Research Article - Research on Chronic Diseases (2018) Volume 2, Issue 1
Onset patterns of chronic fatigue syndrome and myalgic encephalomyelitis
- *Corresponding Author:
- Leonard A. Jason
Center for Community Research 990 W. Fullerton Ave., Suite 3119 Chicago
DePaul University, USA
E-mail: LJASON@depaul.edu
Abstract
The onset of Chronic fatigue syndrome (CFS) and Myalgic Encephalomyelitis (ME) is considered a key area of inquiry. Case criteria for ME and CFS and much of the academic literature suggest that patients typically experience one of two possible onset patterns: sudden or gradual. The current study provided an in-depth investigation of ME and CFS onset in order to provide insight into early symptoms, onset duration, and the progression of functional disability. We collected qualitative descriptive data to gain a rich description of illness onset from the patients’ point of view. Overall, qualitative findings revealed detailed descriptions of ME and CFS onset experiences. Major themes that emerged from the data included: onset/illness progression patterns, illness causes, methods of adapting and coping, hardworking and active lives prior to onset, healthy lives prior to onset, prior health problems, comorbid health conditions, emotional responses to onset, exertional effects, the illness as life limiting, stress, traumatic experiences, lack of support, support, and treatment limitations. A closer examination of the onset/illness progression patterns that emerged from the data provided evidence that individuals with ME and CFS experience complex onset patterns. Furthermore, the study findings suggest that the method of categorizing individuals into sudden versus gradual onset groups fails to capture the more nuanced and varied onset experiences. Prospective research studies that capture the onset period as it is developing could lead to improvements in the way we define and assess ME and CFS onset, and may also lead to methods for early detection, prevention, and individualized treatment approaches.
Introduction
Chronic fatigue syndrome (CFS) is a highly complex illness that results in significant disability and a considerably diminished quality of life [1]. Due to continued questions regarding etiology, the period of onset for the illness is of considerable interest to researchers in the field. Much of the literature on ME and CFS related to onset suggests that individuals experience either a sudden/acute onset in which symptoms appear over a short period of time (e.g. a few hours, days, or weeks) or a slower, gradual onset in which symptoms may develop over a period of months or even years [2]. There is not yet a universal definition for assessing mode of illness onset (sudden versus gradual), and this is reflected in the varying language used across ME and CFS studies and case definitions.
There is controversy as to whether the illness labels CFS [2], ME [3], ME/CFS [4], myalgic encephalopathy [5], and SEID [6] represent one distinct condition, whether they are part of an illness spectrum, or whether they are simply different terms used to describe the same condition. CFS, ME, ME/CFS, and the recently named SEID, are often associated with different case criteria. Each case definition provides a description of onset, and while there are similarities across these descriptions, there are some key differences regarding how onset duration is defined across these various definitions.
Early case criteria developed by Holmes et al. [7] specify that the illness must have a “new onset of persistent or relapsing, debilitating fatigue” without any previous history of similar problems (p. 388). Additionally, Holmes et al [7] stipulate that the main symptoms of CFS must occur over a few hours or days, indicating a sudden or acute onset. According to Holmes et al. [7], symptoms are only met if they begin at the time of the fatigue onset or following onset. Another case criteria for CFS is referred to as the Oxford Criteria [8] which stipulate that CFS involves a “definite” onset as well as clear evidence of infection at the time of onset or first symptoms. Similar to the Oxford Criteria [8], the Fukuda [2] criteria describe the onset of the fatiguing illness as “new” and “definite” (p. 956). The Institute of Medicine (IOM) recently developed a new case definition [6] and renamed the illness as Systemic Exertion Intolerance Disease [SEID]. Similar to the Oxford Criteria [8] and the Fukuda [2] criteria, the case criteria for SEID specifies that the fatiguing illness is of a “new or definite onset” and not “lifelong.” These vague terms were included in the case criteria in order to exclude individuals who have experienced lifelong fatigue. Reeves et al. [9] later clarified that the purpose for the requirement of “new and definite onset” fatigue was to exclude those individuals with a primary personality or somatization disorder, which are both characterized as lifelong with unexplained somatic symptoms. Additionally, Reeves et al. [9] indicated that it is clinically difficult to identify whether fatigue is “new and definite.” The requirement for an “acute onset” of CFS was left out of the Fukuda criteria [2], as Fukuda did not find that the presence of infection differentiated individuals with CFS from those without the illness.
The terms Myalgic Encephalomyelitis (ME) and Myalgic Encephalomyelitis/chronic fatigue syndrome (ME/CFS) have corresponding case criteria [3,4], which are different from the Fukuda [2] criteria in that they require what are considered by many to be key symptoms of the illness (e.g. post exertional malaise and cognitive dysfunction). The [4] criteria for ME/CFS specify that an individual must have a “significant degree of new onset” fatigue (p. 11). Similar to the Holmes [7] criteria for CFS, the case criteria for ME/CFS stipulate that symptoms can only be counted as meeting criteria if they occur or become significantly worse after the onset of the illness. Carruthers et al. [4] describe onset as “distinct” and assert that most individuals experience an acute onset; however, they also assert that some individuals are unhealthy prior to their ME/CFS onset and may not be able to identify a specific trigger for the development of ME/CFS, or they may experience a more “gradual” or “insidious” onset (p. 12). Furthermore, Carruthers et al. [4] suggest that many individuals who experience immune dysfunction experience it most profoundly in the “acute onset stage” and that these symptoms of immune dysfunction fade or come and go as the illness becomes more chronic. According to Carruthers et al. [4] individuals with a viral acute onset show more symptoms of immune dysfunction compared to those who report a more gradual onset.
Similar to CFS and ME/CFS, past case definitions of ME have presented varying descriptions of onset. For instance, an early definition for ME by Ramsay et al. [10] asserts that the onset may be sudden without an identifiable cause, and may be accompanied by acute vertigo. Ramsay reports that there is often a history of infection of the upper respiratory track or sometimes in the gastrointestinal tract in patients with ME. While Ramsey suggested that most individuals with ME experience an acute onset, he suggested that a subset of individuals experience an insidious onset [11].
Hyde’s Nightingale Definition of ME stipulates that ME is both chronic and disabling and is characterized by an acute onset. Additionally, Hyde describes ME as an epidemic or an endemic occurring in two phases (Primary infectious Phase and Primary Chronic Phase). Additionally, Hyde [12] indicates that ME often follows multiple, minor infections in individuals with susceptible immune systems or immune systems that are weakened by severe stressors (e.g. contact with infectious persons, exhaustion, trauma, immunizations, epidemic disease, travel and exposure to virulent agents). Hyde describes the initial phase of ME as the Primary Infection Phase, which is characterized as an epidemic or endemic infectious disease with an incubation period of between four and seven days. He describes the second phase as the Secondary Chronic Phase, occurring with two to seven days of the Primary Infection Phase. In this phase, Hyde asserts that there are measurable changes in the central nervous system (CNS) of an affected individual and that this phase is the chronic form of the disease that is most commonly depictive of ME. Understanding the cause of ME can be complicated, as Hyde asserts that all cases of epidemic and primary ME result from an infectious or autoimmune agent, but he also suggests that there are often other potential causes that may go unnoticed prior to the onset of illness or as part of the illness. With regard to epidemic and primary ME, Hyde suggests that there is a lack of consensus regarding whether there is a viral or infectious etiology of the illness. He suggests that this lack of consensus may be due to the indication that there are patients who experience an acute onset and those that experience a more gradual onset. Hyde suggests that an acute onset is always indicated in a Primary ME patient group whereas a gradual onset may be more indicative of the chronic fatigue syndrome label. Additionally, he expressed the belief that ME is caused by an enterovirus and that those individuals that he has tested for viral infection all experienced an acute onset. While it is not emphasized, Hyde discusses the potential for the development of ME as a result of non-infectious agents (termed Secondary ME), such as exposure to toxic chemicals, which he reported observing in his medical practice. He reports that like Primary ME groups, Secondary ME affects the CNS, and in contrast he suggests that Secondary ME can be more severe.
The International Consensus Criteria for ME [3] also provide information regarding illness onset. Carruthers et al. [3] assert that most patients have an acute infectious onset with flulike and/or respiratory symptoms, but they also acknowledge that a gradual onset does occur in a subset of individuals. Additionally the ME-ICC criteria recommend that patients are classified into subtypes based on whether their onset is acute/infectious or gradual, as well as their severity of onset, as this may predict the severity of the chronic course of the illness.
The lack of a universal definition for mode of illness onset may be contributing to the inconsistencies in the percentage of individuals experiencing sudden versus gradual onset reported in the literature. In his study of pediatric CFS, Bell [13] found that approximately 45% of children and adolescents with CFS experienced an acute onset. There may be multiple reasons for the varying reports of onset duration across these studies, including the samples studied (community-based samples versus tertiary care samples). Levine, Jacobson, and Pocinki [14] evaluated individuals who experienced CFS-related symptoms from four separate communities in different parts of the country that had experienced outbreaks of CFS between the years 1984 and 1986, and found that in three of the communities, the majority of individuals experienced an acute onset, whereas in the fourth community, only 33% experienced acute onset. Other possible reasons for these discrepancies are the use of different case criteria used to select individuals with CFS and different definitions for mode of illness onset. Bell [13] suggested that the definition used to define sudden and gradual onset may influence the number of participants placed in each group.
There is not one universally used or empirically derived definition of onset duration for ME and CFS. Researchers interested in assessing mode of illness onset have used various definitions. Often, the distinction is made between sudden/ acute onset and insidious/gradual onset, but the duration length prescribed to each category differs. For instance, DeLuca et al. [15] defined sudden onset for CFS as an unrecoverable “viral-like illness” that could be traced to a definite date. Gradual onset was described as a “slow progression of symptoms over a period of weeks to several months” or longer (p. 85). Participants were classified under these onset definitions based on independent assessments from a physician and a psychologist. When there was disagreement about an onset category they came to an agreement through discussion. In his study of CFS in an adolescent sample, Bell [13] defined sudden/acute onset as an “abrupt onset of constant and debilitating fatigue that could be dated to a specific event or illness” (p. 45). He described all other onset patterns as gradual. Zhang et al. [16] utilized a very specific definition of acute infectious-like onset, defining it as occurring over a period of no longer than 48 hours. Mawle et al. [17] evaluated sudden versus gradual onset CFS groups and defined sudden onset as “flu-like” and abrupt, over the course of one to two days. Similar to DeLuca [15], Cukor, Sky, and Natelson [18] defined gradual onset as a “slow progression of symptoms over a period of weeks to months or greater” and sudden onset as a “viral-like illness with a specific date of onset from which the patient did not recover” (p. 37). Others simply state that individuals with CFS were grouped by sudden versus gradual onset without providing any description of how sudden and onset groups were determined [17]. Based on much of the research presented above, there appear to be more specific definitions of acute or sudden onset groups; whereas, gradual groups are often seen as an ‘other’ onset category for onset types that cannot be clearly dated or defined.
Mode of illness onset may also be useful in differentiating ME and CFS from illnesses that have some overlapping symptoms, including severe fatigue. Linder et al. [19] used neural networks to classify patients with chronic fatigue syndrome, idiopathic chronic fatigue, lupus erythematosus, and fibromyalgia. The authors [19] attained 95% accuracy in correctly identifying individuals with their given diagnosis (sensitivity) and 85% accuracy in correctly identifying individuals who were negative for a specific diagnosis (specificity). Symptoms that had the highest differentiating accuracy for CFS were those with an acute onset and sore throats. Furthermore, it has been suggested that a sudden onset can help differentiate individuals with CFS from those with primarily depression [4,20] and those with a psychosomatic disorder [4], as these often involve a slower and more gradual onset.
Researchers in the ME and CFS field have also utilized a qualitative approach to better understand a variety of experiences related to ME and CFS, including the experience of illness onset. One study by McCue [21] examined the illness narratives of 14 women who had reported significant improvement or total recovery from CFS. McCue [21] investigated the personal experiences of illness onset that these women recounted, which included their difficulties receiving a diagnosis, the lack of attention to physical symptoms by their doctors, the tendency to assume a psychological etiology, and the significant stigma they experienced by the medical community.
Others have investigated illness narratives of ME and CFS onset with an emphasis on how people account for the initial unfolding of their illness in terms of patterns of onset and the perceived etiological factors and stressful events that co-occurred with onset. Ray et al. [22] interviewed 60 adults out of a larger subset of 147 adults who met the Oxford Criteria [8] for CFS and asked them to describe their illness onset. Ray et al. prompted participants by asking whether they had a gradual or sudden onset and asked them to describe in detail their onset duration as well as perceived contributory factors. Three separate groups of participants were identified when coding for CFS onset. One group was characterized by a gradual onset of CFS in which there was a slow, worsening of symptoms over time, culminating into the attribution that the illness was serious (n=15). A second group was identified as having an acute onset, which was characterized by a sharp increase in symptoms (n=31). Lastly, a third group was identified as having a two-phase illness onset, which began with a sharp deterioration and subsequent improvement in phase one, that was then followed by another deterioration of symptoms in phase two (n=14).
Ray et al. examined differentiating themes across the onset groups and found that individuals in the gradual onset group attributed their illness to an infection or a series of infections [22], antibiotics, and the immune system “breaking down.” More complex accounts included a combination of infection, overexertion, and stress. The sharp onset group described a clear transition between health and becoming ill, and one participant described going “down with a bang” (p. 103). Individuals in the sharp onset group listed similar contributory factors as the gradual group. The phased onset group largely reported that an infection was the trigger of their illness. Within this group, one participant reported that “there was a gap and then it reappeared” (p. 104). In 11 cases, a worsening of illness was attributed to “overdoing things” (p. 104). Some reported a failure to allow themselves time to recover. Additional findings by Ray et al. [22] revealed strong and significant associations between illness duration and onset characteristics, as longer duration of illness (56- 72 months) was associated with a gradual onset, and shorter duration (seven to 22 months) of illness was associated with sudden onset. This suggests illness duration can influence an individuals’ representation of their onset pattern.
