Research Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 2
Metabolic syndrome,hematological markers of inflammation and disease activity in rheumatoid arthritis
- *Corresponding Author:
- Hany M. Aly
Department of Rheumatology & Rehabilitation, Al-Azhar University Faculty of Medicine, Cairo, Egypt
E-mail: hanyaly79@azhar.edu.eg
Abstract
Background: The coexistence of Rheumatoid Arthritis (RA) and Metabolic Syndrome (MetS) is common.
Aim: The present study assessed the effect of MetS on disease activity and hematological markers of inflammation to Platelets/Lymphocyte Ratio (PLR) and Neutrophil/Lymphocyte Ratio (NLR) in RA patients.
Study design: A cross-sectional case control study using STARD reporting guideline.
Methods: The present study included 5 groups: treatment-naïve RA patients with MetS (GI, n=50), treated RA patients with MetS (GII, n=50), treatment-naïve RA patients without MetS (GIII, n=50), treated RA patients without MetS (GIV, n=50) and healthy age and sex-matched controls (GV, n=50). RA was diagnosed based on the 2010 ACR/EULAR criteria. Disease activity of RA patients was calculated using the DAS-28 score. For the diagnosis of MetS, we adopted the Harmonized Joint Scientific Statement (HJSS) on metabolic syndrome recommendations.
Results: Patients in GI had significantly higher DAS28 when compared with other groups. GII and GIII patients had significantly higher DAS28 when compared with GIV. It was also shown that GI patients had significantly higher PLR and NLR when compared with GIII. Similarly, GII patients had significantly higher PLR and NLR when compared with GIV. Logistic regression analysis identified presence only MetS [OR (95%CI): 8.66 (1.34-56.1), p=0.024] and increased PLR (OR (95%CI): 0.98 (0.96-0.99), p=0.002) as independent predictors of high disease activity in treatment-naïve patients while increased PLR was the only independent predictor of disease activity in treatmentexperienced patients.
Conclusion: Metabolic syndrome is associated with elevated hematological markers of inflammation and disease activity in treatment-naïve RA patients. Both PLR and NLR are risk factors for RA activity in treatment-naïve patients with PLR being more strongly correlated with disease activity.
Keywords
rheumatoid arthritis, metabolic syndrome, platelets/lymphocyte ratio, neutrophil/ lymphocyte ratio
Key points
• Rheumatoid arthritis and metabolic syndrome commonly coexist.
• Coexistence of both conditions is associated with increased inflammatory activity.
• This can be expressed as increased PLR and NLR.
• Increased PLR and/or NLR are associated with increased disease activity.
Introduction
Rheumatoid Arthritis (RA) is a chronic disease that primarily affects joints. It characterized bya profound inflammatory state and autoimmune nature [1]. The recent years have witnessed increasing global disease incidence and prevalence with evidently associated morbidity and mortality [2]. The risk of developing RA combines genetic and environmental factors including age, low physical activity, and increased body weight [3]. Moreover, many studies recognized a significant relation between RA and Metabolic Syndrome (MetS). In fact, many components of MetS e.g. obesity and dyslipidemia are frequently seen in RA patients [4]. While the prevalence of MetS in RA patients showed wide variation among different studies [5], the cofounding of MetS in RA patients was associated with more disease activity [6,7].
The inter-relation between RA and MetS is probably attributed to the underlying inflammatory state they share in common [8]. It is understood that innate and adaptive immune cells play an essential role in the production and enhancement of the chronic inflammatory state associated with RA [9]. Also, MetS is regarded as an inflammatory disorder in which inflammatory pathways are implicated in the disease pathogenesis [10].
Besides the traditional inflammatory markers, new hematological markers of inflammation including Neutrophil/Lymphocyte Ratio (NLR) and Platelet/ Lymphocyte Ratio (PLR) were linked to RA [11] and MetS [12,13]. However, no studies assessed how the coexistence of RA and MetS can affect the levels of these markers.
The present study aimed to evaluate the inflammatory state in patients with co-existing RA and MetS as expressed by the hematological markers NLR and PLR.
Subjects and methods
The present study is a cross-sectional case-control study. The study protocol was approved by the local ethical committee and all participants gave informed consent before enrollment.
The present study included 5 groups: treatment-naïve RA patients with MetS (GI, n=50), treated RA patients with MetS (GII, n=50), treatment-naïve RA patients without MetS (GIII, n=50), treated RA patients without MetS (GIV, n=50) and healthy age and sex-matched controls (GV, n=50). Patients were included in the present study if they were diagnosed with RA or MetS while receiving RA treatment or not according to the study group. Patients were excluded if they had associated diabetes mellitus, autoimmune or allergic diseases,known infections or malignant tumors.
Upon recruitment, all participants were submitted to careful history taking, thorough clinical examination and standard laboratory investigations. RA was diagnosed based on the 2010 ACR/EULAR criteria [14]. Disease activity of RA patients was calculated using the DAS-28 score. The score comprises the number of tender joints (0=28), number of swollen joints (0-28), ESR (mm/ hr) and visual analog scale for perceived pain (0-100). Disease activity assessed by DAS-28 was interpreted as low (DAS-28=2.6–3.2), moderate (DAS-28>3.2 to 5.1) or high (DAS28>5.1). Patients were considered in remission if DAS-28 was less than 2.6 [15]. RA patient's quality of life was evaluated using the Health Assessment Questionnaire (HAQ). The questionnaire investigates patients’ ability to perform daily activities [16].
