Review Article - Interventional Cardiology (2013) Volume 5, Issue 6
Pacemaker reutilization for those in underserved nations: examining preliminary data and future prospects
- Corresponding Author:
- Thomas C Crawford
University of Michigan Hospitals
1500 East Medical Center Drive SPC 5852
Ann Arbor, MI 48109-5853, USA
E-mail: thomcraw@med.umich.edu
Abstract
The scope of the problem ▪ Many individuals in underserved nations are subjected to symptomatic bradycardia resulting in increased morbidity and mortality due to a lack of access to pacemakers. Promising solution ▪ Pacemaker reutilization promises to provide an alternative solution to overcome the high cost of new devices. Perceived challenges to pacemaker reutilization ▪ Infection and device malfunction rate must be high in reused devices. ▪ The public may not be willing to have their devices explanted. ▪ There are not enough devices to explant and reuse. ▪ It is unethical to reuse devices as this is below standard of care. ▪ The US FDA regulation prohibit pacemaker utilization. Current knowledge and potential solutions ▪ Numerous trials have established safety and efficacy on pacemaker reutilization. ▪ A great majority of device patients are willing to donate devices after death with funeral directors willing to assist. ▪ The cremation rate is rising to more than 50% in the USA, resulting in an increased number of devices that need to be explanted. These devices could be the main resource for device reuse. ▪ When compared with eminent morbidity from bradyarrhythmia with no available alternative, reuse of pacemakers becomes an ethical necessity. ▪ Project My Heart Your Heart is working closely with the FDA to create a proper legal framework.
Keywords
medical device donation, pacemaker recycling, pacemaker reuse
Cardiovascular disease is the leading cause of mortality worldwide, accounting for 30% of all deaths [1]. In recent years, advances in technology have dramatically reduced the morbidity and mortality of this epidemic. This benefit is clearly evident in the developed world as opposed to low- and middle-income countries (LMICs) where resources are scarce or often nonexistent [2]. Significant shortages of healthcare providers, as well as an inability to afford costly medical devices, partially account for the drastic disparities in healthcare between the developed world and LMICs [3].
In no other field is this great disparity more evident than in the field of cardiac electrophysiology - specifically pacemaker implantation [4]. Between 1993 and 2009, 2.9 million patients in the USA received a permanent pacemaker, with an overall increase of 55% from the previous decade [5]. This is in stark contrast to countries, such as India, where there are <18 new implants per million people [6]. Moreover, in the industrialized world, the primary indication for pacemaker implantation is sinus node dysfunction [7], while in LMICs, complete heart block is the most common indication, thus leading to greater morbidity and mortality [8,9]. An accurate understanding of the problem in LMICs is lacking; however, it is estimated that more than 1 million individuals globally are in need of pacemaker implantation annually [10]. Currently, Heartbeat International (FL, USA), a not-forprofit organization, is trying to provide a solution by donating cardiac devices that are close to expiration [10,101]. Devices close to expiration are those that are nearing the manufacturer shelf life in terms of guaranteed sterility. Typically, the period between the manufacturing date and the ‘use before’ date is 12-18 months [11]. Although the achievements of Heartbeat International can be characterized as nothing less than extraordinary, the organization’s impact is limited by the fact that they rely upon the limited number of devices that are near expiration. Other options must be explored in order to deliver care to those in great need.
In 2010, Project My Heart Your Heart (PMHYH) Pacemaker Reutilization Initiative was proposed with the intention to alleviate symptomatic bradycardia in LMICs [12]. PMHYH is a joint collaboration between individuals, healthcare professionals and funeral directors, the University of Michigan Cardiovascular Center, and World Medical Relief, Inc. (both based in MI, USA). The goal of the initiative is to create a framework where pacemakers can be acquired from funeral homes, certified and safely resterilized by a third party reprocessing company, and ultimately sent to a LMIC for reimplantation in patients who would otherwise not be able to afford a device, all under the auspices of the US FDA [12]. Recipient hospitals would be required to provide documentation of device implantation expertise, as well as followup capabilities. It is upon the hospitals and their social work departments to determine financial need of a patient in order for them to qualify for a donated device; the hospital is not allowed to charge for a device. All patients receiving devices would have their information entered into an online database. Moreover, follow-up data must be entered into this registry in order to assess for complications, as well as device malfunction. In case of device recall or malfunction, the recipient would immediately be contacted and the hospital sent a new refurbished device. With close monitoring and obligatory follow-up, the hope is to limit ‘black market’ sales of donated devices. The entire cost of the project is currently supported by private foundations, philanthropic individuals and university grants. This initiative has the potential to decrease morbidity and mortality for the millions of individuals who currently have no other option but to live with symptomatic bradycardia.
