Short Article - Interventional Cardiology (2012) Volume 4, Issue 4
New therapeutic option for therapy of refractory arterial hypertension: renal denervation
- Corresponding Author:
- Holger Nef
University of Giessen, Department of Cardiology
Klinikstr. 36, 35392 Giessen, Germany
Tel: +49 6419 855 6684
E-mail: holger.nef@me.com
Abstract
Keywords
renal denervation, resistent hypertension
Arterial hypertension represents a major risk factor for cardiovascular morbidity. The incidence of arterial hypertension in Europe is approximately 45%; however, there may be a large number of unreported cases. A total of A total of 5–15% of these patients have therapy-resistant arterial hypertension. This is defined as an insufficient reduction of systolic and diastolic blood pressure, despite treatment with at least three classes of antihypertensive drugs, including diuretics, at the maximum permissible doses, along with lifestyle changes [1,2]. The persistence of hypertensive blood pressure is partly regulated by the renal sympathetic nervous system [3,4]. Thus, renal sympathetic efferent and afferent nerves (Th10-L1), within and surrounding the walls of renal arteries, play a role in arterial hypertension [5–8]. The afferent sympathetic system is activated by rising adenosine levels and affects regulation of the neurohumoral axis by the CNS. The efferent sympathetic nervous system regulates renin release and renal blood flow. In addition, sympathetic activity modulates glucose metabolism and insulin sensitivity [9].
In the 1950s, a radical surgical method was developed for thoracic, abdominal and pelvic sympathetic nerve denervation. This method was successful in lowering blood pressure; however, it was associated with high perioperative morbidity and mortality [10]. Percutaneous renal sympathetic denervation is currently an interventional treatment option for patients with therapy-resistant arterial hypertension [11]. In this procedure, the sympathetic nervous system is modulated by selective denervation through radiofrequency (RF) ablation [12,13]. Before treatment, it is necessary to exclude a secondary origin of hypertension, including stenosis of the renal arteries and primary hyperaldosteronism. Imaging of the renal arteries can exclude hemodynamic stenosis or an abnormal anatomy of the arteries, while hormonal diagnostics are able to detect primary hyperaldosteronism, pheochromocytoma and Cushing’s syndrome. To rule out pseudo-resistance, taking ambulatory blood pressure measurements is recommended [11].
Renal denervation procedure
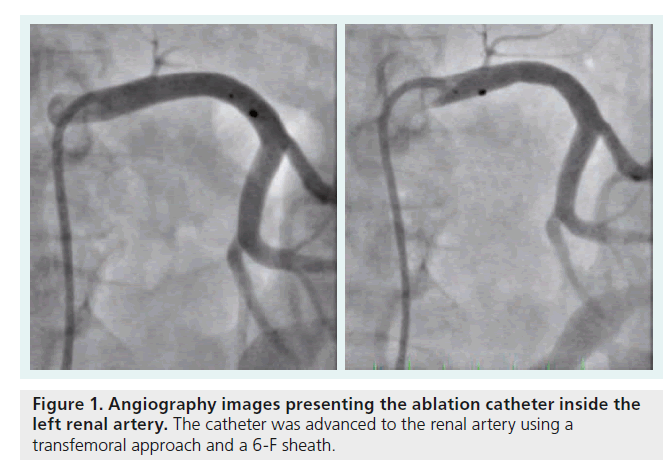
Percutaneous sympathetic renal denervation is performed using a transfemoral approach with a 6-F sheath via the femoral artery. The catheter is then advanced to the renal arteries. The procedure is performed using a specific ablation catheter (Symplicity® Catheter System™, Ardian, Medtronic Inc., CA, USA), with an electrode on the tip for ablation. The catheter is connected to a RF generator. The diameter of the renal artery must be at least 4 mm and the length at least 20 mm to avoid damage due to the temperature. The first RF ablation point should be placed proximal to the first bifurcation of the renal arteries. The catheter should be retracted by approximately 5 mm and the single ablation points will be set in a circumferential formation. In total, depending on the anatomical conditions, RF ablation will be performed four to six times within each renal artery (Figure 1), in the anterior, superior, posterior and inferior positions across the full circumference. The power delivery is controlled by a connected generator and limited to a maximum of 8 W and 40–70°C. The time for energy delivery is limited to 2 min. Systemic anticoagulation is necessary during the intervention and patients are treated with acetylsalicylic acid (100 mg), as an antiplatelet therapy, for 3 months.