In order to fully capture the experience of onset, it may be crucial to interview those who are most directly affected by the illness; the patients themselves. Roth [23] suggests that even in a period of crisis or uncertainty during the illness experience, people are able to note time markers and place them within their illness chronology. Charmaz [24] suggests that illness narratives allow for the identification of nuances of the illness experience that may otherwise not be captured. Illness narratives have also been used to better understand a variety of illness experiences as well as CFS [25,21]. A more in-depth look at this early stage of CFS may provide insight into how individuals with CFS account for and describe their illness onset. Based on previous research documenting the rich information that can be gained from personal narratives of illness experience, the current study involved interviews with individuals with CFS to determine how they describe their illness onset.
Method
▪ Research participants and procedures
The current study involved two phases. In the first phase, participants completed the DePaul Symptom Questionnaire (DSQ), a survey that assesses demographic information, ME and CFS symptomatology, and illness history [26]. In the second phase of the study a subset of phase one participants were asked to complete a semi-structured phone interview regarding their illness onset.
An international convenience sample of adults self-identifying as having CFS, ME/CFS, or ME was recruited [27,28]. In order to be eligible, individuals had to be at least 18 years old, capable of reading and writing English, have a selfreported current diagnosis of ME, CFS, or ME/ CFS, and meet the Fukuda [2] case criteria for CFS. Following approval by DePaul University’s Institutional Review Board, participants were recruited from a variety of sources including postings on internet forums and support group visits. Additionally, some participants who participated in previous studies conducted by the DePaul research team or who emailed the research team’s email address with an interest in participating in future studies were re-contacted.
Participants were given three options for completing the surveys: an electronic survey, a hard-copy survey, or a verbal survey over the telephone. All participants were given the opportunity to complete these surveys at home or in person at the Center for Community Research at DePaul University. Participants were not given a timeline for survey completion, as this illness can be fluctuating in nature, and a rapid decline of functioning can occur on any given day. The first 100 individuals who completed the survey received a $5.00 gift card to Amazon.com for their participation.
Of the original 217 individuals who completed the DSQ, 181 participants were included in the present study. Twenty-eight participants were excluded due to active medical conditions, active psychological conditions, and/or the endorsement of lifelong fatigue, all of which preclude a diagnosis of CFS based upon the Fukuda [2] case definition. Seven participants were excluded due to not meeting full criteria for Fukuda criteria [2]. One participant did not answer the question regarding onset duration and was excluded. Although there was no formal psychiatric interview, Torres-Harding et al. [29] have demonstrated that individuals with CFS are capable of validly self-reporting psychiatric comorbidity information.
Demographically, the sample of 181 participants was 83.3% female and 16.7% male. 97.8% of the sample identified as Caucasian, 0.6% as Asian or Pacific Islander, and the remaining 1.6% identified as “Other.” Of those participants who identified as “Other”, three participants identified as Hispanic or Latino origin, and one identified as multi-racial. One participant identified as American Indian or Alaska Native. With regard to marital status, 57.6% identified as married, 0.6% identified as separated, 18.1% identified as divorced, and 23.7% identified as never married. 43.5% of participants endorsed having children. 55.6% of the sample stated that they were currently on disability, with only 11.7% of the sample working part or full-time. With regards to educational level, 40.0% of the sample held a professional degree, 35.0% held a standard college degree, 17.8% attended college for at least one year, and 7.2% completed high school or had a GED. The mean age was 51.53 (SD = 11.30).
The second phase of the study involved qualitative interviews with a subset of the larger sample of 181 participants. A total of 14 adults were recruited from the larger sample. Participants in phase two were identified using stratified purposeful sampling [30] based on onset duration. Participants responded to an item on the DePaul Symptom Questionnaire (DSQ) [26] that assesses onset duration (i.e. the period of time in which their illness developed). Possible responses included: within 24 hours, over one week, over one month, over two to six months, over seven to 12 months, over one to two years, and over three or more years. Two people from each of the seven onset duration categories were recruited to participate in phase two of the study in order to incorporate a broad range of onset experiences.
All 14 participants (13 females and 1 male) identified as Caucasian, nine (64.3%) identified as married, two (14.3%) identified as divorced, two (14.3 %) identified as never married, and one individual (7.1%) left the marital status item blank. Six (42.9%) participants reported having children. With regard to work status, seven (50%) participants were on disability, one (7.1%) identified as a student, two (14.3%) identified as homemakers, one (7.1%) was retired, one (7.1%) identified as unemployed, and two (14.3%) reported that they were working part-time. With regard to educational level, four (28.6%) held a professional degree, six (42.9%) held a standard college degree, two (14.3%) attended college for at least one year, and two (14.3%) completed high school or had a GED. The mean age was 53.21 (SD = 9.31).
At the start of the phone interview, the interviewers explained to participants that they would be asked to discuss their health and illness experiences. They were told that the interview would take approximately one hour to complete. Additionally, they were told that the interviewer would ask them follow up questions in order to obtain more detailed information about a particular experience or event. They were also reminded that they did not have to answer any question that they did not feel comfortable answering and that they could take breaks at any time during the interview. Additionally, participants had the option of breaking up the phone interview into two separate interviews as the one-hour time commitment was too taxing for some. Participants were also encouraged to tell the interviewer or PI about any questions or concerns they had throughout the study period.
Following the initial introductory statement by the interviewer, interviewers asked participants which illness label (e.g. ME, CFS) they preferred to use when describing their illness. This label was then used throughout the interview. The first study question of the interview was open-ended and read as follows: “Please tell me about the period of time when you first became sick with ME/CFS” (or CFS or ME depending on participants preferred illness label). Interviewers proceeded with an open-ended line of questioning (e.g. “What else do you remember about that experience?”) in order to get rich, detailed information about participants’ perceptions of their onset period. The interview also included an open-ended question that read as follows: “Please tell me about the period of time before you became sick with ME/CFS” (or ME or CFS). Interviewers followed up further with an open-ended line of questioning in order to receive the most rich and detailed account possible from participants: “Please tell me about the year before you became sick with ME/CFS” and/or “Please tell me more about that experience.” The open-ended questioning was adapted for each participant’s unique illness experience; therefore, the interview protocol was semi-structured to allow for flexibility.
Following these open-ended questions, interviewers proceeded with more direct questioning for the purpose of filling in gaps of information that was not provided from the initial open-ended questions and for determining more objective measures of participants’ functioning and disability prior to and following the onset of the illness. Specifically, interviewers asked participants over what period of time their first symptoms developed and what year and month (if remembered) participants became ill with CFS. Interviewers asked participants to indicate their level of disability and functioning using a CFS Disability Scale [13] which was emailed to participants prior to the interview. The CFS Disability Scale is an 11-point scale with possible response values from 0-100, where 100 represents normal, fully active functioning and 0 represents severe disability/unresponsiveness. Participants were asked to rate their functioning level during the time of onset or first sign of symptoms, prior to onset/first symptoms, and the period following onset/first symptoms. In addition to questions about functioning, interviewers asked participants to indicate which symptoms they may have experienced before, during and after onset. Furthermore, interviewers assessed for other significant personal, work, or other health related events that occurred during these timeframes. Interviewers also asked participants to recall significant life events including holidays as well as information regarding the time of year (e.g. seasons) in order to aid participants’ recall of their functioning and symptoms at onset. These recall aids are a major component of the widely used and reliable Timeline Follow Back Interview method for the assessments of past alcohol use [31] and has also been used for retrospective recall of early symptoms in patients with cancer before diagnosis [32]. The interview allowed for considerable flexibility in questioning, as it was important for interviewers to ask questions based on each participant’s unique timeline and illness history.
Following completion of the phone interview, participants were debriefed on the purposes of the study and they were provided with contact information for any further inquiries. The audio taped phone interviews were transcribed verbatim and entered into the qualitative data analysis software program NVivo 10.0.
▪ Qualitative method
The interview transcripts were analyzed using qualitative content analysis. The general analysis steps taken were based on an approach summarized by Zhang and Wildemuth [33]. Following full transcription of the audio taped interviews, the unit of analysis was identified. The interview text was coded by themes, which were expressed in words or phrases. The analysis began with reading and re-reading the interview text in order to gain a full sense of the data. During initial thematic analysis, key words and phrases were identified using an “open coding” approach [34] in order to allow for patterns and themes of onset experience to emerge from the data [30]. The text was read repeatedly this way in order to define and develop categories that were included in the coding scheme. This approach is consistent with the naturalistic inquiry that is characteristic of qualitative description. A coding manual was developed in order to clearly define and outline categories as they emerged and to enhance reliability across coders [33]. The coding manual included definitions and rules for assigning categories to the text, and each category included examples of text from transcripts. Coding and category development was ended once the categories were deemed saturated and new information was no longer contributing to the development of new categories or to category refinement [30]. A three-stage method for establishing intercoder reliability and agreement [35] was used. In the first stage, the PI and second trained coder implemented the coding scheme on a randomly selected sample of transcripts and then calculated intercoder reliability. In the second stage, coding disagreements were discussed and resolved through a negotiation process among the PI and the second coder, in order to establish a high level of intercoder agreement. In the third stage, the PI then implemented the coding scheme on the remaining transcripts. Campbell et al. [35] recommend this three-stage method for situations in which one coder has more expertise on the topic being investigated.
Participant responses to interview questions related to functioning/ability levels over time (using the CFS Disability Scale) were used to develop a visual graph of onset chronology. Lifeline interviews have been used to construct life timelines that require respondents to draw “up and down” lines that represent the positive and negative periods and events of their lives on a visual graph. Okma and Hopman [36] utilized and adapted the lifeline interview method in order to gain a richer understanding of characteristics associated with the onset of generalized osteoarthritis in women. In traditional lifeline studies, the visual graphs are completed by the participant or co-constructed by the participant and interviewer. The current study involved phone interviews; therefore, this author completed the onset lifelines after the interviews with participants were completed. The “ups and downs” on the onset graph were graphed on the Y-axis and were constructed using the participants’ responses to disability/ functioning questions (participants responses using the 0-100 disability scores that from the CFS Disability Scale) as well as their report of symptoms and significant life (personal and health) experiences. The visual graphs are different than typical lifeline graphs, as they do not cover a person’s entire life course, rather they focus on the onset period, the year leading up to onset, and the time following the onset period. Some individuals’ histories began years before the onset and others began a year or a month prior to illness onset; therefore, the interview protocol and the visual graphs allowed for these differences in illness experience. The graphs were created using Microsoft Excel. After the graphs were completed, they were emailed to the respondents in order to check for accuracy. Respondents had the opportunity to provide corrections by replying to the email with a list of corrections and/or additions to the graph, or they could provide their corrections over the phone by communicating to the author which aspects of the graph needed correction.
Following data analyses, the first author emailed participants a summary of the major themes and key findings across the overall sample as well as copies of their individual illness timeline graphs. After the summaries were received, participants were provided the opportunity to provide their impressions of the overall themes and findings by replying to the authors’ email with thoughts and reflections or by opting to have a second 15-30 minute phone interview. The phone conversation was informal and allowed for a back and forth reflection between author and participant regarding the study and overall impressions.
Measures
▪ DePaul symptom questionnaire
All participants completed the DePaul Symptom Questionnaire (DSQ) [26], a self-report measure of CFS, ME, and ME/CFS symptomatology, demographics, and occupational, psychiatric, medical and social history. The DSQ was developed to classify individuals on a variety of CFS, ME, and ME/CFS case definitions; however, the symptom list was based upon a revised approach to the Clinical Canadian criteria for ME/CFS [4]. The DSQ includes questions related to CFS symptoms (including symptoms that preceded the CFS onset), diagnosis, treatments, and psychiatric/medical diagnoses. Participants are also asked to indicate whether they have family members with CFS. Additionally, participants are asked to identify the duration of their illness onset period, the degree to which their illness was caused by physical versus psychological factors, and specific difficulties related to energy, fatigue, and post-exertional malaise. The majority of items on the DSQ have evidenced good to excellent correlation coefficients, suggesting that the overall instrument is a reliable measure for examining symptoms and illness constructs within the patient community [26]. For the purposes of the study, only questions that specifically assessed aspects related to onset duration, infectious events preceding CFS onset, psychiatric comorbidity, and illness attributions regarding the cause of illness were examined. These items are presented below in more detail.
▪ Onset duration
Participants were asked to respond to the following question on an 8-point likert scale: “over what period of time did your fatigue/energy related illness, develop?” Possible responses include: 1= within 24 hours, 2= over one week, 3= over one month, 4= over two to six months, 5= over seven to 12 months, 6= over one to two years, 7= over three or more years, and 8= I am not ill. No participants endorsed that they were not ill. “This time demonstrated excellent testretest reliability with a kappa coefficient of .76 when completed by individuals with ME and CFS [26]”
▪ Events preceding CFS onset
On the DSQ, participants were asked to indicate if they experienced a significant event prior to developing CFS. Specifically the item asks: “did your fatigue/energy related illness start after you experienced any of the following? (Check one or more and please specify)”: an infectious illness, an accident, a trip or vacation, an immunization, surgery, severe stress (bad or unhappy event), other, I am not ill. No participants included in the current study endorsed that they were not ill. This study will focus on responses to the infectious illness category of this item. This item category demonstrated excellent test-retest reliability with a kappa coefficient of .90 [37]”
▪ Psychiatric comorbidity
With regard to psychiatric comorbidity, participants were asked the following questions: “Have you ever been diagnosed and/or treated for any of the following: Major depression, Major depression with melancholic or psychotic features, Bipolar disorder (Manic-depression), Anxiety, Schizophrenia, Eating Disorders, Substance Abuse, Multiple chemical sensitivities, Fibromyalgia, Allergies, Other (Please specify), No diagnosis/treatment. Participants are instructed to check all responses that apply and to also write in the year the condition was experienced, years it was treated, and medication if applicable. For the purposes of the current study, only responses involving psychiatric diagnoses were examined. “This item demonstrated excellent test-retest reliability with kappa coefficients ranging from .76 to .92 for psychiatric diagnoses as reported by individuals with ME and CFS. [37]”
▪Medical outcomes study short-form 36 survey (SF-36)
All participants completed the SF-36 [38], a 36- item self-report measure of disability comprised of eight subscales: physical functioning, role physical, bodily pain, general health, role emotional, social functioning, vitality, and mental health. The composite score for each subscale ranges from 0-100, with higher scores indicating better functioning. This measure is frequently used in research to assess disability brought on by illness. The SF-36 had good internal reliability and convergent validity in a sample of individuals with CFS [39]. It was also able to distinguish individuals with CFS and chronic fatigue from individuals with major depression, acute mononucleosis, and from healthy controls.