The concept of pacemaker reutilization is a logical solution to overcome the cost barrier of treating bradyarrhythmias in LMICs. In this review, we examine the safety and feasibility of pacemaker reutilization, as well as ethical issues surrounding this controversial practice.
Safety of device reutilization
The major safety concerns regarding pacemaker reutilization include a perceived increased risk of infection and device-related malfunction. In all studies, refurbished devices were implanted with new leads.
In some series, cardiac device infection can result in all-cause 6-month mortality up to 18% [13]. On the other hand, larger series reported no device infection-related mortality [14]. However, despite the current concern for pacemaker reutilization, this concept was commonplace approximately 25 years ago [15]. In 1998, Linde et al. provided evidence that pacemaker reutilization, which at the time accounted for 5% of all devices in Sweden, was safe and effective [16]. Furthermore, in 2002 the American College of Cardiology, American Heart Association and the North American Society of Pacing and Electrophysiology stated in an update that “reuse of explanted pacemakers may eventually add significantly to the cost-effectiveness of cardiac pacing” [17,18].
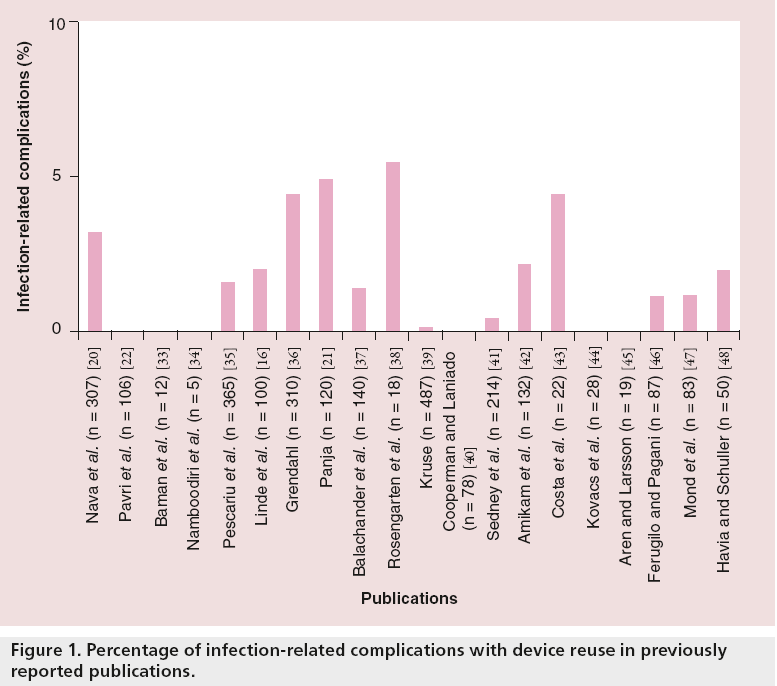
Many studies have examined the safety of pacemaker reuse over the last 20 years, but have been limited by small sample size and limited follow-up duration. However, the majority of these studies have shown pacemaker reutilization to be an overall safe practice (Figure 1). A recent University of Michigan meta-analysis of 18 studies, which included 2270 patients with reused devices, found an overall infection rate of 1.79% and device malfunction rate of 0.68% [19]. Although there was not a higher risk of infection in the pacemaker reutilization group versus the new implantation cohort, the pooled analysis did show a higher risk of device malfunction. The majority of these malfunctions were due to set-screw abnormalities, presumably occurring when the leads were removed from the device themselves during the extraction process. This observation was further confirmed in the most recent device reuse analysis by Nava et al. [20]. The authors suggested that patients with highly symptomatic bradycardia would gladly accept this increased 0.68% risk with hopes of receiving a device that may reduce mortality and improve quality of life [19].