Clinical trials
▪ Proof of principle study (HTN-1)
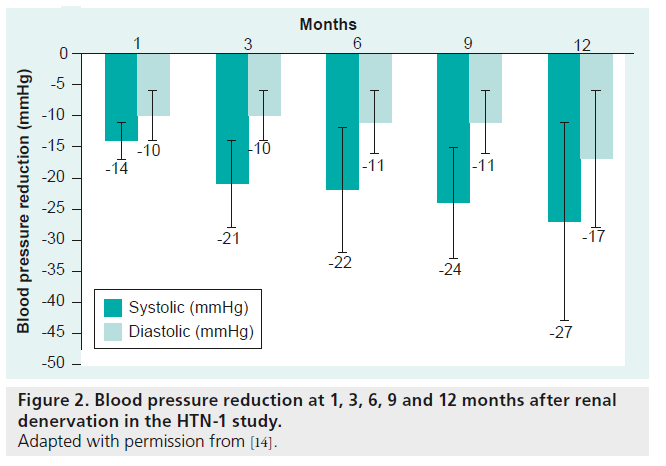
The safety and effectiveness of catheter-based sympathetic renal denervation was approved by a proof-of-principal study (HTN-1) [14]. The procedure was assessed in 45 patients and no complications occurred in 43 of them. Patients developed diffuse visceral pain due to the energy application; therefore, they were treated with narcotics and sedative drugs during the procedure. One patient underwent a renal arterial dissection during catheter placement and another patient had a pseudoaneurysm at the femoral artery. After the application of energy, focal edemas occurred at the renal arteries, which were not flow limiting. Single ablation points were applied in a circumferential formation to prevent blood flow limitations. After a period of 6 months, no stenosis of the renal arteries was detected by magnetic resonance angiogram. There was a significant reduction in systolic blood pressure (SBP), defined as >10 mmHg, after 4 weeks that was sustained for 12 months (after 1 month: -14 mmHg; 3 months: -21 mmHg; 6 months: -22 mmHg; 9 months: -24 mmHg; and 12 months: –27 mmHg) (Figure 2). In addition to the office blood pressure measurement, ambulatory 24 h blood pressure values were measured in 20 patients. The differences between measuements using these two methods were documented after 12 months (office SBP reduction: 27 mmHg vs 24 h SBP reduction: 11 mmHg). There was no heart rate change compared with baseline at any time point [14].
Effects on blood pressure
▪ Symplicity II (HTN-2)
The blood pressure-lowering effect was further investigated by an international, randomized multicenter study. This study enrolled 106 patients with a systolic blood pressure of >160 mmHg (>150 mmHg for patients with Type 2 diabetes mellitus) despite intake of at least three antihypertensive drugs (including diuretics), at the maximum tolerable doses [11]. A total of 49 patients were randomized into the denervation group. Office blood pressure, creatinine and cystatin C were measured at the 4-week and 3- and 6-month follow-ups. In addition, the kidney arteries were imaged by ultrasound, MRI or CT angiogram at 6 months follow-up [11].