▪ DePaul onset interview
A semi-structured interview was developed by this author and colleagues at the Center for Community Research at DePaul University. The Interview includes open ended and close-ended questions that ask participants to describe their illness onset and the year leading up to onset. The questionnaire also includes guidelines for assessing participants’ level of disability and functioning at onset, prior to onset, and following onset. For participants who are not able to identify a clear onset, interviewers asked participants about the period of time in which they experienced the first symptoms/signs of the illness. The interview also assesses any significant personal and health-related events, as well as symptoms experienced before, during, and after illness onset. The questionnaire allows for flexibility and for follow up questions in order to capture each participant’s unique illness timeline and to gain detailed information on onset and functioning.
▪ CFS disability scale
The CFS Disability Scale was developed by Bell [13] as a tool for physicians and other health clinicians to assess disability level and activity reduction. The CFS Disability scale is a modified version of the Karnofsky performance scale [40], which was developed for the purpose of quantifying the functional status of individuals with cancer. Similar to the Karnofsky scale, the CFS Disability Scale is based on an 11-point scale from 0-100 (with 10 point increments), where 0= unresponsive and 100=fully active/ normal.
▪ Results
For the larger sample, the percentage of participants who reported that an infection preceded their illness did not differ by onset group, χ2 (1, N = 181) = 1.10, p =.29 (Table 1). The percentage of participants who reported that the cause of their illness was “Definitely Physical” or “Mainly Physical”, did not differ by onset group, χ2 (1, N = 178) = .91, p =.34. The percentage of participants who endorsed at least one lifetime psychiatric diagnosis did not differ by onset group, χ2 (1, N = 181) = .42, p = .52.
| Sudden | Gradual | |||
|---|---|---|---|---|
| DSQ Item | (n=98) | (n=83) | χ2 | p |
| % (n) | % (n) | |||
| Infectious Onset | 73.5 (72) | 66.3 (55) | 1.11 | 0.29 |
| Physical Attribution | 96.9 (93) | 93.9 (77) | 0.91 | 0.34 |
| Lifetime Psychiatric Dx | 39.8 (39) | 44.6 (37) | 0.42 | 0.52 |
Table 1. Participants with sudden versus gradual onset endorsing viral onset, physical illness attribution, and at least one lifetime psychiatric diagnosis (n=181)
Qualitative analyses were employed in order to investigate how individuals with ME and CFS describe their illness onset, specifically with regard to the early days, weeks, or months in which their illness emerged. Intercoder reliability across two coders (the PI and an undergraduate level research assistant) was calculated on three randomly selected transcripts using the qualitative data analysis software program NVivo 10.0. After utilizing the three-stage method of establishing intercoder reliability and agreement as described by Campbell et al. [33], the overall intercoder reliability was found to be excellent, with an average overall Kappa of 0.98 across the three coded transcripts.
Timeline Graphs
A total of 14 illness timeline graphs were constructed based on the participant interviews completed for the qualitative portion of the study. Seven participants (50%) have provided corrections and feedback on their illness timelines and their graphs can be found in Figures 1-8. The illness timeline graphs provided a detailed chronology of each individual’s functioning over the course of their illness including the year(s) leading up to the onset and the initial month(s) and year(s) of onset. The illness timeline graphs reveal periods of severe disability, remission, and fluctuating illness patterns in a biographical context. The graphs are presented in Figures 1-8 the period of time in which individuals reported on the DSQ that their illness developed (24 hours, over 1 week over 1 month, over 2-6 months, over 7-12 months, over 1-2 years, over 3 or more years). Areas shaded in green signify functioning levels above 50 and areas shaded in red signify functioning levels below 50.
A summary of themes can be found in Tables 2 and 3. Each theme’s meaning as it relates to the way in which participants described the onset and development of their illness is discussed below. The superordinate themes found in the analysis are as follows: onset/illness progression, illness cause, adapting and coping, hardworking, active lives prior to onset, healthy prior to onset, health problems prior to onset, comorbid health conditions, emotional response to onset, exertional effects, life limiting, stress, traumatic experiences, lack of support, support, and treatment limitations. The majority of superordinate themes include more specific subordinate themes and they are described in detail below. Whenever a direct quote is used from a participant, the unique participant number (1 through 14) is attributed to that person as well as the period of time in which their illness developed (as reported on the DSQ onset duration item). The DSQ onset period is shown in parentheses directly following the participant’s number.
| Themes | Total |
|---|---|
| % (n) | |
| Onset/Illness Progression | 100 (14) |
| Sudden | 50 (7) |
| Exact Date | 21 (3) |
| Definitive Turning Point | 36 (5) |
| Realization that Something is Wrong | 50 (7) |
| Steady Progression | 43 (6) |
| Wax and Wane | 64 (9) |
| Unnoticed Progression | 14 (2) |
| Illness Cause | 93 (13) |
| Viral | 93 (13) |
| Mono/EBV | 43 (6) |
| Immune | 36 (5) |
| Stress | 14 (2) |
| Adapting and Coping | 64 (9) |
| Behavioral Coping | 57 (8) |
| Change in Mindset | 29 (4) |
| Hardworking | 64 (9) |
| Active Prior to Onset | 100 (14) |
| Healthy Prior to Onset | 64 (9) |
| Health Problems to Onset | 79 (11) |
| Frequent Sicknesses | 36 (5) |
| Comorbid Health Conditions | 71 (10) |
| Fibromyalgia | 43 (6) |
| POTS | 21 (3) |
| IBS | 14 (2) |
Table 2. Themes pertaining to onset/progression, illness cause, coping, work, and health (n=14).
| Themes | Total |
|---|---|
| % (n) | |
| Emotional Response to Onset | 64 (9) |
| Fear | 14 (2) |
| Depression | 21 (3) |
| Confusion | 14 (2) |
| Anger | 14 (2) |
| No emotional response | 14 (2) |
| Exertional Effects | 79 (11) |
| Life Limiting | 86 (12) |
| Stress | 86 (12) |
| Traumatic Experience | 36 (5) |
| Lack of Support | 50 (7) |
| Support | 21 (3) |
| Treatment Limitations | 43 (6) |
Table 3. Themes pertaining to emotional health, exertion, limitations, stressors, and treatment limitations (n=14).
▪ Onset/Illness Progression
Fourteen study participants described the period of time in which they first became ill. Different themes emerged within this larger category of illness onset/illness progression. Descriptions of onset and illness progression were often described in conjunction with one another, and thus, they comprise one superordinate theme. Below are the various subordinate themes that emerged from the larger onset/illness progression category. Notably, many participants were included in more than one category.
▪ Sudden
Seven participants described the onset of their illness as occurring suddenly and they used words such as “sudden,” “suddenly,” “rapidly,” “overnight,” and “immediately.” A sudden illness onset was described by individuals who endorsed a range of onset periods on the DSQ including: 24 hours (n=2), over 1 week (n=2), over one month (n=1), over 2-6 months (n=1), and over 3 or more years (n=1).
Participant 4 (onset over 1 week) stated: “It was like something had suddenly happened.”
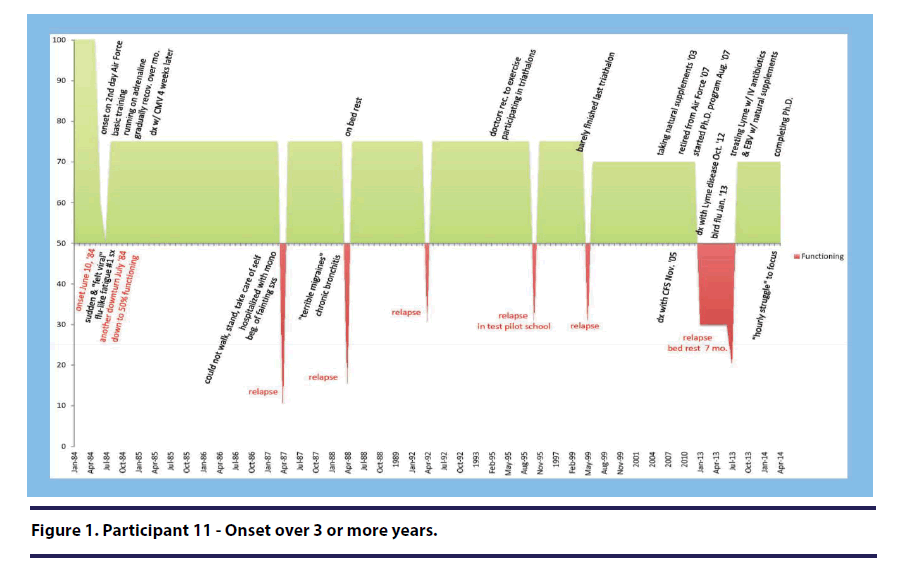
Flipping a light switch. Two of the seven participants within the “sudden” category used the analogy of flipping a light switch to describe the experience of their sudden onset. For example, participant 11 (onset over 3 or more years) stated, “it was a sudden onset” and “so it really was like someone had flipped a light switch and made me sick and never switched it off(see Figure 1).”
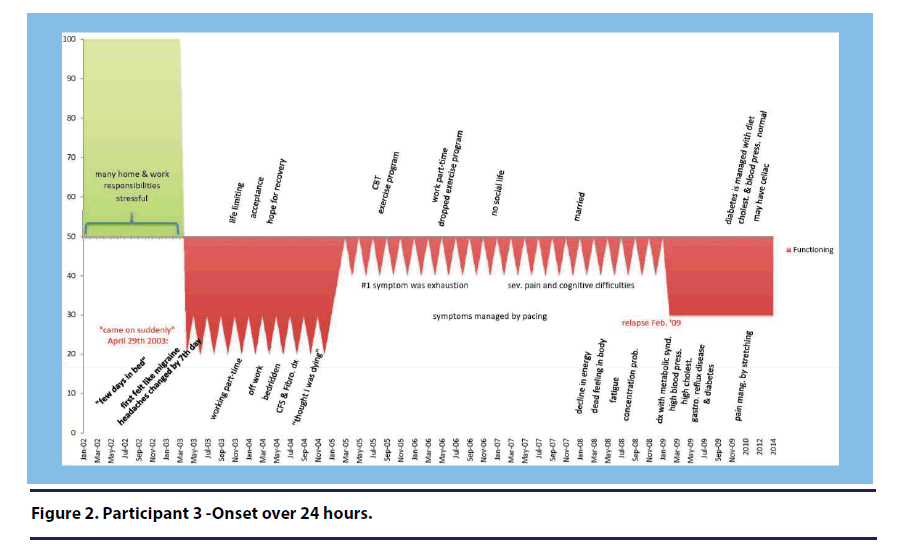
Time. Three of the seven participants described their sudden onset in the context of time. Participant 3 (onset over 24 hours) stated: “my CFS came on suddenly” and “you know, it seemed overnight to me(see Figure 2).”
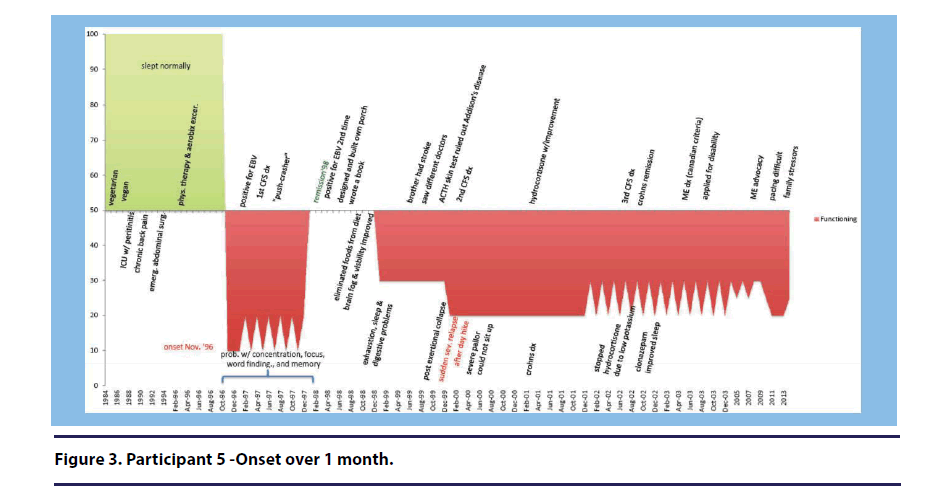
Participant 5 (onset over 1 month) described how her illness began suddenly following a case of gastroenteritis. She stated, “suddenly, in November, I had, this um, in a week’s time, I had this gastroenteritis. The initial insult was a few days. I started feeling the gastroenteritis, you know, within a week I had to go to the ER. (see Figure 3).”
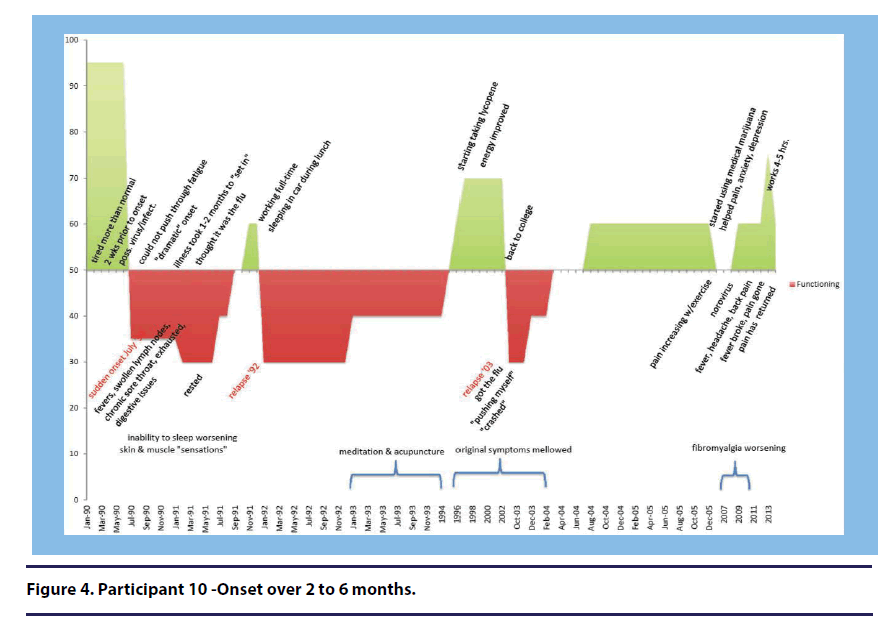
Participant 10 (onset over 2-6 months) perceived her sudden onset as developing over a slightly longer time period than participant 5, stating, “very rapidly, over the series of like 2 months! Maybe 2 months at the most. I was normal and then I was sick(see Figure 4).”