Panja et al. found an overall 5% rate of infection in the reutilization group; however, pacemakers reused in the same patient showed a higher rate of infection of up to 12% [21]. These were mainly devices that were extracted, resterilized and reimplanted in the opposite side of the same patient due to infection. Thus, one must consider internal spread of infection through blood or lymphatic drainage, rather than the implantation of the previously explanted device as the key risk factor.
Pavri et al. reported their experience of reimplantation of 106 cardioverter defibrillators with a mean follow-up of 825 days [22]. There were no device-related infectious complications and 60% of the reimplanted devices delivered life-saving shocks or antitachycardia pacing to the recipient.
Most recently, Nava et al. studied a group of 603 patients in order to determine the safety and efficacy of refurbished pacemakers compared with a control group of new device implants. Their results showed no significant difference between infection rates or device malfunction between the two groups [20].
Feasibility of device reutilization
Alhough the practicality of pacemaker reutilization may seem challenging, it has previously been demonstrated to be feasible in many countries [15]. Many studies have examined the views of patients and funeral home directors, and the steps involved in device extraction and reuse.
▪ Resources for potential device acquisition
In the USA alone, there are more than 225,000 pacemakers implanted each year [6]. Brunner et al. found that median patient survival after pacemaker implantation was 8.5 years; however, only 65.6% of patients were still alive at 5 years after first implantation [23]; they also noted an increase in the average age of patients at the time of implant. The cremation rate in North America is currently estimated to be 45% and is expected to increase to 57% in 2025 [102]. Therefore, it is reasonable to estimate that a majority of patients with pacemakers will wish to be cremated in the near future. Prior to cremation, pacemakers and defibrillators must be removed to prevent device explosion [24]. Thus, their devices will be explanted per routine measures. This large number of available devices could prove to be a valuable source of reused pacemakers.
PMHYH has already started to acquire explanted pacemakers from funeral homes and crematories across the country. All devices are examined by a trained device nurse in order to assess structural integrity (specifically the integrity of device headers), as well as battery longevity. More than 10,000 devices have been donated to the program. Of these devices, 21% were found to have an adequate battery life for potential reutilization in a LMIC. The authors defined adequate battery life as ≥75% battery life or ≥4 years expected longevity [25]. Moreover, a study of 328 implantable defibrillators, explanted for reasons other than reaching elective replacement interval, revealed that the majority had greater than 50% of battery life remaining [26].
As previously discussed, Nava et al. is the most recent large trial to examine the safety of device reutilization [20]. In the reused-device cohort, the average device longevity was 6 years compared with 8 years longevity of a new device. Moreover, only 11 reused devices displayed signs of premature depletion. This study supports the current PMHYH definition of an adequate battery life to be >75%.