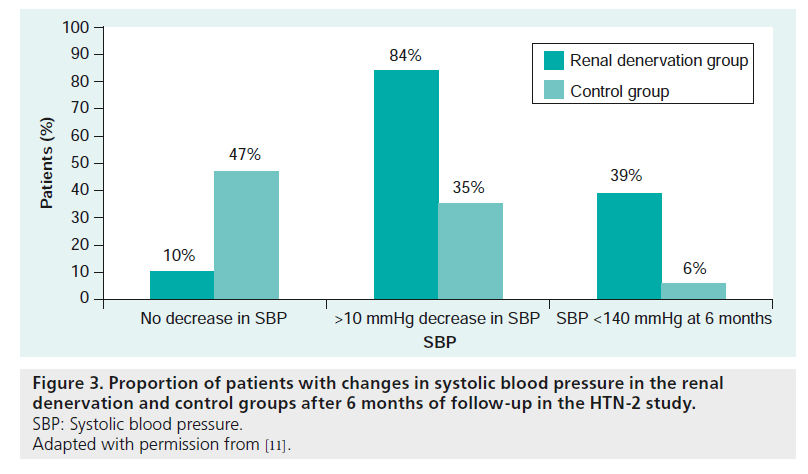
The systolic blood pressure was significantly reduced at all follow-up time points in more than 84% of the patients (Figure 3). Blood pressure was reduced 20 mmHg at 4 weeks, 24 mmHg at 3 months and 32 mmHg 6 months after renal denervation when using office blood pressure measurements. In 20 patients, ambulatory 24-h blood pressure measurements were made to document differences in the 6-month blood pressure assessment, which was revealed to vary substantially depending on the measurement method used (difference of 21/5 mmHg at the 6-month follow-up) [11]. The authors explained in a letter of correspondence that these differences may have occurred owing to incomplete blood pressure data and that 24-h blood pressure measurements were only completed in 20 of 49 patients. Data deficiency could have caused the mean ambulatory blood pressure values to become biased. It is also common that ambulatory clinical blood pressure changes are often lower than office blood pressure changes [12]. No serious complication was reported after the procedure. Imaging of the renal arteries 6 months after the denervation showed no evidence of renal artery stenosis, aneurysmatic dilatation or damage. However, one patient had an atherosclerotic lesion that may have progressed, but did not require intervention. The stenosis was not at a location where RF was applied [10,11]. There were no changes in renal function observed, even in cases with moderately reduced renal function, which suggests the procedure was safe. Renal denervation selectively reduced the efferent sympathetic activity, which was confirmed by a reduction of the noradrenalin levels in the spillover measurement. A following study demonstrated a significant blood pressure reduction in patients who were initially randomized to the control group and crossed over to the renal denervation group after 6 months [15].
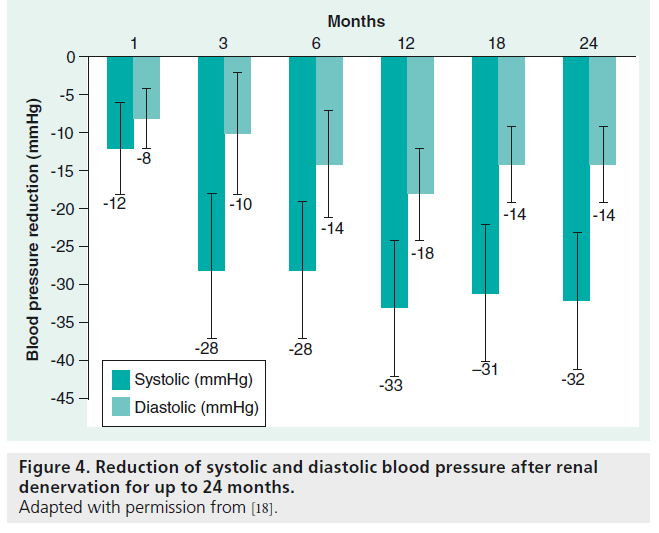
The persistence of the blood pressure-lowering effect was then examined and the blood pressure reduction was sustained for an observation period of 24 months [16]. This long-term proof-of-principle study included 153 patients. The inclusion and exclusion criteria were chosen according to the Symplicity HTN-2 Trial and the primary end point was a significant change in office blood pressure measurements. Follow-up visits were set for 1, 3, 6, 12, 18 and 24 months [15]. However, only 18 patients were observed for the full 24 months. Regarding the blood pressure- lowering effect, the mean SBP was reduced after renal sympathetic denervation at all time points (4 weeks: 20/10; 3 months: 24/11; 6 months: 25/11; 9 months: 23/11; 12 months: 26/14 and 24 months: 32/14 mmHg) (Figure 4). The use of central sympatholytic medications and an increased SBP before renal denervation were identified as predictors for significant blood pressure reduction. Sympathetic reinervation was considered to attenuate the long-term effect of blood pressure reduction after renal denervation [14]. Nerval regrowth of sympathertic fibers was described after heart transplantation [15]. However, regrowth of renal sympathetic nerve fibers has not been previously described in humans [17]. Furthermore, the consistent blood pressure reduction over a time period of 2 years makes renal sympathetic reinnervation less likely. Furthermore, there were no major complications reported, such as stenosis, aneurysm or cholesterol emboli after a post-interventional time period of 24 months [18].
