Mini Review - Interventional Cardiology (2023)
New technologies in echocardiography enable timely management of NSTE-ACS today
- Corresponding Author:
- Fabio Capasso
Department of Cardiology and Cardiac Surgery, Casa di Cura San Michele, Maddaloni, Italy,
E-mail: 2fabiocap65@libero.it
Received date: 13-Nov-2023, Manuscript No. FMIC-23-119901; Editor assigned: 15-Nov-2023, PreQC No. FMIC-23-119901 (PQ); Reviewed date: 29-Nov-2023, QC No. FMIC-23-119901; Revised date: 06-Dec-2023, Manuscript No. FMIC-23-119901 (R); Published date: 13-Dec-2023, DOI: 10.37532/1755- 5310.2023.15(S20).502
Abstract
An occluded vessel is the primary cause in one-third of Non-ST-segment Elevation Acute Coronary Syndrome (NSTE-ACS). Consequently, the identification of patients with a suspected occluded vessel as a distinct pathological scenario within the NSTE- ACS spectrum assumes essential importance to avert potentially detrimental delays in reestablishing coronary blood flow. The lack of electrocardiographic sensitivity in diagnosing an Acute Coronary Occlusion (ACO) conflicts with the need to determine the optimal timing for invasive therapy in NSTE-ACS patients. Echocardiography is an effective imaging tool for evaluating global and regional left ventricular myocardial function but the reproducibility of its mainly used conventional parameters as the ejection fraction and regional motion score is poor, reducing the accuracy of clinical application, especially in the earliest stages of ischemia. Over the past two decades, 2D Speckle Tracking Echocardiography (2D-STE) has been shown to delineate accurately, by means of the regional Longitudinal Strain (LS) measurement, the Functional Risk Area (FRA) allowing identification of high risk NSTE-ACS patients, surpassing the capabilities of traditional echocardiography and Electrocardiogram (ECG) indicators. However, the loading dependence of the LS has been shown in many studies and was overcome more recently, by regional analysis of myocardial Pressure-Strain Loop (PSL) and Myocardial Work (MW) which implemented and empowered it, demonstrating greater specificity in determining the extent and localization of the FRA during an ACS. Particularly, a regional LS with shortening less than 14% and a myocardial work below 1700 mmHg% demonstrate high sensitivity and specificity in identifying segments affected by ischemia from the earliest stages.
Keywords
Non-ST-segment elevation acute coronary syndrome• Acute coronary occlusion• Functional risk area • Myocardial work
Introduction
Coronary artery disease manifests in a spectrum of ways, from silent ischemia and stable angina pectoris to Acute Coronary Syndrome (ACS) and death. The presence of acute ST-segment elevation in the Electrocardiogram (ECG) predicts Coronary Artery Occlusion (ACO) with high specificity [1], and it is recommended to initiate mechanical Percutaneous Coronary Intervention (PCI) or pharmacological reperfusion as early as possible [2]. However, the sensitivity of the ECG in diagnosing ACO is suboptimal, and 30% of ACO patients do not develop ST-segment elevation [3,4]. These patients are diagnosed with Non-ST-Segment Elevation Acute Coronary Syndrome (NSTE-ACS), a more heterogeneous patient group. The ST-segment elevation ECG phenotype commonly correlates with acute coronary thrombosis and is more common in transmural infarctions, whereas NSTE-ACS, defined as non-transmural infarctions, frequently occurs in the presence of severely narrowed but still patent coronary arteries.