▪ Patients’ perspectives
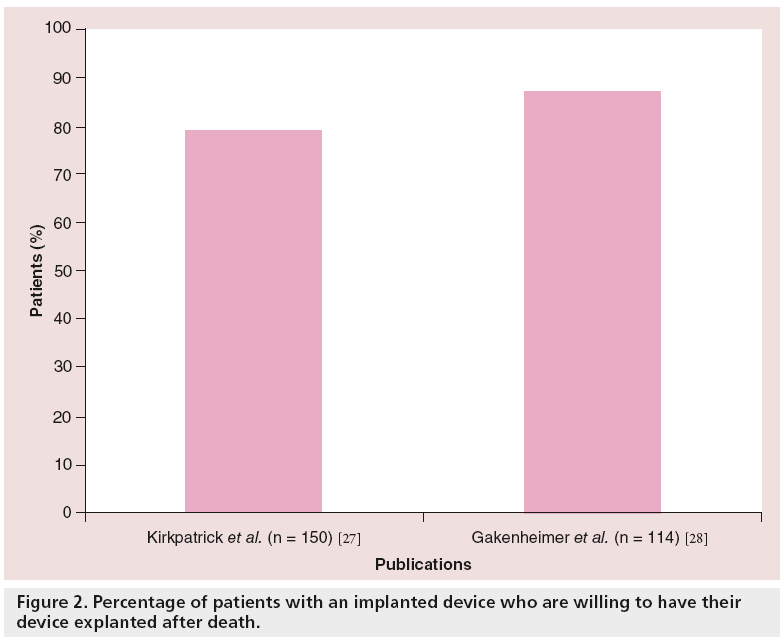
In a survey study carried out in the Chicago (IL, USA) area, Kirkpatrick et al. noted that, among 150 patients with implanted devices, 87% did not know how devices were handled after death [27]. The majority of the patients (79%) were willing to have their devices returned to manufacturers after death. A total of 72% of device patients indicated they would sign a device advanced directive if asked and, of those, 92% were willing to have their devices removed and donated for use in developing countries [27]. Similar findings were seen in a more recent survey performed at the University of Michigan that included 114 patients with devices and 1009 members of the general population [28]; 87% of the patients with implantable devices and 71% of the general population supported pacemaker reutilization if given the opportunity (Figure 2). Therefore, it is logical to discuss with patients how to handle their devices upon death, similar to the concept of organ donation. This concept may be even more important than organ donation, as all device recipients can be potential donors.
▪ Funeral home directors’ perspective
Despite recommendations by the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines stating that funeral directors should notify the physician monitoring the device immediately and return the patient’s device to the manufacturer after a patient’s death [29], present studies show that most funeral directors do not perform this practice routinely [27,28]. These guidelines also recommend that patients or family members should be asked to consent for post-mortem device evaluation, including interrogation and removal [29]. Funeral home directors reported that 85% of patients are buried with their device. Retrieval of devices mainly occurred for reasons such as cremation or family request. Of those devices, 84% were discarded as waste or stored with no intended purpose [28]. However, when asked, the majority of funeral home directors (89%) expressed willingness to donate the device to be used in underserved areas [28].
The current literature supports that pacemaker reutilization is a feasible practice with support from device patients, as well as members of the general public, including funeral directors.
▪ Device acquisition and performance measures
PMHYH provides a framework for those interested in participating in a program to acquire, sterilize and redistribute devices to those in underserved nations. After identifying a device for potential reuse, signed consent must be obtained from the family in order to proceed with device removal. The majority of funeral directors have had experience and are comfortable with explanting devices, despite only a third of them undergoing prior formal training in device removal [28]. This training is of utmost importance as reuseddevice malfunction was mainly due to set-screw abnormalities during the extraction process [19]. The donated device should then be sent to a center of excellence for investigation, which includes interrogation to assure adequate battery life and other performance-testing specifications. Based on prior case series of pacemaker reuse, a cutoff of ≥70% of battery life was suggested by Baman et al. for proper reuse [12]. Others have suggested the reuse of pacemakers implanted <3 years ago may possess an average longevity of 7 years [16]. Devices that pass all quality-control measures would then undergo a process to erase all patient identifiers, followed by sterilization and suitable packing. The device should then be sent to nonprofit charitable organizations that specialize in delivering medical equipment for distribution to LMICs, where the device will be implanted with new unused leads [12].
Cost-effectiveness
Very little data exist regarding the cost-effectiveness of reutilization of pacemakers in LMICs. Linde et al. preformed a cost-benefit analysis in the 1990s that revealed a >US$900,000 saving with reutilization of 317 devices (~US$2800 per device) [16]. However, a major limitation of this analysis was that the cost of resterilizing the device was examined only while other factors, such as hospital and physician fees, were excluded. In 1986, however, Myers concluded that the cost saving with reutilizing devices is almost negligible when factoring hospitals and implantation fees [30].