The results of this long-term proof-of-concept study were limited because there was no comparison with a control group and only a small number of patients were followed for the full 24 months [18]. Yet the blood pressure reduction observed was consistent with the findings of the HTN-1 and HTN-2 Trials at 3, 6 and 12 months after renal denervation; therefore, the authors suggested the results would be similar if a greater number of patients had reached the 24-month follow up [18].
Recently presented data exposed that patients who were initially considered to be nonresponders finally responded after a follow-up time of 3 years (blood pressure reduction of >10 mmHg) [19]. A significant reduction of blood pressure variability was shown at 24-h ambulatory blood pressure monitoring at 6 months after the renal denervation procedure [20].
▪ Symplicity III (HTN-3)
The Symplicity HTN-3 study – a prospective, randomized, single-blind, masked procedure trial – will be published soon [21]. Primary end points will be defined as changes in office systolic blood pressure reduction at a 6-month follow-up. Furthermore, major adverse events such as allcause mortality, end-stage renal disease (estimated glomerular filtration rate < 15 ml/min/m2 or need for renal replacement therapy), significant embolic events with end organ damage and renal artery perforation and/or dissection requiring intervention, as well as vascular complications requiring surgical repair, will be examined. Patients will be blinded and procedural pain will be treated with opiates and sedatives. Subjects will not be informed about procedural details and duration of the procedure. In the renal denervation group, the procedure will be performed according to standard clinical practice and controls will undergo only an angiography of the renal arteries [21].
More international trials on heart failure, insulin resistance, chronic kidney disease and endstage renal disease, as well as sleep apnea, will be expected in the near future.
Physiological background
The effects of the CNS on the kidneys are regulated by efferent and afferent sympathetic fibers that are located within and around the walls of the renal arteries. The efferent sympathetic fibers increase the renin secretion and activate the renin-angiotensin–aldosterone system, leading to water and sodium retention. The afferent sympathetic fibers, activated from renal mechano- and chemo-receptors, influence the general sympathetic activity via feedback mechanisms involving the vascular tone [3–5,8]. Furthermore, the sympathetic nervous system modulates glucose metabolism, insulin sensitivity and metabolic syndrome. Regarding this aspect, the sympathetic nervous system induces gluconeogenesis, increases glucagon secretion and aggravates the glucose tolerance. Insulin itself increases the sympathetic activity. The effect of moxonidin on glucose metabolism was previously described. Owing to reduced sympathetic activity, moxonidin influences glucose metabolism and consequently affects diabetes mellitus. After renal denervation, reduction of sympathetic activity was detected by the noradrenaline spill-over measurement [14]. Hence, the positive effect of the renal denervation on glucose metabolism could be shown; however, the exact mechanism has not yet been identified. Further clinical trials need to be conducted.