▪ Exact date
Three participants were able to report the exact date of their illness onset. When asked about the period of time her first symptoms developed, Participant 3 (onset over 24 hours) stated, “It was April 29th, 2003(see Figure 2).”
▪ Definitive turning point/downturn
Five individuals described a definitive turning point/downturn during the period of time that their illness developed. This was often described as a point in the illness development when their health and functioning took a clear turn for the worse and symptoms became significantly more severe and debilitating. A definitive turning point was described by individuals who endorsed an onset period of one week (n=1), 7-12 months (n=2), and 1-2 years (n=2) on the DSQ.
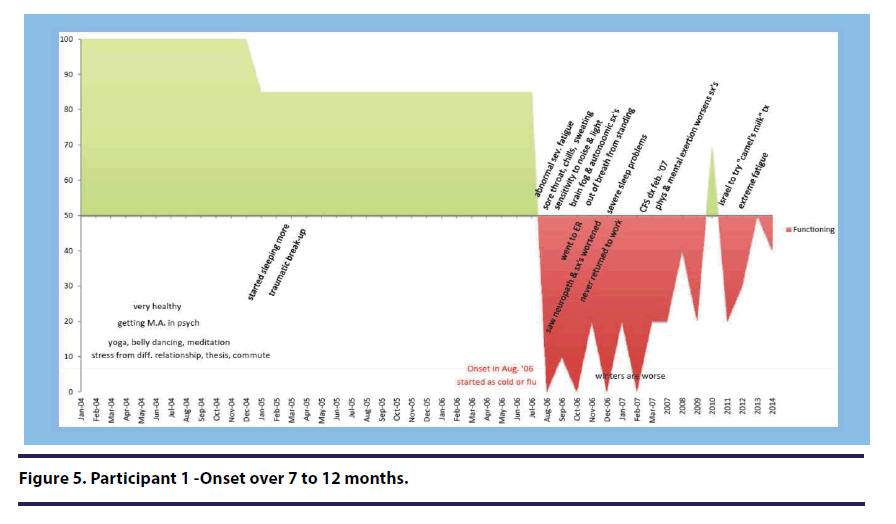
For Participant 1 (onset over 7-12 months), her “definitive turning point/downturn” was the day that she also identifies as her illness onset (see Figure 5).
She used the exact phrase “definitive turning point,” stating, “there is a definitive turning point august 22nd, 2006. After that, my life was never the same.” She elaborated further stating “after that one [illness episode] I did not recover. I never returned to work. Yeah, so everything changed from that point on.”
Tipping point. Two participants described the theme in terms of a tipping point. For example, Participant 12 (onset over 7-12 months) stated “whatever happened in March, I had an infection or whatever it was, just kind of tipped me over the edge.”
Participant 13 (onset over 1-2 years) describes a series of infections and a colonoscopy as the tipping point of her illness stating, “2006 was sort of when the sinus infections and all those infections started. And then 2008 was when I had my colonoscopy and it just kind of pushed me over the edge.”
▪ Realization that something is wrong
Seven participants described a moment or period of time in which they understood that their illness was more than an ordinary sickness such as the flu, and there was something seriously wrong with them medically. A realization that something was wrong was described by individuals who endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=1), over one month (n=1), over 2-6 months (n=2), over 7-12 months (n=1), and over 1-2 years (n=1).
Participant 3 (onset over 24 hours) stated “I thought there was something seriously wrong with me and was sure that the blood tests would come up with some horrible news (see Figure 2).”
Participant 6 (onset over 1 month) stated, “the notion that there was something seriously wrong started creeping in.”
Participant 1 (onset over 7-12 months) reported that she knew something was seriously wrong with her in the early stage of her illness stating, It was in the first two weeks actually. Week one, after a week of cold you think it would get better. You know what getting better feels like, and it just wasn’t happening. When I went into the second week, I was thinking, this is not normal, this is not normal(see Figure 5).”
▪ Steady progression
Six participants described their illness as a steady progression in which the illness and accompanying symptoms accumulated and worsened over time. A steady illness progression was described by individuals who endorsed a range of onset periods on the DSQ including: over 1 week (n=2), over one month (n=2), over 2-6 months (n=1), and over 1-2 years (n=1).
This theme is conveyed in a quote from Participant 6 (onset over 1 month) who described his illness progression in terms of a slow decline, stating “I had this initial hit and then there’s just been this constant chipping away.” He also used the exact phrase “steady progression” stating, “From the initial illness it was this steady progression and I’d say it’s been an accelerating one as of the last six or eight years have gone.”
Participant 4 (onset over 1 week) stated, “I was getting progressively worse.”
When referring to symptoms related to her illness Participant 14 (onset over 1 week) stated, “all these things were increasing over the following years.”
▪Wax and wane/illness episodes
When describing the period of time in which initial symptoms developed, nine participants described their illness as something that waxed and waned. They often described this experience in terms of “phases” “cycles” and “illness episodes.” These illness periods were more or less severe at times during the development of the illness. A wax and wane/illness episode onset pattern was described by individuals who endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=1), over 2-6 months (n=2), over 7-12 months (n=1), and over 1-2 years (n=2).
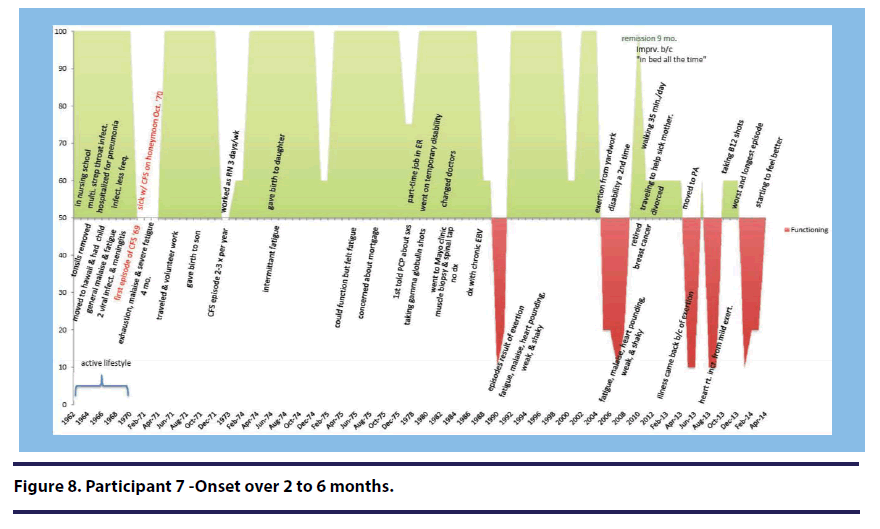
Participant 7 (onset over 2-6 months) provided a quote that specifically included the phrase “wax and wane.”
It was kind of uh... wax and wane. Uh...again, it would maybe last a week or two...after the birth of my son. I...I didn’t feel good for four months. And after the birth of my daughter it took about three months.
Cycles. Two participants described this theme as “cycles” or “cyclical.” When describing her illness cycles, Participant 1 (onset over 7-12 months) stated, “They would last for hours and hours and hours, and day after day after day… [they] would come in cycles(see Figure 5).”
Participant 14 (onset over 1 week) described her illness cycles as variable in nature, stating, “eventually it went away and it would come back but it wasn’t constant”
Improvement. A sub-theme within the larger category of wax and wane was the specific description of periods in which illness improvement was noted. Six participants described periods of improvement with regard to their illness progression; however, this improvement was cyclical and always temporary. Periods of improvement may have been signified by either a brief or long period of symptom resolution, or a reduction in symptom severity.
Participant 4 (onset over 1 week) stated, “I would start to get better and then by midsummer I would be feeling really pretty good. So the first year when this happened, I was...I thought you know, ok, you know I’ve gotten better”
▪ Long-term improvement
Only one participant described a “slow” improvement of her illness over time; therefore, it is not considered a theme within the data, rather a category that separates this participant from the others.
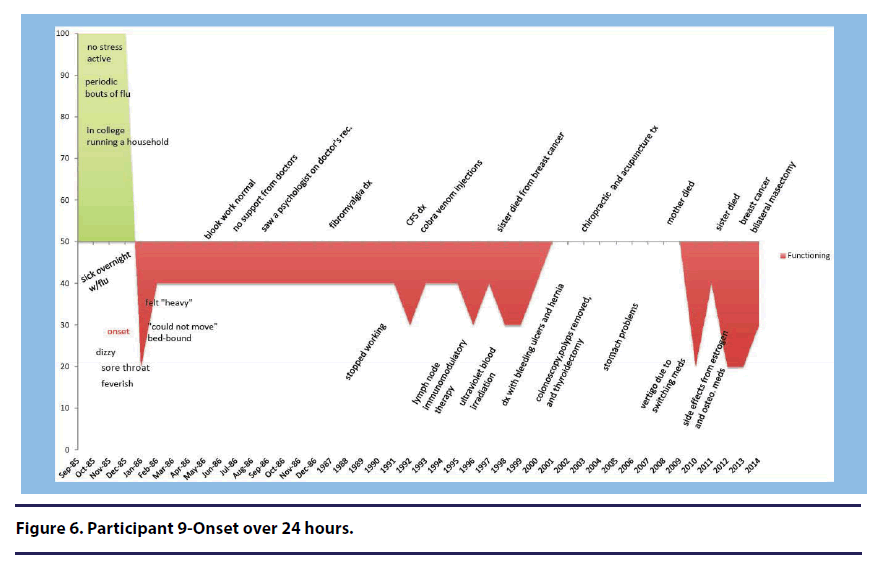
Participant 9 (onset over 24 hours) described how her illness has been slowly improving since 1986(see Figure 6).”
Well you know I got somewhat better over the years. I mean, obviously, it’s been since ’86, so there have been periods where I’m somewhat, but I’m certainly better than I was then, but um it’s never you know gotten… really gotten better.
▪ Unnoticed progression
Two participants indicated that their illness progression was unnoticed at first and that it was only years later, and in hindsight that they realized that their illness had been developing for a long period of time.
This theme was conveyed by Participant 6, (onset over 1 month), who now believes his illness started in his teen years. He reported that as a teen he did not have as much energy as other male peers his age; however, because of the large amount of energy that adolescent males have, he did not realize anything was wrong.
I was able to do everything else and that energy that I had lost [from the illness], knocked off a piece of you know of the vast amount [of energy] a 15 year old has, so it wasn’t necessarily noticed. It was, you know, as such… It’s really only in hindsight that I’ve realized, you know how significant it was at the time and how it would, how it would progress.
Participant 2 (onset over 1-2 years) described how she initially perceived her illness as part of the normal aging process. She stated:
It took me ages to realize, because at that stage I said maybe this is what getting old is about, because I’m 60 this year, you know, so at 57, I thought maybe this is the way life is just gonna be, you know what I mean?
▪ Illness cause
Thirteen participants described their perceived cause of illness. Participant 12 (onset over 7-12 months) was the only participant who did not describe a perceived cause of illness. Subordinate themes within the larger theme of illness cause are listed below. It is important to note that many individuals reported that there was more than one possible cause of their illness, and therefore, they are included within more than one sub-theme. Three sub-themes emerged from the larger theme of illness cause: 1. infectious/ viral, 2. immune component, and 3. stress as a precursor. Other causes also emerged from the data but were not considered themes, as they were endorsed by one person. The additional illness causes described include the belief that the illness was caused by an adrenal problem, autonomic problems, diet, mosquito pesticides, mitochondrial disease, mold, and physical trauma.
▪ Infectious/viral
One sub-theme that emerged within the larger category of illness cause was the belief that the illness was caused by a virus or an infectious agent. Thirteen individuals reported that the cause or partial cause of their illness was viral or infectious in nature.
When describing the onset period of her illness, Participant 4 (over 1 week) stated “I had something that felt to me like a cold, or you know, a virus, it felt to me like a virus.”
Mono/Epstein-Barr virus (EBV). Six participants specifically believed that the development of mono and/or the Epstein-Barr virus (EBV) was the cause or the partial cause of their illness. For example, this sub-theme was conveyed by Participant 6 (over 1 month) who stated, “I got mono and never fully recovered.”
▪ Immune component
Five participants specifically described an immune component to their illness onset, development, and/or progression.
Participant 11 (onset over 3 or more years) stated, “when you got mono on top of carrying Lyme, which is affecting the immune system, of course you’re never going to get better(see Figure 1).”
Participant 13 (over 1-2 years) stated:
I do think that like uh... a series of illnesses, like stuff growing up sort of contributed and I just...I guess I just want to mention, I had um mono, which was in the mid 90’s. I had bronchitis in college and the late 90’s, I was in a bad car accident in 2000… um, so I did have like a couple other significant things that I...I personally think weakened my [immune] system a little bit each time along the way.
▪ Stress as a precursor
While the majority of participants discussed stressful events leading up to or following their illness onset, two participants believed that stress played a significant role in the development of their illness. Participant 2 (onset over 1-2 years) stated, “so I think you know I keep looking for precursor things. I think that you’ve gotta add stress to the possible things.” When describing the cause of her illness development, Participant 5 (over 1 month) stated, “it was probably overworking and the stress of moving(see Figure 3).”
▪ Adapting and coping
Nine participants described ways in which they coped and adapted to their illness onset. Adaptations and coping strategies in response to the illness were described by individuals who endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=1), over 2-6 months (n=2), over 7-12 months (n=1), over 1-2 years (n=1), and over 3 or more years (n=1).
▪ Behavioral coping
Within this larger theme, eight participants described behavioral forms of coping such as pacing, reducing work hours, reducing social activities, attending support groups, and creating symptom lists to keep track of the illness progression.
Participant 2 (over 1-2 years) discussed the benefits of pacing stating:
Two years in [to the illness] I attended a multidisciplinary chronic pain program which was eight hours a day for a month, and that was sort of enormously helpful in helping me come to terms with the fact that I couldn’t do stuff, and in working out what my limits were and what I could do about it, and I think as a result of that program, I was able to sorta work more effectively, and I ended up getting tenure… and I think it’s as clear as that. Without that program I think I probably would not have got tenure.