PMHYH and the University of Michigan group believe that, despite having low-cost pacemakers available in the market, the cost of these devices is still simply unattainable for those in LMICs. PMHYH estimates that the cost of shipping and resterilizing a device would amount to approximately US$100, with private donations and foundations covering the majority, if not all, of this cost. Specific LMICs, such as the Philippines and Vietnam, with government-run hospitals that cover implanting costs would benefit most from such a program. The patient would be responsible for the device lead, which would cost approximately US$200 [12]. Overall, we believe that pacemaker reuse can be performed in a cost-effective manner if used in the appropriate setting.
Legal and regulatory aspects
The reuse of pacemakers was initially supported by the National Association for Sport and Physical Education with a conclusion in 1985 stating that pacemaker reuse is reasonably safe [15]. Moreover, the American College of Cardiology /American Heart Association/National Association for Sport and Physical Education later acknowledged that pacemaker reuse “may eventually add significantly to the cost-effectiveness of cardiac pacing” [18].
However, the current FDA regulations prohibit device reutilization as it is regulated as a class III device that is not to undergo resterilization with subsequent distribution [103]. Proposed methods to address the regulatory concerns included shipping the device ‘unprocessed’ as a hazardous material with no intention of human use, thus leaving the onus of sterilization, testing and manufacturing for the overseas recipient institutions. This approach may place a heavy burden on the less-able and underfinanced party. Another approach is to file for FDA approval for potential exportation of resterilized devices only to those in need in undeserved nations [104,105]. Currently, PMHYH is working with the FDA in order to provide a legal framework in the form of an investigational device exemption for those interested in providing this valuable resource to those in underserved nations.
Ethical aspects
According to the principle of distributive justice, pacemaker reuse has an ethical justification. In his essay entitled ‘Famine, Affluence and Mortality,’ philosopher Peter Singer based an argument of global distribution on two main principles; the first is that “suffering and death from lack of food, shelter and medical care are bad,” and the second is that “if it is in our power to prevent something bad from happening, without thereby sacrificing anything of comparable moral importance, we ought, morally, to do it” [31]. Based on this, one could conclude that a moral duty exists to donate used pacemakers to those in need [32]. One important ethical concern is that the reutilization of pacemakers in LMICs may create a practice that is below the standard of care in the receiving countries. Numerous studies have already established the safety of pacemaker reuse [16,20,21,33-48]. However, a meta-analysis of these studies raised a concern that these devices may have a slightly higher malfunction rate, as well as a potential increase in the need for more generator replacements, since explanted pacemakers have a less than full battery capacity [19].
Aragam et al. argue that reuse of pacemakers is no different to the concept of expanded criteria donor organs for solid organ transplantation [32]. Expanded criteria donor organs, as opposed to standard criteria donor organs, are those that carry higher risks of failure due to associated donor comorbidities. Expanded criteria donor organs are usually offered to patients in need that may never receive a standard criteria donor organ. This concept is similar to implanting ‘substandard’ reused pacemakers to those in LMICs who cannot utilize ‘standard’ new pacemakers.
Ultimately, it is vital to establish protocols for sterilization, proper device extraction and reimplantation, with an oversight of the whole process to prevent diversion or resale. In addition, acquiring informed consent by both donors and recipients ensures respect for autonomy [49]. Failure to achieve these obstacles could result in decreased safety and efficacy of device reuse, as well as potential ‘black market’ distribution of devices that were initially intended to help those in need.
Conclusion
We believe that pacemaker reutilization is a highly effective, safe and ethically responsible method to deliver a resource to LMICs that would otherwise be unattainable. Previous studies have shown that infectious risk of device reuse is similar to new device implantation. Moreover, the feasibility of device acquisition has been established with the support of physicians, funeral directors and patients, as well as their families. Finally, we believe that it is our ethical obligation to provide this currently wasted resource to LMICs. It is the goal of PMHYH to work together with the FDA in order to create a framework to deliver this life-saving therapy to those in greatest need.
Future perspective
Our group believes that in the near future, PMHYH, in conjunction with the FDA, will be delivering reutilized devices to many implantation centers in underserved nations across the world. All device recipients will be closely followed to fully determine the safety and feasibility of a large-scale device reutilization program. Once the ‘roadmap’ for device reutilization is established, other centers of excellence across the country will be able to create their own regulated device-reutilization programs in order to save thousands or even millions of lives in LMICs.