As obstructive sleep apnea syndrome (SAS) is thought to be caused by an activated sympathetic system or sympathetic overdrive, the effect of renal denervation on the syndrome was examined [22], although no mechanism has been identified as of yet. It is hypothesized that chronic fluid overload aggravates sleep apnea. Thus, a reduction of the efferent sympathetic activity by renal denervation will reduce the total body fluid and the venous pooling, and alleviate the severity of SAS. In addition, renal denervation modulates the central control of baroreceptor and chemoreceptor activity and is considered to influence sleep apnea [22]. Furthermore, the reduced sympathetic activity affects the central ventilatory and airway control. While these mechanisms seem to play a role in the maintenance of SAS, the exact mechanisms are still unclear.
Effects on glucose metabolism
Resistant arterial hypertension is associated with metabolic comorbidities, such as insulin resistance and dysregulation of glucose metabolism due to sympathetic over activity. After renal denervation, sympathetic activity was reduced and positive effects on glucose metabolism, such as insulin production associated with blood pressure reduction, were observed in a prospective study that included 50 patients [9]. The inclusion and exclusion criteria were equal to those in the HTN-2 Trial and 37 patients were randomized to the treatment group. Fasting glucose, insulin, C-peptide and HbA1c were measured before, as well as 1 and 3 months after, renal denervation. The oral glucose tolerance test was performed 1 and 3 months post-procedure. The treatment group demonstrated a significant reduction in fasting glucose after 3 months (118 ± 3.4 mg/dl to 108 ± 3.8 mg/dl; p = 0.039). Furthermore, insulin levels decreased (from 20.8 ± 3 μIU/ml to 9.3 ± 2.5 μIU/ml; p = 0.006) along with C-peptin levels (from 5.3 ± 0.6 ng/ml to 3.9 ± ng/ml; p = 0.002). In comparison, no significant differences were observed in the control group after the 3 months of follow-up [9].
Effects on renal function
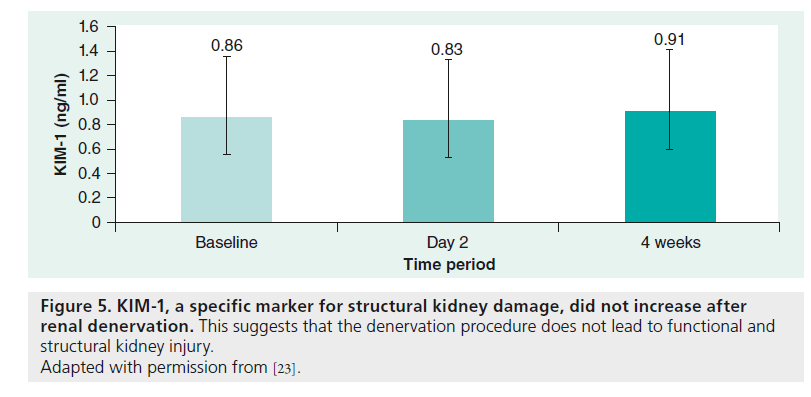
Investigations on renal denervation excluded patients with progressive chronic kidney disease (estimated glomerular filtration rate <45 ml/min/1.73 m²) because of potential kidney damage after ablation [11]. Based on our own experience, the renal function (estimated glomerular filtration rate) tends to increase. However, it is unknown whether this is an effect of the blood pressure reduction or a direct effect of the reduced sympathetic nerve system of the kidneys. Cholesterol emboli or thermal damage did not appear or were not described [18]. In a recent study, KIM-1, a sensitive, specific and highly predictive marker for damage of the proximal renal tubule system, was examined to analyze acute kidney injury after renal denervation. KIM-1 did not increase after renal denervation (baseline: 0.86 ng/ml [interquartile range: 0.52–1.88]; second day: 0.83 ng/ml; at 4 weeks: 0.91 ng/ml [interquartile range: 0.63–1.35]; p = 0.29), which suggests that the denervation procedure does not lead to functional and structural kidney injury (Figure 5) [23].