Participant 1 (over 7-12 months) discussed how she adapted by changing her daily routine to accommodate the illness(see Figure 5). She stated, “I had to learn to schedule to take a shower, and have at least two hours before getting up and doing something else. I needed to rest just to take a shower.”
▪ Change in mindset
Four participants discussed adapting to or coping with the illness by using internal and cognitive strategies, such as engaging in mindfulness/ meditation, adopting a philosophical sense of acceptance of the illness, and optimistic thinking.
This theme is conveyed in a quote by Participant 2 (onset over 1-2 years) who discussed the benefits of mindfulness and meditation as forms of coping. She stated:
Meditation, where you also watch your thoughts and try to be detached about them…and the very day I was diagnosed happened to be a day for that. I found that ability to be a bit detached just enormously helpful and it has continued to be a huge coping strategy.
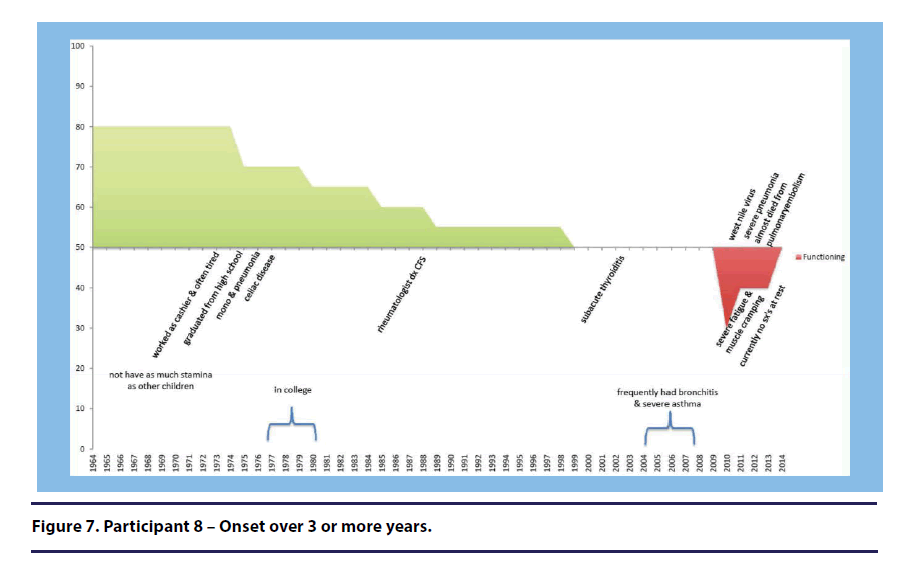
Participant 8 (onset over 3 or more years) discussed the value of acceptance of her illness stating, “I accepted it pretty philosophically. I didn’t do a lot of chest beating. (see Figure 7).”
▪ Hardworking
Nine participants described themselves as hardworking or overworking in the year leading up to their illness onset and/or during the early development of their illness. Individuals who described working hard in the year leading up to their illness onset or during the early development of their illness endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=2), over 2-6 months (n=1), over 1-2 years (n=2), and over 3 or more years (n=1).
Two participants specifically used the term “overworking.” For example, Participant 3 (onset over 24 hours) stated, “at the time I was a single mom with two teenagers and a mortgage. I worked full time. I loved my job um but I was indeed overworking for sure(see Figure 2).
Participant 6 (onset over 1 month) discussed the price he paid for working too hard at the time that his illness was developing.
I think that I worked longer than I should have, um for my health, for sure… um but I think I pushed myself you know a lot further than I think a lot of people might. Um which just kinda made my decline that much worse um you know and so I’ve had loss of function since then.
▪ Active prior to onset
All 14 participants described having active lifestyles prior to the onset of their illness. They described their engagement in sports, social activities, and work related activities.
When describing the year prior to her illness onset, Participant 1 (onset over 7-12 months) stated, “it was great, I did yoga, belly dancing, meditation, you know all sorts of things. I was finishing my bachelor’s degree in psychology(see Figure 5).” Participant 5 (onset over 1 month) described her many physical activities prior to her illness onset (see Figure 3).” She stated, “I was an avid hiker and climber and biker” Participant 7 (onset over 2-6 months) described her active lifestyle with fondness stating, “I was working as a nurse full time during those times, and felt pretty good. And was active in helping my brother take care of their kids, and going on day trips and then dating um...and just enjoying life(see Figure 8).”
Healthy prior to onset
Nine participants considered themselves healthy prior to their illness onset. Many of these participants also identified health conditions or problems prior to their illness development; however, they still considered themselves as relatively healthy individuals. Individuals who described themselves as healthy prior to onset endorsed a range of onset periods on the DSQ including: 24 hours (n=2), over 1 week (n=1), over one month (n=2), over 7-12 months (n=1), over 1-2 years (n=1), and over 3 or more years (n=2).
Participant 11 (onset over 3 or more years) discussed her health prior to onset stating, “I was in the best shape of my life(see Figure 1).”
▪ Health problems prior to onset
While the majority of participants described themselves as relatively healthy prior to the onset of their illness, many of these same participants identified health problems and ailments in the months or year leading up to the onset of the illness. Eleven participants described one or more health problems leading up to their illness onset. Participants who described themselves as having health problems prior to onset endorsed a range of onset periods on the DSQ including: 24 hours (n=2), over 1 week (n=1), over one month (n=1), over 2-6 months (n=2), over 7-12 months (n=1), over 1-2 years (n=2), and over 3 or more years (n=2). For example, Participant 1 (onset over 7-12 months) described health symptoms she experienced in the months leading up to her illness(see Figure 5).”
I also noticed that kind of fatigue, and uh being much more tired than usual…. I noticed that. And then in the summer, July of 2006, July, August, I started noticing that when I stood for ten to fifteen minutes I would get out of breath and I would almost faint, I would have to sit down, it was so extreme I would break out into this sweat. I would feel extremely weak. I would need to sit down. That was very unusual but that definitely started happening around July, August.
▪ Frequent sicknesses
Five participants described experiencing frequent sicknesses, such as colds or persistent strep throat prior to their illness onset.
Participant 7 (onset over 2-6 months) described a series of sicknesses while she was studying in nursing school and prior to the development of her illness (see Figure 8).”
I recall that I was sick a lot in nursing school but it seemed to be more viruses because I had not been exposed, especially when I was in pediatrics. I was like sick all the time. I was hospitalized with pneumonia…um, but again, I thought I was just...that was just my resistance building up. I had several episodes of strep throat.
Participant 5 (onset over 1 month) also described frequent sicknesses in the year leading up to her illness onset (see Figure 3).
…maybe the year or year or two prior in hindsight you seem to have a little bit more um problems than you normally would, um you know, like flus or things that you didn’t have before.
▪ Comorbid health conditions
Eleven participants reported that they had comorbid health conditions during their ME and CFS progression. Three subordinate themes emerged from the data: 1. Fibromyalgia, 2. Postural orthostatic tachycardia (POTS), and 3. Irritable bowel syndrome (IBS). Additional comorbidities were reported; however they were not included as themes as only one participant endorsed having each condition. These comorbidities included migraines, temporomandibular disorders (TMD/TMJ), multiple chemical sensitivities, Lyme disease, thyroiditis, degenerative eye disease, ulcers, asthma, and deep vein thrombosis. Individuals who described comorbid health problems endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=2), over 2-6 months (n=1), over 7-12 months (n=1), over 1-2 years (n=2), and over 3 or more years (n=2).
▪ Fibromyalgia
Six participants reported that they had a diagnosis of fibromyalgia in addition to ME/ CFS. Participant 4 (onset over 1 week) discussed how when she was diagnosed with fibromyalgia she was not surprised, as she had wondered since she was a teenager if she had the condition.
I also saw a rheumatologist who thought I had fibromyalgia and I kind of dismissed the diagnosis, because I thought I had fibromyalgia but I thought I had fibromyalgia you know, ever since I was a teenager…and I mean it wasn’t diagnosed then, but when I first...when I first came...when I first learned what it was, which was several years prior to this time, I thought I probably had fibromyalgia, but I didn’t think it was a big deal, but it didn’t stop me from doing anything.
▪ Postural orthostatic tachycardia
Three participants reported that they experienced Postural Orthostatic Tachycardia (POTS). Participant 6 (onset over 1 month) described how he was initially diagnosed with activity induced asthma when he was younger, but later realized he has been suffering from POTS all along. He stated, “the only diagnosis I got at the time was um activity induced asthma, and I think that, what was really going on was POTS, but nobody…POTS wasn’t even in the lexicon in 1980. Nobody looked for that.”
▪ Irritable bowel syndrome
Two participants reported that they had irritable bowel syndrome (IBS). Participant 13 (onset over 1-2 years) described how she was diagnosed with multiple conditions including IBS stating, “I was diagnosed with IBS, TMJ, migraines, chronic fatigue syndrome, and fibromyalgia.”
▪ Emotional response to onset
Nine participants described their emotional response to their illness onset. A range of responses were noted, including fear, depression, confusion, and anger. Individuals who described an emotional response to onset endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=1), over 2-6 months (n=2), over 1-2 years (n=1), and over 3 or more years (n=2).
▪ Fear
Two participants described the feeling of fear in response to their illness onset. Participant 2 (onset over 1-2 years) described telling her family that she would never recover. She stated: I’m calling up one of my sisters...my family is in Australia, so is my husband, and telling her about how scared I was that I wouldn’t get well and I’d have to give up my job. I remember being just very, very freaked about the possibility that I had this disease that wouldn’t go away.
Depression/sadness
Four participants described experiencing periods of depression following the onset of their illness. Many participants described how the depression came once they came to the realization that the illness may never resolve.
Participant 10 (onset over 2-6 months) described how the depression hit a year after her illness onset. (see Figure 4) She stated: Then after about a year, I’m starting to realize that this might not go away. This might take a while to go away. And I just started getting really depressed.” She elaborated further stating, “It was just like, this is insane, and you start to get really depressed. She also described the belief that her depression was not wholly psychological. She suggested that the depression was partly a physiological response to her illness and partly due to environmental reasons such as invalidation from others.
It really lingered. I mean it’s been up and down for the past 25 years of the depression, and I think part of it is physiological. I think there is something about the illness that pushes people into the depression, and then I also think that it’s environmental, you know. I think that when your life has been limited in such a way and you’re not being validated as someone who has an illness that’s a very depressive situation.
▪ Confused
Two participants described the feeling of confusion in response to their illness onset. Participant 11 (onset over 3 or more years) described feeling “perplexed” about how she could become sick in a matter of a day(see Figure 1). She stated, “so a lot of it was denial, um, but you know just being totally perplexed by how you could go from totally healthy one day to being totally sick the next and not even know what happened.”
▪ Angry
Two participants described feeling angry during their illness onset. Participant 10 (onset over 2-6 months) described anger about getting negative feedback from her doctors and their inability to tell her how to treat her illness(see Figure 4). She stated, “I was getting angry! I was like don’t tell me that I’m crazy, just tell me what I need to do.”
▪ No emotional impact
Two participants reported that their illness onset and early progression did not significantly impact them emotionally. Participant 11 (onset over 3 or more years) stated, “Mentally, I was still emotionally there(see Figure 1).”
▪ Exertional Effects
Eleven participants described how exertion, whether physical or mental, triggered or worsened their symptoms. Mild to severe exertion was described as causing a further decline in health. Individuals who described an exertional effects endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=2), over 2-6 months (n=1), over 7-12 months (n=2), over 1-2 years (n=2), and over 3 or more years (n=1).
Participant 4 (onset over 1 week) described the difficulty of going to the grocery store. She stated, “It wouldn’t be uncommon for me to go to the grocery store and have to rest in the car for about 20 minutes before I would go in and doing grocery shopping.”
Participant 6 (onset over 1 month) described the impact that mental exertion had on his illness. He described an instance in which he had severe exertional effects following the completion of a neuropsychological evaluation for his disability assessment. He stated:
I had to do an interview for disability, a neuropsych evaluation… an all-day thing… um and I was in bed for three weeks. I was in horrible shape after that. I was essentially sitting at a desk for 8 hours.
▪ Life limiting
Twelve participants described how the illness limited their lives during their illness onset. They also discussed how the illness continues to limit their lives. Participants described ways in which their family, social, and work lives were negatively affected by the illness. They also discussed a decline in their functional abilities. Individuals who described exertional effects endorsed a range of onset periods on the DSQ including: 24 hours (n=2), over 1 week (n=2), over one month (n=2), over 2-6 months (n=1), over 7-12 months (n=1), over 1-2 years (n=2), and over 3 or more years (n=2).
Participant 3 (onset over 24 hours) described the negative impact the illness had on her family and on her social life(see Figure 2).
I was pretty much unable to take care of my kids and work at the same time, so between coming home and just dropping at the door… um my kids were old enough to sorta help out, and uh they would sorta throw together some sorta of a dinner and we would have dinner together but I couldn’t really socialize. I was so dead by the end of the day, I was just like a plasma, and that went on again I guess until about October, so I was sleeping at every coffee break lunch break, I was going home and going straight to bed, um I wasn’t eating well, my kids weren’t eating well.
Participant 6 (over 1 month) specifically used the term “life limiting” and he described how he eventually became so limited that he could not drive and was mostly confined to his bed. He stated, “I got to the point where going to the doctor and then dropping off prescriptions off at a pharmacy was a limit, and I’m not driving basically… not driving at all right now, and you know mostly bed bound.”
▪ Stress
Twelve participants described experiencing stress in the year leading up to and/or following their illness onset. Individuals who described stressful events or experiences endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=2), over one month (n=2), over 2-6 months (n=2), over 7-12 months (n=2), over 1-2 years (n=2), and over 3 or more years (n=1).
Participant 5 (onset over 1 month) described multiple stressors leading up to her illness onset, including negotiating to buy a house with her husband, participating in a big art show, and the sudden death of her mother(see Figure 3).
When we first moved, since it was pretty stressful doing all the negotiating to get the house, stuff like that. It was at also at the same time, that we had our biggest art show of the year that we had to do, the resorts festival, so we were getting ready to do a seventeen day sting there at the same time that we were closing on our house, um so it was all that going on… I also had uh oh, oh, geez, I almost forgot, April my mother died suddenly um, how could I forget that…um we weren’t terribly close but she was my mom, and we had a big family.