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
The scope of the problem
▪ Many individuals in underserved nations are subjected to symptomatic bradycardia resulting in increased morbidity and mortality due to a lack of access to pacemakers.
Promising solution
▪ Pacemaker reutilization promises to provide an alternative solution to overcome the high cost of new devices.
Perceived challenges to pacemaker reutilization
▪ Infection and device malfunction rate must be high in reused devices.
▪ The public may not be willing to have their devices explanted.
▪ There are not enough devices to explant and reuse.
▪ It is unethical to reuse devices as this is below standard of care.
▪ The US FDA regulation prohibit pacemaker utilization.
Current knowledge and potential solutions
▪ Numerous trials have established safety and efficacy on pacemaker reutilization.
▪ A great majority of device patients are willing to donate devices after death with funeral directors willing to assist.
▪ The cremation rate is rising to more than 50% in the USA, resulting in an increased number of devices that need to be explanted. These devices could be the main resource for device reuse.
▪ When compared with eminent morbidity from bradyarrhythmia with no available alternative, reuse of pacemakers becomes an ethical necessity.
▪ Project My Heart Your Heart is working closely with the FDA to create a proper legal framework.
References
Papers of special note have been highlighted as:
▪ of interest
- Mendis S, Lindholm LH, Mancia G et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J. Hypertens. 25(8), 1578-1582 (2007).
- Joshi R, Jan S, Wu Y, MacMahon S. Global inequalities in access to cardiovascular health care: our greatest challenge. J. Am. Coll. Cardiol. 52(23), 1817-1825 (2008).
- Chaturvedi V, Talwar S, Airan B, Bhargava B. Interventional cardiology and cardiac surgery in India. Heart 94(3), 268-274 (2008).
- Mond HG, Irwin M, Ector H, Proclemer A. The world survey of cardiac pacing and cardioverter-defibrillators: calendar year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. Pacing Clin. Electrophysiol. 31(9), 1202-1212 (2008).
- Greenspon AJ, Patel JD, Lau E et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009 increasing complexity of patients and procedures. J. Am. Coll. Cardiol. 60(16), 1540-1545 (2012).
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009 - a World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 34(8), 1013-1027 (2011).
- Birnie D, Williams K, Guo A et al. Reasons for escalating pacemaker implants. Am. J. Cardiol. 98(1), 93-97 (2006).
- Thomas MO, Oke DA, Ogunleye EO, Adeyanju FA. Bradypacing: indications and management challenges in Nigeria. Pacing Clin. Electrophysiol. 30(6), 761-763 (2007).
- Millar RN; Cardiac Arrhythmia Society of South Africa. 1998 survey of cardiac pacing in South Africa - report of the working group on registries of the cardiac arrhythmia society of South Africa (CASSA). S. Afr. Med. J. 91(10), 873-876 (2001).
- Mond HG, Mick W, Maniscalco BS. Heartbeat International: making ‘poor’ hearts beat better. Heart Rhythm 6(10), 1538-1540 (2009).
- Senaratne J, Irwin ME, Senaratne MP. Pacemaker longevity: are we getting what we are promised? Pacing Clin. Electrophysiol. 29(10), 1044-1054 (2006).
- Baman TS, Kirkpatrick JN, Romero J et al. Pacemaker reuse: an initiative to alleviate the burden of symptomatic bradyarrhythmia in impoverished nations around the world. Circulation 122(16), 1649-1656 (2010).
- Baman TS, Gupta SK, Valle JA, Yamada E. Risk factors for mortality in patients with cardiac device-related infection. Circ. Arrhythm. Electrophysiol. 2(2), 129-134 (2009).
- Nery PB, Fernandes R, Nair GM et al. Devicerelated infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J. Cardiovasc. Electrophysiol. 21(7), 786-790 (2010).