However, numerous pathophysiological studies have shown that an occluded vessel is the primary cause in one-third of these patients. In recent years, the incidence and fatality rate of NSTE-ACS have gradually increased and tended to affect younger individuals. Urgent Percutaneous Coronary Intervention (PCI) is essential to restore normal coronary artery blood flow and should be performed as early as possible. In patients with a working diagnosis of ST-segment Elevation Myocardial Infarction (STEMI), a primary invasive strategy (i.e., immediate angiography and PCI as needed) is the preferred reperfusion therapy, provided it can be performed in a timely manner [5]. Recent guidelines recommend an immediate invasive strategy with emergency angiography and PCI if required for very high-risk NSTE-ACS patients. High-risk NSTE-ACS patients are recommended to undergo an inpatient invasive strategy and should be considered for an early invasive strategy (i.e., within 24 hours). For patients who do not meet any of the very high-risk or high-risk criteria (generally patients with clinical suspicion for NSTE-ACS and non-elevated troponins or patients with elevated troponins not meeting the criteria for MI), the strategy can be customised based on the degree of clinical suspicion [5]. Consequently, the identification of patients with a suspected occluded vessel as a distinct pathological scenario within the NSTE-ACS spectrum assumes paramount importance to avert potentially detrimental delays in reestablishing coronary blood flow. In fact, effectively and early reopening an acutely occluded coronary artery is an advisable treatment for NSTE- ACS, as it can restore myocardial perfusion and promptly improve the myocardial systolic function of patients. Timely recognition of these conditions holds significant clinical importance since they are amenable to urgent or early revascularization treatments, leading to improved long-term patient outcomes.
Literature Review
A new potential role of advanced echocardiography in identifying very high-risk NSTE-ACS patients beyond conventional diagnostic criteria
The recognized lack of electrocardiographic sensitivity in diagnosing ACO conflicts with the need to determine the optimal timing for invasive therapy in NSTE-ACS patients, which varies according to different risk cohorts [6]. Previous studies have shown that a 24- hour delay to PCI was an independent predictor of short and late mortality in patients with NSTE-ACS [7]. Therefore, additional diagnostic tools are needed to identify patients who require prompt reperfusion therapy. In recent years, the evaluation of left ventricular structure and function changes after an acute coronary occlusion and during the ischemic cascade has been a significant research focus. Echocardiography is an effective imaging method for evaluating global and regional left ventricular myocardial function due to its advantages of being noninvasive, easy to operate, cost-effective, and highly operable. For a long time, the evaluation of left ventricular systolic function has been essential for the initial diagnostic approach in the acute setting of coronary artery disease. However, Left Ventricular Ejection Fraction (LVEF) remains the most commonly used parameter but has a well-recognized limitation of subjectivity. At the same time, the echocardiographic analysis of regional myocardial motion is often affected by the pull of surrounding tissues, resulting in segmental tethering. Therefore, the reproducibility of ejection fraction and regional motion score is poor, reducing the accuracy of clinical application, especially in the earliest stages of ischemia. In fact, it is known that the first myocardial fibers involved in ischemia are longitudinal subendocardial ones, representing the majority of the cardiac wall structure, which leads to an early longitudinal myocardial regional shortening impairment. The earliest LV regional longitudinal contraction abnormalities can be missed in the visual assessment of 2D-echocardiographic analysis, which is better at detecting radial motion but less effective at detecting the later compromise of other myocardial layers (i.e., mesocardial circumferential and epicardial helical fibers). Furthermore, the mesocardial layers’ fibers contribute to compensating for the initial LVEF impairment by enhancing circumferential strain in the earliest phases of an ischemic injury, appearing to preserve systolic function of the Left Ventricle (LV). In contemporary clinical practice, the use of advanced echocardiographic technologies, such as 2D speckle tracking analysis, has enabled the rapid and noninvasive identification of very high-risk phenotypes in patients with Non-ST Elevation Acute Coronary Syndrome (NSTE-ACS). ACO causes ischemia and systolic dysfunction in the area known as the ‘ischemic risk area,’ later defined as the ‘Functional Risk Area’ (FRA) by Eek et al. [8], which is an independent predictor of death and a major determinant of final infarct size in patients with ACO [9,10]. Experimental studies have shown a good correlation between the FRA and regional systolic dysfunction [11,12]. Regional strain analysis by Speckle Tracking Echocardiography (STE) has previously identified patients with ACO with good sensitivity. STE can be used to quantitatively analyze LV systolic function by tracking myocardial echo spots from the perspective of myocardial mechanics. In one case report, urgent revascularization by PCI in an NSTE-ACS patient, who had a low-risk score and mild electrocardiographic ischemic signs at admission, was