schwartz BG, Ludeman DJ, Mayeda GS et al. High-risk percutaneous coronary intervention with the TandemHeart and Impella devices: a single-center experience. J. Invasive Cardiol. 23(10), 417–424 (2011).
- Thomas JL, Al-Ameri H, Economides C et al. Use of a percutaneous left ventricular assist device for high-risk cardiac interventions and cardiogenic shock. J. Invasive Cardiol. 22, 360–364 (2010).
- Thiele H, Sick P, Boudriot E et al. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur. Heart J. 26(13), 1276–1283 (2005).
- Kirklin JK, Westaby S, Blackstone EH et al. Complement and the damaging effects of cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 86, 845–857 (1983).
- Kar B, Gregoric ID, Basra SS et al. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J. Am. Coll. Cardiol. 57, 688–696 (2011).
- Gibbon JH Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn. Med. 37, 180–185 (1954).
- Lillehei CW. A personalized history of extracorporeal circulation. Trans. Am. Soc. Artif. Intern. Organs. 28, 5–16 (1982).
- Kirklin JW, Donald DE, Harshbarger HG, Hetzel PS, Patrick RT, Swan HJ. Studies in extracorporeal circulation. I. Applicability of Gibbon-type pump-oxygenator to human intracardiac surgery: 40 cases. Ann. Surg. 144(1), 2–8 (1956).
- Koutouzis M, Kolsrud O, Albertsson P, Matejka G, Grip L, Kjellman U. Percutaneous coronary intervention facilitated by extracorporeal membrane oxygenation support in a patient with cardiogenic shock. Hellenic J. Cardiol. 51(3), 271–274 (2010).
- Spina R, Forrest AP, Adams MR et al. Veno-arteral extracorporeal membrane oxygenation for high-risk cardiac catheterization procedures. J. Heart Lung Circ. 19(12), 736–741 (2010).
- Thiagarajan RR, Brogan TV, Scheurer MA et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann. Thorac. Surg. 87, 778–785 (2009).
- Peberdy MA, Kaye W, Ornator JP et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 58(3), 297–308 (2003).
- Aziz TA, Singh G, Popjes E et al. Initial experience with CentriMag extracorporal membrane oxygenation for support of critically ill patients with refractory cardiogenic shock. J. Heart Lung Transplant. 29(1), 66–71 (2010).
▪▪ The SHOCK trial was a seminal trial showing improved mortality at 6 months for those patients who were referred for percutaneous coronary intervention upon presenting with cardiogenic shock.
▪▪ The REMATCH trial was the first randomized trial of the original HeartMate® XVE versus medical therapy. This trial showed improved outcomes of left ventricular assist device therapy over inotropes and ushered in the current era of mechanical circulatory support.
▪▪ Large meta-analysis that puts the current recommendation of using intra-aortic balloon pump (IABP) in doubt for cardiogenic shock.
▪▪ Randomized, open, multicenter trial assessing effects of the IABP on myocardial infarct size as documented by cardiac MRI, showing no additive effect on reducing infarct size when IABP was used.
▪▪ Largest case series of acute decompensated heart failure treated with the TandemHeart®. This case series included a very acutely ill patient population that was treated with the TandemHeart with good 6-month outcomes and minimal complications.
▪▪ Large registry of extracorporeal membrane oxygenation-assisted cardiopulmonary resuscitation.
▪ Website
101. Miller R. PROTECT II study halted for futility: Abiomed explanation meets skepticism. www.theheart.org/article/1161463.do (Accessed 17 May 2012)