- Boal BH, Escher DJ, Furman S et al. Report of the policy conference on pacemaker re-use sponsored by the North American Society of Pacing and Electrophysiology. Pacing Clin. Electrophysiol. 8(2), 161-163 (1985).
- Linde CL, Bocray A, Jonsson H, Rosenqvist M, Radegran K, Ryden L. Re-used pacemakers - as safe as new? A retrospective case-control study. Eur. Heart J. 19(1), 154-157 (1998).
- McGregor M. The reuse of cardiac pacemakers. Can. J. Cardiol. 8(7), 697-701 (1992).
- Gregoratos G, Abrams J, Epstein AE et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices - summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to update the 1998 Pacemaker Guidelines). J. Am. Coll. Cardiol. 40(9), 1703-1719 (2002).
- Baman TS, Meier P, Romero J et al. Safety of pacemaker reuse: a meta-analysis with implications for underserved nations. Circ. Arrhythm. Electrophysiol. 4(3), 318-323 (2011).
- Nava S, Morales JL, Marquez MF et al. Reuse of pacemakers: comparison of short and long-term performance. Circulation 127(11), 1177-1183 (2013).
- Panja M, Sarkar CN, Kumar S et al. Reuse of pacemaker. Indian Heart J. 48(6), 677-680 (1996).
- Pavri BB, Lokhandwala Y, Kulkarni GV, Shah M, Kantharia BK, Mascarenhas DA. Reuse of explanted, resterilized implantable cardioverter-defibrillators: a cohort study. Ann. Intern. Med. 157(8), 542-548 (2012).
- Brunner M, Olschewski M, Geibel A, Bode C, Zehender M. Long-term survival after pacemaker implantation. Prognostic importance of gender and baseline patient characteristics. Eur. Heart J. 25(1), 88-95 (2004).
- Gale CP, Mulley GP. Pacemaker explosions in crematoria: problems and possible solutions. J. R. Soc. Med. 95(7), 353-355 (2002).
- Baman TS, Crawford T, Sovitch P et al. Feasibility of postmortem device acquisition for potential reuse in underserved nations. Heart Rhythm 9(2), 211-214 (2012).
- Baman TS, Gakenheimer L, Romero J et al. ICD reutilization: can referral centers provide devices for donation to developing world countries? Eur. Heart J. 31, Abstract 238 (2010).
- Kirkpatrick JN, Ghani SN, Burke MC, Knight BP. Postmortem interrogation and retrieval of implantable pacemakers and defibrillators: a survey of morticians and patients. J. Cardiovasc. Electrophysiol. 18(5), 478-482 (2007).
- Gakenheimer L, Lange DC, Romero J et al. Societal views of pacemaker reutilization for those with untreated symptomatic bradycardia in underserved nations. J. Interv. Card. Electrophysiol. 30(3), 261-266 (2011).
- Carlson MD, Wilkoff BL, Maisel WH et al.; American College of Cardiology Foundation, American Heart Association, International Coalition of Pacing and Electrophysiology Organizations. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm 3(10), 1250-1273 (2006).
- Myers GH. Is reuse financially worthwhile? Pacing Clin. Electrophysiol. 9(6), 1288-1294 (1986).
- Peter Singer. Famine, affluence, and morality. Philos. Public Aff. 1(1), 229-243 (1972).
- Aragam KG, Baman TS, Kirkpatrick JN et al. The ethics of pacemaker reuse: might the best be the enemy of the good? Heart 97(24), 2005-2006 (2011).
- Baman TS, Romero A, Kirkpatrick JN et al. Safety and efficacy of pacemaker reuse in underdeveloped nations: a case series. J. Am. Coll. Cardiol. 54(16), 1557-1558 (2009).
- Namboodiri KKN, Sharma YP, Bali HK, Grover A. Re-use of explanted DDD pacemakers as VDD - clinical utility and cost effectiveness. Indian Pacing Electrophysiol. J. 4(1), 3-9 (2004).
- Pescariu S, Stiubel M, Cozma D et al. [The reutilization of pacemakers, an alternative for poor seniors: a retrospective study]. Stimucouer 31, 186-189 (2003).