guided by 2D-Strain analysis, leading to complete LV function recovery with normalized Global Longitudinal Strain (GLS) and minimal infarct size on contrast Magnetic Resonance Imaging (MRI) performed after 6 months [13]. In recent years, various new echocardiographic technologies have been proven to be applicable to evaluating myocardial regional abnormalities during ischemia. Over the past two decades, 2D Strain Imaging has emerged as a highly sensitive diagnostic tool for accurately delineating FRA within the context of ACS, surpassing the capabilities of traditional echocardiography and Electrocardiogram (ECG) indicators. However, the STE has good repeatability, but it is still affected by afterload dependency. Furthermore, previous studies have shown that increased afterload reduces the strain value, sometimes leading to a misinterpretation of the true systolic function of the left ventricle and an erroneous conclusion of reduced myocardial function [14,15]. More recently, in similar clinical scenarios, regional analysis of myocardial Pressure- Strain Loop (PSL) (Myocardial Work; GE Vingmed Ultrasound, Horten, Norway) has become a supplementary tool to 2D Strain. Noninvasive left ventricular PSL is a new technology based on two-dimensional Speckle-Tracking Echocardiography (2D-STE) in recent years. The Myocardial Work (MW) obtained by the PSL provides a new parameter for quantitatively evaluating LV systolic function from the perspective of myocardial mechanics. PSL technology simultaneously combines the GLS of 2D-STE and LV pressure (replaced by brachial artery systolic and diastolic blood pressure measured by a noninvasive cuff sphygmomanometer) to overcome the influence of GLS load conditions [14]. A recent study showed that left ventricular PSL provides a unique method of quantifying MW, superior to conventional EF and GLS [16]. MW demonstrates greater specificity in determining the extent and localization of the ischemic area due to its reduced dependence on loading conditions compared to 2D Strain alone. This approach is grounded in pathophysiological insights, indicating that a myocardial segment with elevated afterload can exhibit decreased systolic deformation (strain) even in the absence of ongoing ischemia, while still maintaining a healthy energetic and metabolic state. In the study by Eek et al., a peak strain value of 14% was used to dichotomize segments into normal or dysfunctional. In the same manner, a cut-off for regional MWI was determined by using the number of dysfunctional segments as the threshold variable in Receiver Operator Curve (ROC) analysis to identify ACO. A wide range of values were applied to determine the optimal cut- off for dichotomizing segments. A regional MWI value of 1700 mmHg % provided the largest Area Under the Curve (AUC) at identifying patients with ACO and divided segments into functional or dysfunctional. The number of adjacent dysfunctional segments constituted the FRA by MWI [8]. Particularly, a regional Longitudinal Strain with shortening less than 14% and a myocardial work below 1700 mmHg% demonstrate high sensitivity and specificity in identifying segments affected by ischemia right from the onset of acute coronary occlusion, often preceding the detection of clear mechanical impairment (Figure 1) [17,18].
Figure 1: Data from a very high-risk NSTE-ACS patient with an acute proximal occlusion in the Left Anterior Descending coronary artery and subocclusion of Left Circumflex Artery in the proximal-mid trait. Note: (A) Two myocardial strain curves from one normal  and one dysfunctional
and one dysfunctional segment; (B) Two estimated PSL
segment; (B) Two estimated PSL  from the same patient. The top PSL shows a normal segment (Basal Inferior) with a MWI of 2152 mmHg%. The bottom PSL shows a dysfunctional segment (Basal Anterior) within the functional risk area, with a MWI of 727 mmHg%. The Left Ventricular PSL (top and bottom) is depicted
from the same patient. The top PSL shows a normal segment (Basal Inferior) with a MWI of 2152 mmHg%. The bottom PSL shows a dysfunctional segment (Basal Anterior) within the functional risk area, with a MWI of 727 mmHg%. The Left Ventricular PSL (top and bottom) is depicted  ; (C) A bull’s eye plot showing segmental work in an 18-segment model. Most of the segments had impaired systolic function with an MWI less than 1700 mmHg% (segmental work cut-off). The wide FRA comprises 15/18 of all segments and is demarcated
; (C) A bull’s eye plot showing segmental work in an 18-segment model. Most of the segments had impaired systolic function with an MWI less than 1700 mmHg% (segmental work cut-off). The wide FRA comprises 15/18 of all segments and is demarcated 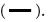
Conclusion
Several studies have supported early angiography and intervention (within 24 hours) in NSTE-ACS patients, particularly among high- risk patients (GRACE score>140). The use of risk stratification models is helpful but relies on biochemical markers that are elevated several hours after an ACO. Immediate intervention, similar to primary PCI for STEMI patients, has also been evaluated for NSTE-ACS patients without an overall positive effect. Nevertheless, subpopulations within this heterogeneous patient group may benefit from immediate PCI. We believe that 2D-STE implemented by MW, novel analytical tools in Echocardiography, may serve as the foundation for an innovative diagnostic algorithm that could revolutionize decision-making in interventional cardiology, going beyond the current recommendations of the latest guidelines.