Effects on sleep apnea
Obstructive SAS is associated with cardiovascular risk factors, such as resistant hypertension, insulin resistance and metabolic syndrome, as well as with cardiovascular diseases. It was considered that obstructive SAS could be a consequence of, as well as a reason for, increased sympathetic activity [22]. Obstructive SAS is reported to occur in <80% of patients with resistant hypertension [24–27].
A recent study showed that there is a correlation between reduced blood pressure levels and improvements in sleep apnea [25]. In this study, the diagnosis of SAS was made by a standard attended polysomnography according to the standard criteria of the American Academy of Sleep Medicine Task Force. The electro-encephalogram, electrooculogram and electromyogram of chin muscles, as well as an ECG were simultaneously recorded. The apnea/hypopnea index (AHI) was calculated using the number of apneic and hypopneic episodes per hour of sleep and the oxygen desaturation levels. Obstructive SAS was diagnosed in eight of the ten included patients, and two patients had mixed SAS. According to the AHI, five patients had mild SAS (AHI < 15 per h) and five patients had moderate SAS (AHI > 15 per h). After 3 months of follow-up, no significant changes in AHI were observed, although there was a tendency (p = 0.059) towards an improvement in AHI at 6-month follow-up in eight of ten patients. However, no changes were observed in the oxygen desaturation index. A significant correlation was found between an improvement in AHI and daytime/night-time blood pressure reduction in the 24-h ambulatory period [22].
The association between catheter-based blood pressure reduction and improvement in sleep apnea has not yet been evaluated and the mechanism for the positive effect on SAS after renal denervation was not identified in the observational study discussed above. It was suggested that the reduced fluid retention after renal denervation might play an important role. Blood pressure reduction might affect SAS and reduced sympathetic activity could potentially affect the central ventilatory and airway control mechanisms.
Effect on the cardiorespiratory response
Reduced SBP was demonstrated during exercise after renal denervation [27]. Therefore, cardiopulmonary exercise was evaluated in 46 patients and the following vital parameters were recorded: heart frequency and rhythm by 12 lead ECGs, maximum work rate, peak oxygen consumption and oxygen uptake at the anaerobic threshold, minute ventilation, ventilatory efficiency for carbon dioxide (VE/VCO2) slope, and the respiratory exchange ratio. The maximum work rate increased slightly, along with a reduced SBP during the exercise stages. Furthermore, the heart rate recovery improved compared with the baseline and dysregulation of blood pressure or heart rate did not occur after blood pressure adaption. Despite a slight improvement in the maximum work rate, oxygen consumption (VO2 peak) was not significantly altered. In addition, there were no changes in minute ventilation or ventilatory efficiency after renal denervation [27].
Effect on left ventricular function & remodeling
The effect of the sympathetic denervation on left ventricular (LV) function and hypertrophy was evaluated in a recent study [28]. Transthoracic echocardiography was performed in 64 patients, at baseline and after 1- and 6-month followups. A total of 18 of 46 patients that underwent renal denervation were compared with a control group. The LV mass was calculated using the Devereux formula and indexed to the body surface area [29,30]. In addition, the relative wall thickness was measured. In the treatment group, the LV mass index significantly decreased from baseline at 3- and 6-month follow-up (p < 0.001). LV hypertrophy was most evident when there was regression of the LV mass, and the LV mass index was noticeably reduced. The systolic LV function significantly improved after renal denervation and was associated with a significant increase in the LV ejection fraction (baseline: 63.1 ± 8.1% vs 70.1 ± 11.5% at 6 months; p < 0.001) [28]. In addition, diastolic dysfunction, myocardial relaxation and end diastolic pressure improved after renal denervation. These parameters indicate that the renal sympathetic denervation had a positive effect on cardiac remodeling and a prognostic benefit in patients with therapy-refractory arterial hypertension. An improvement in the diastolic function was observed in correlation with regression of the LV mass [29].