Participant 11 (onset over 3 or more years) described the stress she was experiencing concurrently with the onset of her illness. Specifically she discusses the stressors she experienced during her basic training to be an air force pilot(see Figure 1).
unfortunately it happened on the second day of four weeks of basic training and I had no clue what had gone on other than you know it was a very stressful time. I was uh, I had to do very well at basic training because at the time, the pilot slots for women were very uh rare, and so you had to do very well on your application in order to get selected, and a lot of personal pressure on me, and then that first night, of course they overload on purpose because they are trying to make you quit and um, I stayed up all night, pulled an all-nighter essentially because your socks had to be exactly such and such length, and you know, all the certain way and all of that and they had you go to meetings and all this other stuff during the first day that you never had a chance to put this together.
▪ Traumatic experiences
Five individuals reported that they experienced trauma prior to the onset of their illness. The traumatic experiences that were discussed included a car accident, rape, falling down a staircase, severe childhood burns, and the traumatic delivery of one of their children. Three out of the five participants experienced the trauma as adults and closer in time to the onset of their illness. Individuals who described traumatic experiences endorsed the following onset periods on the DSQ: 24 hours (n=1), over 2-6 months (n=1), over 7-12 months (n=1), over 1-2 years (n=1), and over 3 or more years (n=1).
Participant 3 (onset over 24 hours) suggested that the physical trauma she experienced after falling down stairs had a role in her illness development(see Figure 2).
December 2002, I had a fall, um I fell down some stairs and was knocked out, so that was… you know, often you’ll hear about people who got fibromyalgia, they say that it can happen after some sort of traumatic event, physical event or emotional event, so I wonder if that played a part of it.
Participant 13 (onset over 1-2 years) described a car accident she experienced prior to her illness development.
Yeah, so I um...I guess the most recent thing before all of the um...the...sinus infections and stuff was a car accident in 2000. Where I was rear-ended and I got very bad whiplash, and it took a long time to get over that. I’m...my neck is still not the same. It still gets really tight. I developed scar tissue and stuff, so it was pretty significant for me.
This participant also suggested that the car accident along with a “series of illnesses” weakened her immune system.
I had um mono, which was in the mid ‘90’s. I had bronchitis in college and the late ‘90’s. I was in a bad car accident in 2000. Um, so I did have like a couple other significant things that I...I personally think weakened my system a little bit each time along the way.
▪ Lack of support
Seven participants described a lack of support following the onset of their illness. This included a lack of support from family, friends, and physicians. Individuals who described a lack of support following their illness onset endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=1), over one month (n=2), over 2-6 months (n=1), over 1-2 years (n=1), and over 3 or more years (n=1).
Participant 10 (onset over 2-6 months) describes the lack of support she received from her family, friends, and her boyfriend during the onset of her illness(see Figure 4). She stated:
you’re alone, usually, right! You’re… you’re completely alone right? I did not have a support system. My parents are not supportive people. My boyfriend was not a supportive person! (laughs) so I did not have support to encourage me… that… you know, maybe you can get over this. You know, maybe life will get better. That didn’t happen, you know. I was alone most of the time. I was just trying to Figure things out and then not getting a lot of help from the world.
Participant 5 (onset over 1 month) described the lack of support she received from doctors. She stated, “I kinda toughed it out on my own, because my past experiences with doctors(see Figure 3), including the gastro I did see in December, kinda just blew me off.”
▪ Support
Three participants described the support that they received during the onset and early progression of their illness. Individuals who described support following their illness onset endorsed the following onset periods on the DSQ including: over one month (n=1) and over 1-2 years (n=2).
Participant 2 (onset over 1-2 years) described the significant support that she received from her primary care physician during the period of time that she became ill.
I consulted my own primary care physician who is someone who always believes me when I tell her how I’m feeling. She’s great. She didn’t blow me away which is I think the important thing. I think that I sort of totally proved myself as being a good dooby before I got sick because nobody thought I was faking and they have incredibly understood.
Participant 6 (onset over 1 month) described the support he received from his general practitioner. He stated, “I guess I have to say, you know, as far as the way some people get treated by doctor’s, I’ve been lucky. My GP has been very supportive. He also described the support he received from his wife stating, “My wife, you know cooks and cleans and takes care of the kids, and I do what I can for moral support essentially.”
▪ Treatment limitations
Six participants described limitations of the treatments that they were receiving during the period of time when they became ill. Individuals who described treatment limitations endorsed a range of onset periods on the DSQ including: 24 hours (n=1), over 1 week (n=1), over one month (n=2), and over 1-2 years (n=2).
Participant 5 (onset over 1 month) described how her doctor told her that she could exercise, which only worsened her condition. She described how she learned later on that exercise could worsen her prognosis(see Figure 3).
Exercising and activity after the onset of illness to worsen your prognosis so… I read that too late (laughs) and my doctors told me that I had CFS which you are allowed to exercise… and if they had known about any of …they would have said hey, cut this out, lay down.
Participant 6 (onset over 1 month) described the antiviral treatment his doctor has tried. He stated, “she’s done you know antivirals and other things and we haven’t had any real luck. Basically getting treatment is either helping symptoms a little bit or it doesn’t help.”
▪ Symptoms
All fourteen participants identified symptoms that were experienced during the onset of their illness. Symptoms primarily fell in the following broad categories: flu-like symptoms, digestive symptoms, pain symptoms, autonomic symptoms, fatigue, post exertional malaise (PEM), sleep difficulties, and cognitive impairment.
Impressions
Feedback and impressions have been shared by eight out of the 14 participants interviewed for phase two of the study. Overall, the feedback has been positive and all participants have indicated that the themes and illness timeline graphs effectively summarize their experience.
Five out of the eight participants provided minor corrections and additions to their illness graphs. Three reported that their graphs did not require any corrections/changes. After reviewing the overall themes and key quotes that conveyed each theme, one participant commented, “I found that I could relate to the other participants as well. Each quote might as well have come from me too.” Two participants elaborated on some themes. One participant who had described stress as a partial precursor for her illness development elaborated on this theme by describing how her stress was “good stress” that involved positive milestones in life (e.g. raising children and buying a house). She stated “I was having the time of my life.” Another participant commented on the exertional effects theme and described how many individuals with the illness “realize too late the benefits of pacing.” She discussed how participants often realize the importance of reducing activities after the exertional effects have already taken a severe toll on the body. One participant noticed that she was initially categorized in the “onset over 3 or more years” onset group based on her answer to the onset question on the DSQ. However, she stated that her illness was sudden and developed over one day. This was reflected in her qualitative interview as well. A few participants described an emotional reaction to reading the overall themes and from receiving their illness timeline graphs. One participant stated, “reading through my narrative made me very emotional. There is something about looking at your own words that makes it very validating. With that, comes an incredibly strong and direct connection to the suffering I am having to minimize each and every day. It’s like the floodgates open, and it’s hard to contain all the emotions that are normally tucked away.”
Discussion
The current study serves as an investigation of onset patterns associated with ME and CFS. The qualitative study provided rich descriptions of onset experiences across participants who endorsed a range of onset timeframes on the DePaul Symptom Questionnaire (DSQ; from 24 hours to over 3 years). These rich descriptions provide insight into the symptoms, onset patterns, and early characteristics associated with the initial phase of the illness.
The qualitative interviews in the current study yielded rich descriptions that provide insight into the way people with ME and CFS describe their illness onset, including perceptions of mode of onset, illness progression, functional, social, and treatment limitations, emotional responses, degree of support from others, and early health problems and symptoms. Within the category of onset/illness progression, 50% of participants endorsed a sudden onset of ME and/or CFS. These findings are consistent with the qualitative study by Ray et al. [22], which revealed that 50% of a sample of CFS participants reported a sudden illness onset. Another onset theme that emerged within the current study was the experience of a steady progression of symptoms that accumulated over time (endorsed by 43% of participants). This theme is also consistent with Ray et al. [22] who found that 25% of their study population described a gradual illness onset in which people reported a slow, worsening of symptoms over time. A third theme that emerged within the onset/illness progression category of the current study was the experience of a wax and wane progression in which there were periods of improvement/ remission and periods of worsening symptoms accompanied by a noticeable decline in functioning. This wax and wane pattern was described by 64% of participants, who used terms such as illness episodes, phases, and cycles. This theme is similar to the two-phase illness onset group previously described by Ray et al. [22]. Ray describes this phased onset as a sharp deterioration of health followed by improvement in phase one, which is then followed by another deterioration of symptoms in phase two. Ray et al. found this pattern in 23% of their sample. The wax and wane pattern found in the current study differs from the two-phase, as it is not limited to “two phases.” Many participants in the current study described numerous illness phases throughout the course of their illness progression. Additionally, the onset themes of the current study are not mutually exclusive. Specifically, some participants endorsed a sudden onset followed by a steady progression of the illness. Other participants described a sudden onset and a wax and wane illness course rather than a steady progression of symptoms.
Additional onset/illness progression themes emerged from the data. For example, a subset (21%) of participants reported the exact date of illness onset. As might be expected, all of these individuals described their onset as sudden. The qualitative findings of the current study reveal that the majority of individuals who endorsed a sudden illness onset did not name the exact date of onset. This finding suggests that requiring a specific date of onset could be too strict for determining mode of illness onset.
A subset (14%) of participants described the experience of an unnoticed illness progression. These individuals also described a steady progression of their illness in which symptoms and functional limitations slowly increased over time. Previous qualitative studies have not specifically identified or described this experience of an unnoticed illness progression. These findings have clinical implications, as individuals who do not recognize the progression of their illness until years later likely will not seek medical care and support as quickly as others who identify that something is wrong earlier in the illness development. This could ultimately impact the course, treatment, and prognosis of the illness Thirty-six percent of participants also described a definitive turning point/downturn in their illness progression in which symptoms and functional limitations significantly worsened. Furthermore, 50% described a moment in the illness progression in which they realized that something was seriously and medically wrong with them. These experiences were significant for participants as they signified a period of the illness development in which there was gained insight on the seriousness of the illness. These experiences could have clinical significance for patients, as they potentially mark a point in time in which they feel the need to seek medical treatment and make steps to receive a diagnosis.
The majority of participants (93%) from the qualitative sample reported that a virus or infection was the cause of or partial cause of the ME or CFS onset. This finding is consistent with the quantitative findings in phase one. The majority of participants from the larger quantitative sample reported on the DSQ that an infection or virus preceded the illness onset. Furthermore, mode of illness onset did not differentiate individuals based on viral/infectious etiology. These results are also consistent with Ray et al. [22] who found that a subset of all three onset groups identified in their study (sudden, gradual, and phased) endorsed a viral/ infectious onset.
Additionally, Ray et al. [22] found that a portion of participants attributed their illness development to their immune system “breaking down.” A subset (36%) of participants in the current study also endorsed an immune component to their illness cause. These individuals typically described a series of infections (one individual described infections in combination with a physical trauma) that negatively impacted the immune system over time. These findings are consistent with an immune component theory posed by Hyde [12] who asserts that ME often follows multiple, minor infections in individuals with susceptible immune systems or immune systems that are weakened by severe stressors (e.g. contact with infectious persons, exhaustion, trauma, immunizations, epidemic disease, travel and exposure to virulent agents). Additionally, prior research has evidenced immune dysfunction and damage to the CNS in individuals with CFS [41].
Ray et al. found that individuals with a sudden or “sharp” onset were most likely to report that an infection was a trigger for illness onset [22]. Additionally, Ray et al. reported that individuals with a sudden onset more often describe pre-onset factors (e.g. stress, overactivity, predispositions for health problems etc.) that may have built up and contributed to the onset of illness. Additionally, Ray found that individuals in the phased group were more likely to describe exacerbating illness factors that followed the onset of their illness. [22] In contrast, the current study showed that the majority of participants identified an infection or virus as the trigger for their illness regardless of onset type. Furthermore, pre-onset triggers and post-onset exacerbating factors (e.g. overexertion) were endorsed regardless of onset type.
A small subset of participants (14%) reported that stress was a partial cause of their illness onset. Similarly Ray et al. [22] found that some individuals in their sample described “complex” onset contributory factors, which included a combination of infection, overexertion, and stress. Salit found that individuals with CFS (regardless of onset group) reported a higher number of stressful life events prior to CFS onset compared to a control group [42]. In contrast MacDonald et al. [43] did not find an increase in life stress in the year before the onset of CFS.
Thirty-six percent of participants described traumatic events over the course of their lives. Only one participant suggested that the cause of the illness was partially due to the trauma. Overall, the current study revealed that the majority of participants (86%) endorsed stressors in the year leading up to and following illness onset; however, the stress was not described as a precursor to the development of the illness, but rather something that exacerbated the illness.
Many participants in the current study described the experience of working hard, and a small subset within this theme discussed the experience of “overworking” in the year leading up to their illness onset. In an anthropological study of the experience of CFS, Ware [44] writes about individuals’ descriptions of working hard in the year or years leading up to their illness onset. Ware described this hardworking behavior in terms of “type A” and “perfectionistic” characteristics that led many to feel exhausted. [44] While some participants in the current study indicated stress or exhaustion in the year leading up to their illness, the majority described their hardworking styles in a positive manner. Furthermore, this hard work ethic was often described in order to show the stark contrast to their considerably more limited lives (functionally and socially) following illness onset. In fact, all 14 participants in the current study described having active lifestyles including a range of both work and recreational activities. The findings from the current study suggest that when assessing for factors related to stress and functioning leading up to the onset of ME and CFS, it would be beneficial to include questions that assess for whether these activities were deemed stressful or taxing, as this may have implications for illness attributions and whether stressful experiences and lifestyles are truly perceived as contributory to onset.
A majority (79%) of participants in the current study reported that they were relatively healthy prior to the onset of their illness. This finding is consistent with a qualitative study by Lovell [45], which revealed that aid workers who developed CFS when living overseas considered themselves as healthy before the development of the illness. While the majority of participants in the current study considered themselves relatively healthy prior to onset, 79% also described notable health problems in the year or years leading up to the onset of the illness. Within this category, 36% described the experience of being frequently sick with colds or sore throats. A previous study found by Evans et al. [46] found that individuals retrospectively reported experiencing multiple health symptoms prior to the onset of their fatiguing illness. These included Fukuda [2] symptoms, neurological impairments, sensitivities, cardiovascular symptoms, loss of thermostatic stability, pain, sleep disturbances, neurosensory, perceptual, and motor symptoms, neuroendocrine, and mood symptoms. The presence of health problems and symptoms prior to the onset of the illness could potentially be identified as risk factors for ME and CFS. These health problems may also influence illness course and differentiate individuals with ME and CFS into subtypes [44].