- Grendahl H. Pacemaker reuse. Tidsskr. Nor. Laegeforen. 114, 3420-3423 (1994).
- Balachander J. Efficacy and safety of refurbished pacemakers: report on collaborative programme with 140 implantations and 6-year follow-up. Indian Heart J. 41, 430 (1989).
- Rosengarten M, Chiu R, Hoffman R. A prospective trial of new versus refurbished cardiac pacemakers: a Canadian experience. Can. J. Cardiol. 5(3), 155-160 (1989).
- Kruse IM. Experiences from the reuse of implantable pulse generators. J. Cardiovasc. Electrophysiol. 3(1), 61-63 (1985).
- Cooperman Y, Laniado S. The use of resterilized pacemakers: experience of 78 units. Pacing Clin. Electrophysiol. 8, 291 (1985).
- Sedney M, Rodgrio F, Bizot J, Buis B. [Reusing pacemakers]. Ned. Tijdschr. Geneeskd. 130, 399-402 (1986).
- Amikam S, Feldman B, Boal E, Riss E, Neufeld HN. Long term follow-up of patients with reused implanted pacemakers. Cardiac. Pacing 12, 109-111 (1983).
- Costa R, Moreira LF, Pego-Fernandes PM et al. Reuse of pacemaker generators. Arq. Bras. Cardiol. 40(5), 317-318 (1983).
- Kovacs P, Gomory A, Worum F et al. Five years experience with reused pacemakers. Pacing Clin. Electrophysiol. A, 54 (1981).
- Arén C, Larsson S. Reuse of hermetically sealed cardiac pacemakers. Pacing Clin. Electrophysiol. 2, A-73 (1979).
- Ferugilo G, Pagani T. Pacemaker reutilization: a study of biological factors and clinical experience. G. Ital. Cardiol. 8(Suppl. 1), S315-S317 (1978).
- Mond H, Tartaglia S, Cole A, Sloman G. The refurbished pulse generator. Pacing Clin. Electrophysiol. 3(3), 311-317 (1980).
- Havia T, Schuller H. The re-use of previously implanted pacemakers. Scand. J. Thorac. Cardiovasc. Surg. Suppl. (22), 33-34 (1978).
- Kirkpatrick JN, Papini C, Baman TS et al. Reuse of pacemakers and defibrillators in developing countries: logistical, legal, and ethical barriers and solutions. Heart Rhythm 7(11), 1623-1627 (2010).
▪ Explains the work of Heartbeat International organization, which plays a great role in the pacemaker reutilization concept.
▪ Meta-analysis of most of the trials that evaluated the safety of pacemaker reuse, thus providing a comprehensive look.
▪ First case-control prospective trial to compare refurbished pacemakers and new pacemakers.
▪ One of the only two unique articles that closely look at public reception and acceptance of reusing pacemakers.
▪ One of the only two unique articles that closely look at public reception and acceptance of reusing pacemakers.
▪ Describes the current challenges facing pacemaker reutilization and provides solutions to the matter.
▪ Websites
- Heartbeat International Foundation Pacemaker Program (2013). www.heartbeatsaveslives.org
- Cremation Association of North America. Industrial Statistics (2013). www.cremationassociation. org/?page=IndustryStatistics
- US FDA. United States Code of Federal Regulations, title 21/chapter 9/subchapter III/section 331(a) (2006). www.gpo.gov/fdsys/granule/USCODE-2010- title21/USCODE-2010-title21-chap9- subchapIII-sec331/content-detail.html
- US FDA. United States Code of Federal Regulations, title 21/chapter 9/subchapter VIII/section 381 (2013). www.gpo.gov/fdsys/granule/USCODE-2011- title21/USCODE-2011-title21-chap9- subchapVIII-sec381/content-detail.html
- US FDA. United States Code of Federal Regulations: title 21/chapter 9/subchapter VIII/section 382 (2013). www.gpo.gov/fdsys/granule/USCODE-2011- title21/USCODE-2011-title21-chap9- subchapVIII-sec382/content-detail.html