Conclusion
Renal denervation, by selective ablation of the renal sympathetic efferent and afferent nervous systems, represents a new treatment option for patients with resistant arterial hypertension. In addition to the blood pressure reduction, the reduced sympathetic activity also had positive effects on glucose metabolism, such as insulin production, and sleep apnea. It was demonstrated that the blood pressure reduction was sustained for an observation period of 24 months. No major complications, such as stenosis or aneurysm of the renal arteries or cholesterol emboli, were described. This new therapy option had no effect on some patients (8–16%) and predicting parameters have not yet been described in detail for nonresponders. It is still unclear which parameters or biomarkers can identify patients who will benefit from renal denervation. Further clinical trials in a large population are necessary to obtain a better understanding of these issues.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- Mancia G, De Backer G, Dominiczak Aet al. 2007 Guidelines for the managementof arterial hypertension: The Task Force forthe Management of Arterial Hypertension ofthe European Society of Hypertension(ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 28,1462–1536 (2007).
- Deutsche Hochdruckliga e.V. DHL®.Leitlinien zur Behandlung der arteriellenHypertonie. Nieren- und Hochdruckkrankheiten 38, 137–188 (2009).
- Kopp UC, Cicha MZ, Smith LA et al.Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of a1- and a2-adrenoceptors on renal sensory nerve fibers.Am. J. Physiol. Regul. Integr. Comp. Physiol.293, R1561–R1572 (2007).
- Hausberg M, Kosch M, Harmelink P et al.Sympathetic nerve activity in end stage renaldisease. Circulation 106, 1974–1979 (2002).
- DiBona GF. Sympathetic nervous system andthe kidney in hypertension. Curr. Opin. Nephrol. Hypertens. 11, 197–200 (2002).
- DiBona GF, Kopp UC. Neural control of renalfunction. Physiol. Rev. 77, 75–197 (1997).
- Esler M, Jennings G, Korner P et al.Assessment of human sympathetic nervoussystem activity from measurements ofnorepinephrine turnover. Hypertension 11,3–20 (1988).
- Schlaich MP, Lambert E, Kaye DM et al.Sympathetic augmentation in hypertension:role of nerve firing, norepinephrine reuptake,and angiotensin neuromodulation.Hypertension 43, 169–175 (2004).
- Mahfoud F, Schlaich M, Kindermann I et al.Effect of renal sympathetic denervation onglucose metabolism in patients with resistanthypertension: a pilot study. Circulation 123,1940–1946 (2011).
- Smithwick RH, Thompson JE.Splanchnicectomy for essential hypertension;results in 1,266 cases. JAMA 152(16),1501–1504 (1953).
- Symplicity HTN-2 Investigators. Renalsympathetic denervation in patients withtreatment-resistant hypertension (TheSymplicity HTN-2 Trial): a randomisedcontrolled trial. Lancet 376, 1903–1909(2010).
- O’Brien E. Renal sympathetic denervationfor resistant hypertension. Lancet 373(9681),2109–2110 (2009).
- Schlaich MP, Sobotka PA, Krum H,Lambert E, Esler MD. Renal sympatheticnerveablation for uncontrolled hypertension.N. Engl. J. Med. 361, 932–934 (2009).
- Krum H, Schlaich M, Whitbourn R et al.Catheter-based renal sympatheticdenervation for resistant hypertension:a multicentre safety and proof-of-principlecohort study. Lancet 373, 1275–1281(2009).
- Hansen JM, Abildgaard U, Fogh-Andersen N et al. The transplanted human kidney does not achieve functionalreinnervation. Clin Sci. 87, 13–20 (1994).