Participants in the current study identified symptoms experienced during the onset of their illness. Specifically symptoms generally fell in the following categories: flu-like symptoms, digestive problems, pain, autonomic dysfunction, fatigue, post exertional malaise (PEM), sleep difficulties, and cognitive impairment. Two symptoms that were endorsed with the highest frequency were “exhaustion” (57%) and general cognitive difficulties (43%). In congruence with the tenets of qualitative description, the author made an effort to use words to describe symptoms that were also used by the participants themselves. It is notable that a large proportion of participants used the term “exhaustion” rather than only fatigue. Future surveys designed to assess early signs and symptoms within the onset period should consider using the participant’s preferred language for their symptoms. Overall, the identification of early signs and symptoms of the illness could be beneficial for early intervention and treatment.
Many participants described comorbid health conditions including fibromyalgia, POTS, and IBS. These findings are consistent with previous quantitative studies that have revealed that CFS is highly comorbid with fibromyalgia [47-49]. Individuals with highly comorbid conditions may be at risk for developing ME and CFS, and they may also negatively influence the severity of onset and illness trajectory.
A majority (79%) of participants described the negative impact that exertion had on their symptoms and illness course. These exertional effects included both mental and physical exertion. Post exertional malaise (PEM) has been found to elicit a worsening of symptoms (e.g. fatigue, headaches, cognitive dysfunction etc.) following routine daily tasks including going to the grocery store, walking, and showering [50- 51] found up to 93.8% of individuals with CFS endorsed the experience of PEM depending on how the questions on a survey were worded. Furthermore, PEM has also been measured using objective methods [52].
Participants described both behavioral and mental/internal forms of coping in response to the onset of ME and CFS. Behavioral forms of coping included attempts to limit activities to prevent overexertion, attending support groups, and creating symptom lists. In an earlier qualitative study on illness perceptions in individuals with CFS, Clements and colleagues [53] also found that individuals described behavioral forms of coping such as pacing and reducing activities. Findings from the current study as well as by Clements also revealed that these strategies were considered most helpful to symptom management rather than as a cure for the illness. The pacing strategy endorsed by many individuals in the current study has been supported by the energy envelope therapy, which suggests that balancing perceived energy with expended energy can help individuals with ME and CFS conserve energy and reduce overexertion [54-56]. Other forms of coping described in the current study involved internal methods, such as a changing one’s mindset. For example, many individuals described the development of a philosophical acceptance of the illness. This experience of gaining acceptance is consistent with Fennell’s phase theory of CFS [57] in which participants reach acceptance of the illness in phase three following the crisis and stabilization experiences in phase one and two. While the experience of acceptance was described by individuals following the initial crisis phase in the current study, some participants found acceptance relatively early on in their illness progression and even before reaching “stabilization.”
Qualitative findings from the current study suggest that the onset of illness had an emotional impact on more than 50% of participants. Some participants described going through periods of depression, whereas others described fear, anger and a state of confusion regarding the onset of the illness. This is consistent with findings from a mixed method study by Tuck and Wallace [58] who found that compared to a control group, women with CFS reported significantly higher levels of depression, anxiety, anger, and confusion following the onset of their illness. The experience of depression following onset was corroborated in qualitative interviews [58]. These findings suggest that the onset of ME and CFS can have a profound emotional impact on the sufferer. Individuals with ME and CFS could benefit from significant emotional and instrumental support from friends, family, and health providers during the earliest phase of illness development.
A majority of participants described many ways in which the illness limited their lives in terms of work, social life and family responsibilities. Consistent with this finding, others have found that individuals with CFS report significantly impaired quality of life. [59,1] Furthermore, while a small subset of individuals described the support they received during the onset of ME and CFS (21%), half of participants described the lack of support they received from others (friends, family, and doctors) during the onset of the illness. A qualitative study by Dickson et al. [60] found that CFS participants described a sense of loneliness, isolation, and lack of support from friends, family, and general practitioners. A needs assessment by Drachler et al. [61] revealed that individuals with ME and CFS expressed the need for support in understanding and receiving a diagnosis, validation from health providers and family, as well as support in finding ways to engage in social activities. Additionally, mixed method study revealed that individuals with CFS lack social support and their degree of perceived social support was correlated with quality of life factors. [62] Furthermore, Jason, Witter, and Torres-Harding have provided evidence that perceived social support is correlated with physical health outcomes in individuals with CFS [63]. Taken together, these suggest that individuals with ME and CFS are severely lacking a sense of support from others (health providers and family/friends) during the onset of illness and in the years following onset.
In addition to the limited perceived support, 43% also described limitations of the many treatments that they tried during the early development of their illness. Currently there is not a gold standard treatment for the illness due to the multidimensionality of the illness, the absence of a confirmed etiology, and the variability in case criteria for diagnosing the illness [64]. A lack of social support and an absence of effective treatments available in the early stages of the illness likely has a negative impact on the course of illness and overall quality of life in individuals with this debilitating illness.
Overall, the qualitative findings provide insight into how individuals with ME and CFS describe and reconstruct their illness onset and progression. Findings of the current study revealed many commonalities with Ray et al. [22]. Most notably, both studies reveal that ME and CFS onset is likely more complex than the dichotomous categorization of onset (sudden versus gradual) that is commonly described in the literature. Both studies found evidence for the experience of sudden, steadily progressing/ gradual, and phased onset patterns. However, the current study findings differ from Ray et al. [22], as participants in the current study were often included in more than one onset group. For example, an individual with a sudden onset could experience a steady progression or a wax and wane illness pattern. These findings suggest that onset and illness progression may be even more complex and dynamic than Ray et al. [22] suggested. Furthermore, the current study findings suggest that onset and illness progression are closely tied together and constructs that are not easily differentiated (e.g. steady progression may describe an illness course as well as a gradual onset). The themes that identify a definitive turning point and the realization that something is wrong medically have significant clinical and research implications, as they denote a period in time in which an individual might pursue medical care and also begin the search for a diagnosis. A better understanding of the signs and symptoms that accompany these moments of insight might lead to interventions that focus on earlier points in the illness trajectory and for individuals who might have otherwise recognized the severity of their illness much later.
The ME and CFS illness timeline graphs of the current study fit nicely within the trajectory framework of chronic illness by Corbin et al. [65]. They describe a trajectory as an illness course that is shaped by the ill individual, his/ her family members, friends, and health care providers, over time. Different illness phases include the biographical and health events that are present before the onset of illness (pretrajectory), the period of time in which symptoms and signs first appear (trajectory onset), life threatening emergencies (crisis phases), “active” illness periods that may require hospitalization (acute phases), periods in which the illness is relatively well managed (stable phases), periods of fluctuating illness that are poorly controlled (unstable phases), periods of illness progression/ decline (downward phases), and lastly, the final phase of life (dying) [65]. These phases (with the exception of the dying phase) are made visible by the illness timeline graphs of the current study.
Corbin et al. discuss implications for thinking about chronic illness in terms of a trajectory [65]. Specifically, an understanding of illness course can help one better manage disability and improve quality of life, help change or shape the course of illness, and more effectively manage symptoms. Furthermore, while health care providers may have an understanding of a patient’s medical course and treatment history, an illness trajectory can provide additional insight into the way in which individuals manage and shape their illness in the context of daily life [65]. Corbin and colleagues point out that many of the strategies that are used to manage illness occur at home and not in medical offices or hospitals. Furthermore, they discuss how a trajectory can reveal how pre-illness and pre-medical intervention experiences shape the way individuals understand and respond to their illness and to their health care providers [65].
Illness timeline graphs have many clinical and research implications within the ME and CFS context. Specifically, they allow for years of illness information to be displayed in a more digestible, visual format. Furthermore, the timeline graphs can be used by patients to track symptoms over time and to identify potential factors that contribute to periods of illness remission or decline. An individual with ME and CFS may identify a period in his/her illness course when a certain medication or behavioral coping strategy (e.g. pacing) was associated with an improvement in functioning and quality of life. [66] have also described how the process of tracking illness trajectories can highlight factors that contribute to illness changes and can allow patients to potentially take control over their illness course. In the current study, one participant, who described herself as a “highly visual person,” reported that the opportunity to see the peaks and valleys of her functioning over time provided her with heightened clarity about her illness experience.
The process of visually tracking ME and CFS illness progression may help to identify different subtypes of the illness, which then may help health care providers tailor treatments to the individual. A person who has had a slow and downward progression of their ME or CFS would likely benefit from different treatments/ recommendations than an individual who has demonstrated a wax and wane and unpredictable illness course. Additional research on pretrajectory, trajectory onset, and illness course patterns may also lead to the identification of early signs/symptoms and methods that focus on early intervention/prevention of the illness.
Visual illness timelines have been used previously in health research. A study by Lunney et al. [67] utilized graphical timelines to show that end of life functioning was highly variable across four different types of end of life trajectories (e.g. sudden death, cancer death, death from organ failure, and frailty). The authors suggested that the health trajectories can be used to tailor end of life interventions to the individual in order to improve overall quality of life. Another study by [68] used graphical timelines to show the trajectory of breathlessness in individuals with chronic obstructive pulmonary disease (COPD). Their findings showed that those who have worsening or fluctuating breathlessness trajectories may have a more difficult time predicting and controlling the symptom and likely require individualized treatment. Similar methods could be used for understanding illness patterns in individuals with ME and CFS.
Further still, illness graphs can be used as communication tools for patients who are meeting with new health care providers and who might not have a full understanding of a patient’s illness history and course. Patients can use the graphs to provide their health care providers with a sense of how their symptoms have changed over time, the various treatments that they have tried, and whether the illness is worsening. Cognitive difficulties including word finding and brain fog difficulties are cardinal symptoms of ME and CFS. Many participants in the current study discussed how these problems make it difficult to have a conversation. Therefore, a visual graph of their illness trajectory may be easier to share with a health provider than providing a verbal account of their illness history. Additionally, the sharing and co-construction of an illness history between patient and health care provider may increase the degree of support and validation that patients receive at their medical visits. Kleinman [69] refers to a patient care model called “empathic witnessing” in which there is an “existential commitment to be with the sick person and to facilitate his or her building of an illness narrative that will make sense of and give value to the experience” (p. 54). This act of witnessing and sharing an illness narrative has been described as a co-construction between patient and physician [70] and is theorized as a way to promote validation and empathic interactions between patients and health care providers. Corbin et al. discuss how patients who present to a health clinic for pain management are often coming with years of health experiences that influence how they react to treatment and to their providers. Specifically, they discuss how a patient who is branded as “difficult” by a health care provider is likely reacting to past experiences and interactions with previous providers as cited in [65]. Knowledge of a person’s illness history and previous interactions within the healthcare system can help providers shape the attributions they make about patients and it can lead to increased empathy and support. Given the lack of support that many participants in the current study experienced from health providers, friends, and family members, the illness timelines may provide a way for health care providers to better connect with their patients on an empathic level. the samples were not selected through random assignment; thus, participants in the current convenience sample may have different qualities than a more representative population of individuals affected by ME and CFS. For instance, participants were largely White women and middle aged. Based on earlier research [71], CFS occurs at higher rates in African-American and Latino samples. Another limitation of the current study is the retrospective nature of the self-report method and qualitative interviews. Participants provided self-reported information on their illness onset, which in many cases occurred many years prior. It is possible that their responses are biased due to recall difficulties that occur when remembering remote events. While the potential for recall bias is a limitation of the current study, highly salient information is often recalled more accurately than less salient information [72-74]. The majority of individuals in the current study described their onset period as a “life changing” and a salient period in their life.
There is significant variability in the ME and CFS literature with regard to the way in which sudden versus gradual onset is defined. Furthermore, differences across samples (e.g., community based versus tertiary) and across case definitions used to select for ME and CFS increases the difficulty in comparing the results of the current study with previous findings. The current study defined sudden onset as occurring between 24 hours to one month, and gradual as any onset greater than one month. This decision was based on previous research, in which sudden onset was defined as up to one month [75]. Unfortunately there is not yet a uniform definition for mode of illness onset, thus contributing to the wide variety of onset definitions in the literature. For example, Salit [42] defined sudden as occurring in conjuction with an “acute precipitating event,” whereas gradual onset was defined as any onset that did not have an “acute precipitating event” (p. 61). Similarly DeLuca and colleagues [15] described sudden onset as viral with a clear onset date and gradual onset was defined as slowly progressing over “weeks to several months or greater” (p. 85). Overall, differences in methodology, onset categorization, diagnostic criteria, and sample selection across studies make the comparability of onset differentiation on key factors of ME and CFS a complicated endeavor.
In summary, the qualitative findings and illness timeline graphs revealed that ME and CFS onset experiences are likely more complex than the dichotomous sudden versus gradual categorization that is ubiquitous in the ME and CFS literature. The findings of the current study are aligned with recommendations for future research that were suggested by Ray and colleagues [22]. Ray et al. [22] recommended that future studies should involve the investigation of different etiological onset patterns as well as the potential interactions between various causal factors (e.g. infection, stress, and overexertion) and the degree of risk that these pose for the future development of the illness. In addition to these recommendations, the current study findings suggest that onset and illness progression may be even more dynamic and complex than it has been previously described in the literature. Specifically, the onset patterns revealed in the current study do not appear to be mutually exclusive (e.g. patients may identify with both sudden and steadily progressing/gradual onsets). These findings point to the need for further assessment of illness onset patterns and progression on larger and more representative populations. Furthermore, it is recommended that surveys designed to assess the onset experience include more than one question to assess mode of illness onset and illness patterns. In order to capture the complex ME and CFS onset experiences, surveys might include questions that assess the period of time in which an individual’s first symptoms were experienced, whether the individual perceived their onset as sudden (regardless of the period of time that their first symptoms developed), whether the illness progression was initially noticed, and whether it progressed in the form of a steady progression or a cyclical “wax and wane” pattern. Furthermore, it would be beneficial to ask individuals about how long it took in days, months, and/or years until they experienced a “definitive turning point” in which functioning significantly decreased, as well as when they experienced a period of reflection in which the illness was perceived as something more serious than a typical sickness such as the flu.