In conclusion, the latest contemporary echocardiographic technologies offer the potential to expedite reperfusion strategies and enhance the long-term prognosis of patients with NSTE-ACS. This progress is achievable through the application of non-invasive diagnostic tools, harnessing the power of modern echocardiography implemented by 2D-STE and Myocardial work analysis.
Acknowledgement
The authors wish extends their special thanks to Mr. Manuel Materiale, Marketing Manager EMEA-AP at Opsense Medical for kindly reviewing the manuscript.
References
- DeWood MA, Spores J, Notske R, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med.303:897-902 (1980).
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33:2569-619 (2012).
- Perron A, Lim T, Pahlm-Webb U, et al. Maximal increase in sensitivity with minimal loss of specificity for diagnosis of acute coronary occlusion achieved by sequentially adding leads from the 24-lead electrocardiogram to the orderly sequenced 12-lead electrocardiogram. J Electrocardiol. 40(6):463-469 (2007).
- Phibbs B, NelsonW. Differential classification of acute myocardial infarction into STand non-ST segment elevation is not valid or rational. Ann Noninvasive Electrocardiol. 15(3):191-199 (2010).
- Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction
- Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guidelines and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guide-lines. Circulation.126:875-910 (2012).
- Sorajja P, Gersh BJ, Cox DA, et al. Impact of delay to angioplasty in patients with acute coronary syndromes undergoing invasive management: Analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. J Am Coll Cardiol.55:1416-1424 (2010).
- Eek C, Grenne B, Brunvand H, et al. Strain echocardiography predicts acute coronary occlusion in patients with non-ST-segment elevation acute coronary syndrome. Eur J Echocardiogr.11:501 (2010).
- Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation. 86(1):81-90 (1992).
- Bello D, Einhorn A, Kaushal R, et al. Cardiac magnetic resonance imaging: Infarct size is an independent predictor of mortality in patients with coronary artery disease. Magn Reson Imaging. 29(1):50-56 (2011).
- Kaul S, Pandian NG, Gillam LD, et al. Contrast echocardiography in acute myocardial ischemia. III. An in vivo comparison of the extent of abnormal wall motion with the area at risk for necrosis. J Am Coll Cardiol.7:383-392 (1986).
- Buda AJ, Zotz RJ, Pace DP, et al. Comparison of two-dimensional echocardiographic wall motion and wall thickening abnormalities in relation to the myocardium at risk. Am Heart J. 111(3):587-592 (1986).
- Capasso F, Pepe M, Severino S, et al. Urgent myocardial revascularization in non ST-segment elevation acute myocardial infarction guided by speckle tracking echocardiography: A challenging interventional decision making. Cardiology. 140(4):222-226 (2018).
- Boe K, Russell C, Eek K, et al. Non-invasive myocardial work index identifies acute coronary occlusion in patients with non ST-segment elevation-acute coronary syndrome. Eur Heart J Cardiovasc Imaging. 16(11):1247-1255 (2015).
- Yingchoncharoen S, Agarwal ZB, Popović T, et al. Normal ranges of left ventricular strain: A meta-analysis. J Am Soc Echocardiogr. 26(2):185-191 (2013).
- Chan N, Edwards B, Khandheria K, et al. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 20(1):31-39 (2019).
- Mehta SR, Granger CB, Boden WE, et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 360(21):2165-2175 (2009).
- Neumann FJ, Kastrati A, Pogatsa-Murray G, et al. Evaluation of prolonged antithrombotic pre-treatment ("cooling-off" strategy) before intervention in patients with unstable coronary syndromes: A randomized controlled trial. JAMA. 290(12):1593-1599 (2003).