- Esler MD, Krum H, Schlaich M,Schmieder R, Bohm M, Sobotka P. Renalsympathetic denervation for treatment ofresistent hypertension: one year results fromthe symplicity HTN-2 randomized controlledtrial. J. Am. Coll. Cardiol. 59, e1705 (2012).
- Kaye DM, Esler M, Kingwell B,McPherson G, Esmore D, Jennings G.Functional and neurochemical evidence forpartial cardiac sympathetic reinnervationafter cardiac transplantation in humans.Circulation 88, 1110–1118 (1993).
- Symplicity HTN-1 Investigators. Catheterbasedrenal sympathetic denervation forresistant hypertension durability of bloodpressure reduction out to 24 months.Hypertension 57, 911–917 (2011).
- Krum H, Barman N, Schlaich M, Sobotka P,Esler M, Mahfoud F. Long term follow-up ofcatheter based renal sympathetic denervationfor resisteant hypertension confirms durableblood pressure reduction. J. Am. Coll. Cardiol.59, e1704 (2012).
- Zuern CS, Rizas KD, Eick C et al. Effects ofrenal sympathetic denervation on 24-hourblood pressure variability. Front. Physiol. 3,134 (2012).
- Kandzari DE, Bhatt DL, Sobotka PA et al.Catheter-based renal denervation for resistanthypertension: rationale and design of thesymplicity HTN-3 trial. Clin. Cardiol.doi:10.1002/clc.22008 (2012) (Epub ahead ofprint).
- Witkowski A, Prejbisz A, Florczak E et al.Effects of renal sympathetic denervation onblood pressure, sleep apnea course, andglycemic control in patients with resistanthypertension and sleep apnea. Hypertension58, 559–565 (2011).
- Dörr O, Liebetrau C, Möllmann H et al.Kidney-injury-molecule-1 (KIM-1) as abiomarker for damage in proximal renal tublesystem after renal denervation. J. Am. Coll. Cardiol. 59, e1717 (2012).
- Logan AG, Perlikowski SM, Mente A et al.High prevalence of unrecognized sleepapnoea in drug-resistant hypertension.J. Hypertens. 19, 2271–2277 (2001).
- Somers VK, White DP, Amin R et al. Sleepapnea and cardiovascular disease: anAmerican Heart Association/AmericanCollege of Cardiology Foundation ScientificStatement from the American HeartAssociation Council for High Blood PressureResearch Professional Education Committee,Council on Clinical Cardiology, StrokeCouncil, and Council on CardiovascularNursing, in collaboration with the NationalHeart, Lung, and Blood Institute NationalCenter on Sleep Disorders Research (NationalInstitutes of Health). Circulation 118,1080–1111 (2008).
- Baguet JP, Barone-Rochette G, Pepin JL.Hypertension and obstructive sleep apnoeasyndrome: current perspectives. J. Hum. Hypertens. 23, 431–443 (2009).
- Ukena C, Mahfoud F, Kindermann I et al.Cardiorespiratory response to exercise afterrenal sympathetic denervation in patientswith resistant hypertension. J. Am. Coll. Cardiol. 58(11), 1176–1182 (2011).
- 28 Brandt MC, Mahfoud F, Reda S et al. Renalsympathetic denervation reduces leftventricular hypertrophy and improvescardiac function in patients with resistanthypertension. J. Am. Coll. Cardiol. 59,901–909 (2012).
- Lang RM, Bierig M, Devereux RB et al.Recommendations for chamberquantification: a report from theAmerican Society of Echocardiography’sGuidelines and Standards Committee andthe Chamber Quantification Writing Group.J. Am. Soc. Echocardiogr. 18, 1440–1463(2005).
- Devereux RB, Alonso DR, Lutas EM et al.Echocardiographic assessment of leftventricular hypertrophy: comparison tonecropsy findings. Am. J. Cardiol. 57,450–458 (1986).