Given the potential for recall bias when conducting a retrospective investigation of ME and CFS onset patterns, future studies might employ prospective methods in order to track individuals’ onset patterns as they develop. There has been an influx of ecological momentary assessment techniques in health research for the purpose of tracking health symptoms and conditions over time [76]. These real time methods for tracking health could also be used to construct visual graphs that map symptoms and functioning over time, which could lead to interventions aimed at prevention. Furthermore, the current study revealed that many participants realized too late that exertion worsened their illness. If illness timeline graphs were developed in real-time, early intervention may be possible, which may also lead to better health outcomes. Future studies might also investigate ways to develop efficient methods that allow patients to develop illness timelines graphs themselves. This would allow them to visualize and monitor their illness trajectory as well as communicate their illness experience to health care providers. Lastly, future studies that focus on onset assessment should utilize survey questions that more closely assesses onset experiences. Specifically, an onset survey should include questions regarding the period of time in which first symptoms are developed, whether participants perceive their onset as sudden, steadily progressing, waxing and waning, or improving, and at what point in the patient’s illness course they realize the need to seek specialized medical treatment. Additionally, the survey should assess for early signs and symptoms, as these may be important risk factors for the development of the illness. A survey specifically designed to assess onset patterns in a large and representative sample of individuals with ME and CFS could provide valuable information about the prevalence of different onset patterns and the potential for these patterns to differentiate patients on key factors including etiology, illness course, and prognosis.
References
- Anderson, JS, Ferrans CE. The quality of life of persons with chronic fatigue syndrome. J. Nerv. Ment. Dis, 185(6), 359-367 (1997).
- Fukuda K, Straus SE, Hickie I, et al. I. C. F. S. S.The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 121 (12), 953-959 (1994).
- Carruthers BM, van de Sande MI, De Meirleir KL,et al. Myalgic encephalomyelitis: International consensus criteria. J. Intern. Med., 270(4), 327-338 (2011).
- Carruthers BM, Jain AK, De Meirleir KL, etal. Myalgic encephalomyelitis/chronic fatigue syndrome: Clinical working case defintion, diagnostic and treatment protocols. J. ChronicFatigue Syndrome, 11(1), 7-116 (2003).
- Shepherd S, Chaudhuri A. ME/CFS/PVFS. An exploration of the key clinical issues. Essex, UK: ME Association (2001).
- IOM (Institute of Medicine) Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. Washington, DC: The National Acadaemies; 2015.
- Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann. Intern. Med, 108(3), 387-389 (1988).
- Sharpe MC, Archard LC, Banatvala JE, et al. A report--chronic fatigue syndrome: Guidelines for research. J. Royal Society of Med., 84(2), 118 (1991).
- Reeves WC, Lloyd A, Vernon SD, et al. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC. Health. Serv. Res. 3(25) (2003).
- Ramsay AM, David AS, Wessely S, et al. Myalgic encephalomyelitis, or what? The. Lancet. 332(8602), 100-101 (1988).
- Dowsett EG, Ramsay AM, McCartney RA, Bell EJ. Myalgic encephalomyelitis--a persistent enteroviral infection? Postgrad. Med. J, 66(777), 526-530 (1990).
- Hyde BM. The nightingale definition of Myalgic encephalomyelitis (M.E.). Ottawa, ON: The Nightingale Research Foundation (2007).
- Bell DS. The doctor’s guide to chronic fatigue syndrome: understanding, treating, and living with CFIDS. Da Capo Press (1995).
- Levine PH, Jacobson S, Pocinki AG, Clinical, Epidemiologic, and Virologic Studies in Four Clusters of the Chronic Fatigue Syndrome. Arch. Intern. Med, 152(8), 1611-1616 (1992).
- DeLucaJ, Johnson SK, Ellis SP, Natelson BH.Sudden vs gradual onset of chronic fatigue syndrome differentiates individuals on cognitive and psychiatric measures. J. Psychiatr. Res. 31, 83- 90 (1997).
- Zhang Q, Natelson B, Ottenweller J, et al. Chronic fatigue syndrome beginning suddenly occurs seasonally over the year. Chronobiology International: The J. Biological & Medical Rhythm Res., 17(1), 95 (2000).
- Mawle AC, Nisenbaum R, Dobbins J, et al. Immune responses associated with chronic fatigue syndrome: a case-control study. J. Infect. Dis,175(1), 136-141 (1997).
- Cukor D, Sky LT, Natelson BH. Psychiatric comorbidity and somatic distress in sudden and gradual onset chronic fatigue syndrome. J. Chronic Fatigue Syndrome, 7(4), 33-44 (2000).
- Linder R, Dinser R, Wagner M, et al. Generation of classification criteria for chronic fatigue syndrome using an artificial neural network and traditional criteria set. In vivo, 16(1), 37-43 (2002).
- Jason LA, Corradi K, Torres-Harding S, et al. Chronicfatigue syndrome: the need for subtypes. Neuropsychology Review, 15(1), 29-58 (2005).
- McCue P. CFS/ME and mental health diagnoses:a qualitative approach to assessing the experiences of women who have now recovered. Clin. Eff. Nurs. 8(3), 194-201 (2004).
- Ray C, Jeffefues S, Weir W, Making sense of chronic fatigue syndrome: Patients’ accounts of onset. Psychology and Health, 13(1), 99- 109(1998).
- Roth JA Timetables; structuring the passage of time in hospital treatment and other careers. Bobbs-Merrill (1963).
- Charmaz K. Good days, bad days: The self and chronic illness in time. Rutgers University Press (1991).
- Hughes JL Illness narrative and chronic fatigue syndrome/myalgic encephalomyelitis: A review. Br. J. Occup. Ther. 65(1), 9-14 (2002).
- Jason LA, So S, Brown, et al. Testretest reliability of the DePaul Symptom Questionnaire. Fatigue : Biomedicine, Health & Behavior, 3(1), 16-32 (2015).
- Jason LA, Brown A, Evans M, et al. Contrasting chronic fatigue syndrome versus myalgic encephalomyelitis/chronic fatiguesyndrome. Fatigue: Biomedicine. Health & Behavior, 1, 168–183 (2013).
- Jason LA, Sunnquist M, Brown A. et al. Are Myalgic Encephalomyelitis and chronic fatigue syndrome different illnesses? A preliminary analysis. J. Health. Psychol. 21(1), 3–15 (2016).
- Torres-Harding SR, Jason LA, Cane V, et al. Physicians’ diagnoses of psychiatric disorders for persons with chronic fatigue syndrome. Int. J. Psychiatry. Med. 32, 109–124 (2002).
- Patton MQ. Qualitative evaluation and research methods. SAGE Publications, Inc (2002).
- Sobell LC, Sobell MB Timeline follow-back. In Measuring alcohol consumption Humana Press pp: 41-72. (1992).
- Corner J, Hopkinson J, Fitzsimmons D et al. Is late diagnosis of lung cancer inevitable? Interview study of patients’ recollections of symptoms before diagnosis. Thorax, 60(4), 314- 319 (2005).
- Zhang Y, Wildemuth BM. Qualitative analysis of content. In B. Wildemuth (Ed.), Applications of Social Research Methods to Questions in Information and Library Science Westport, CT: Libraries Unlimited pp: 308-319 (2009).
- Corbin J, Strauss A. (Eds.). Basics of qualitative research: Techniques and procedures for developing grounded theory. Sage (2008).
- Campbell JL, Quincy C, Osserman J, Pedersen OK. Coding in-depth semistructured interviews problems of unitization and intercoder reliability and agreement. Sociol. Methods. Res. 0049124113500475 (2013).
- Okma Keulen P, Hopman Rock M. The onset of generalized osteoarthritis in older women: a qualitative approach. Arthritis. Care. Res. 45(2), 183-190 (2001).
- Jason LA, Evans M, Brown A, et al. Chronic Fatigue Syndrome Versus Sudden Onset Myalgic Encephalomyelitis. J. Prev. Interv. Community. 43(1), 62-77 (2015).
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form health survey (SF-36): Conceptual framework and item selection. Medical Care 30(6), 473-483 (1992).
- Buchwald D, Pearlman T, Umali J et al. Functional status in patients with chronic fatigue syndrome, other fatiguing illnesses, and healthy individuals. Am. J. Med. 101(4), 364- 370 (1996).
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In C. M. MacLeod (Ed.) Evaluation of chemotherapeutic agents. New York: Columbia University Press pp: 191-205 (1949).
- Broderick G, Fuite J, Kreitz A, et al. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain. Behav. Immun. 24(7), 1209- 1217 (2010).
- Salit IE. Precipitating factors for the chronic fatigue syndrome. Journal of Psychiatric Research, 31, 59-65 (1997).
- MacDonald KL, Osterholm MT, LeDell KH, etal. A case-control study to assess possible triggers and cofactors in chronic fatigue syndrome. Am.J. Med, 100(5), 548-554 (1996).
- Ware, N. Society, mind and body in chronic fatigue syndrome: an anthropological view. Chronic fatigue syndrome 173, 62-82 (1993).
- Lovell DM. Chronic fatigue syndrome among overseas development workers: a qualitative study. J. Travel. Med 6(1), 16-23 (1999).
- Evans M, Barry M, Im Y, et al. An investigation of symptoms predating CFS onset. Journal of prevention & intervention in the community, 43(1), 54-61 (2015).
- Buchwald D, Garrity D. Comparison of patients with chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivities. Arch. Intern. Med. 154(18), 2049-2053 (1994).
- Whitehead WE, Palsson O, Jones KR Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology, 122(4), 1140-1156 (2002).
- Stewart JM, Gewitz MH. Orthostatic I ntolerance in adolescent chronic fatigue syndrome. Pediatrics, 103(1), 116-121 (1999).
- Spotila JM. Unraveling post-exertional malaise. [Weblog post]. Retrieved from
- Jason LA, Richman JA, Rademaker AW, et al. community-based study of chronic fatigue syndrome. Arch. Intern. Med. 159(18), 2129 (1999).
- Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J. Pain. 10, 1099-1112 (2009).
- Clements A, Sharpe M, Simkins S, Borril J, Hawton K Chronic fatigue syndrome: A qualitative investigation of patients’ beliefs about the illness. J Psychosom Res. 42, 615–624 (1997).
- Jason LA, Melrose H, Lerman A et al. Managing chronic fatigue syndrome: overview and case study. AAOHN journal: official J the Am Association of Occupational Health Nur, 47(1), 17-21 (1999).
- Jason LA, Evans M, Porter N, et al. The development of a revised Canadian myalgic encephalomyelitis chronic fatigue syndrome case definition. Am. J. Biochem. Biotechnol. 6(2), 120-135 (2010).
- Pesek JR, Jason LA, Taylor RR. An empirical investigation of the envelope theory. J. Hum. Behav. Soc. Environ. 3(1), 59-77 (2000).
- Fennell PA. The four progressive stages of the ME/CFS experience: A coping tool for patients. J. Chronic. Fatigue. Syndr. 1(3), 69-79 (1995).
- Tuck I, Wallace D. Chronic fatigue syndrome: a woman’s dilemma. Health. Care. Women. In 21(5), 457-466 (2000).
- Schweitzer R, Kelly B, Foran A, Terry D, Whiting J. Quality of life in chronic fatigue syndrome. Soc. Sci. Med. 41(10), 1367-1372 (1995).
- Dickson A, Knussen C, Flowers P. Stigma and the delegitimation experience: An interpretative phenomenological analysis of people living with chronic fatigue syndrome. Psychol. Health. Med. 22(7), 851-867 (2007).
- Drachler ML, Leite JC, Hooper L, et al. The expressed needs of people with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. BMC Public Health. 9(1), 458 (2009).
- Schoofs N, Bambini D, Ronning P, Bielak E, Woehl J. Death of a lifestyle: the effects of social support and healthcare support on the quality of life of persons with fibromyalgia and/or chronic fatigue syndrome. Orthop. Nurs. 23(6), 364-374 (2004).
- Jason LA, Witter E, Torres-Harding S. Chronic fatigue syndrome, coping, optimism and social support. J. Ment. Health. 12(2), 109-118 (2003).
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am. J. Psychiatry. 160 (2), 221-236 (2014).
- Corbin, Juliet MRN, DNSc, Strauss AA nursing model for chronic illness management based upon the trajectory framework. Scholarly Inquiry for Nursing Practice, 5(3), 155-174 (1991).
- Henly SJ, Wyman JF, Findorff MJ. Health and illness over time: The trajectory perspective in nursing science. Nursing research, 60(3), S5 (2011).
- Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA, 289(18), 2387-2392 (2003).
- Bausewein C, Booth S, Gysels M, et al. Individual breathlessness trajectories do not match summary trajectories in advanced cancer and chronic obstructive pulmonary disease: results from a longitudinal study. Palliative Medicine. 24(8), 777-786 (2010).
- Kleinman A. The illness narratives: Suffering, healing, and the human condition. Basic Books (1988).
- Verghese A. The Physician as Storyteller. Ann. Intern. Med. 135(11), 1012 (2001).
- Jason LA, Fennell PA, Klein S, et al. An investigation of the different phases of the CFS illness. J. Chronic. Fatigue. Syndr. 5(3-4), 35-54 (1999).
- Cannell CF, Marquis KH, Laurent A. A summary of studies of interviewing methodology. (DHEW Publication No. (HRA) 77-1343). Vital Health Statistics, Series 2, Number 69. Rockville, Md.: National Center for Health Statistics (1977).
- Dawson EG, Kanim LE, Sra P, et al. Low back pain recollection versus concurrent accounts: Outcomes analysis. Spine, 27(9), 984-993 (2002).
- Stull D, Leidy NK, Prasuraman B, Chassany O. Optimal recall periods for patient-reported outcomes: Challenges and potential solutions. Curr. Med. Res. Opin. 25(4), 929-942 (2009).
- Jason LA, Fennell PA, Taylor RR, Fricano G, Halpert JA. An empirical verification of the Fennell phases of the CFS illness. J. Chronic. Fatigue. Syndr. 6(1), 47-56 (2000).
- Steptoe A, Gibson EL, Hamer M, Wardle J. Neuroendocrine and cardiovascular correlates of positive affect measured by ecological momentary assessment and by questionnaire. Psychoneuroendocrinology, 32(1), 56-64 (2007).