For the diagnosis of MetS, we adopted the Harmonized Joint Scientific Statement (HJSS) on metabolic syndrome recommendations. MetS criteria included increased blood pressure>130/85 mmHg, elevated FBS>100 mg/dL, obesity: waist circumference: >94 cm (male) >80 cm (female) or body mass index >30 kg/ m2, dyslipidemia: triglycerides ≥ 150 mg/dL and high-density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL (male), 50 mg/dL (female). MetS was diagnosed in the presence of any three criteria [17].
Data obtained from the present study were statistically analyzed using SPSS 20 (IBM, USA). Numerical data were expressed as mean ± SD while categorical data were presented as number and percent.
Results
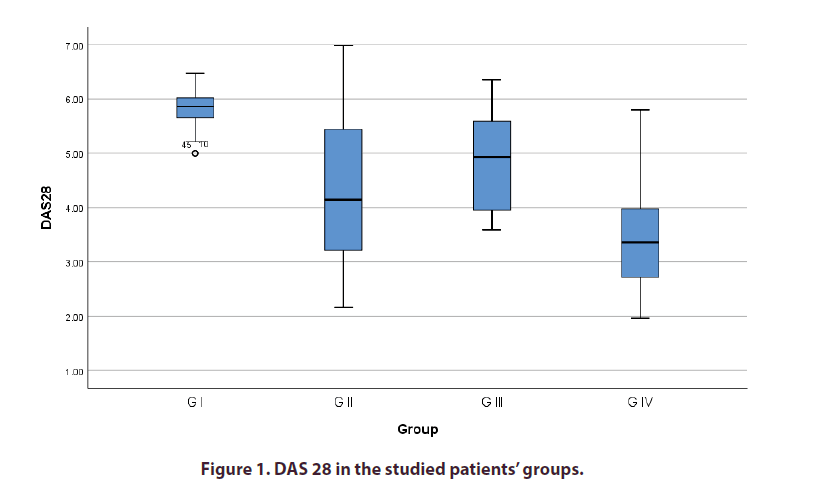
Comparison between the study groups regarding the clinical and laboratory data is shown in Table 1. Patients in GIhad significantly higher DAS28 when compared with other groups. GII and GIII patients had significantly higher DAS28 when compared with GIV (Table 1 and Figure 1). It was also shown that GI patients had significantly higher PLR and NLR when compared with GIII. Similarly, GII patients had significantly higher PLR and NLR when compared with GIV (Table 1). Correlational analysis revealed a significant direct correlation between both PLR and NLR and DAS28 in the studied group. The correlation was weaker for NRL when compared with PLR (Table 2).
| G I Treatment-naïve + MetS N=50 |
G II Treatment-experienced + MetS N=50 |
G III Treatment-naïve N=50 |
G IV Treatment-experienced N=50 |
G V Controls N=50 |
P value | |
|---|---|---|---|---|---|---|
| Clinical findings | ||||||
| Age (years) | 39.9 ± 9.2 | 44.3 ± 9.3 | 40.8 ± 8.5 | 43.4 ± 8.3 | 41.1 ± 11.4 | 0.1 |
| Male/Female | 7/43 | 11/39 | 10/40 | 08/42 | 08/42 | 0.83 |
| Disease duration (months) | 6 (4.8-7.0)*§ | 54.0 (48.0-84.0)# | 5.0 (3.8-5.0)§ | 84.0 (60.0-120.0) | - | <0.001 |
| Tender point count | 6.0 (5.0-8.0)*§ | 2.0 (1.0-3.0)# | 7.0 (6.0-8.0)§ | 2.0 (1.0-5.0) | - | <0.001 |
| Swollen point count | 7.0 (4.0-8.0)*§ | 2.0 (1.0-3.0)# | 8.0 (7.0-9.0)§ | 2.0 (1.0-3.0) | - | <0.001 |
| VAS | 70.0 (65.0-80.0)*§ | 15.0 (10.0-30.0)# | 65.0 (60.0-75.0)§ | 20.0 (10.0-30.0) | - | <0.001 |
| DAS-28 | 5.9 (5.7-6.0)*#§ | 4.1 (3.2-5.5)§ | 4.9 (3.9-5.7)§ | 3.4 (2.7-4.0) | - | <0.001 |
| HAQ | 1.5 (1.2-1.8)*§ | 1.1 (1.0-1.2)# | 1.7 (1.4-2.0)§ | 1.0 (1.0-1.1) | - | <0.001 |
| Laboratory data | ||||||
| ESR (mm/hr) | 50.0 (46.0-52.0)*§¥ | 18.0 (12.0-28.0)# | 48.0 (42.0-50.0)§¥ | 18.0 (12.0-28.0) | 15.0 (12.0-19.0) | <0.001 |
| CRP (mg/dl) | 15.0 (12.0-18.0)*§¥ | 8.0 (4.0-10.0)#¥ | 16.0 (12.0-18.0)§¥ | 6.0 (4.0-8.0)¥ | 0.0 (0.0-0.0) | <0.001 |
| Hemoglobin (gm/dl) | 11.2 (10.9-11.5)§¥ | 11.9 (11.0-12.5)#¥ | 10.8 (10.4-11.1)§¥ | 12.1 (11.0-12.5)¥ | 13.50 (12.8-13.8) | <0.001 |
| WBCs (× 10^3) | 6.8 (5.7-7.8) | 6.4 (5.5-7.6) | 6.3 (5.4-7.8) | 6.2 (5.5-7.6) | 6.1 (5.5-7.2) | 0.17 |
| Platelets (× 10^3) | 319.5 (296.5-351.8)#§¥ | 304.0 (284.8-330.3)# | 276.0 (256.8-304.3) | 293.5 (297.0-312.3) | 298.0 (268.5-309.5) | <0.001 |
| Neutrophils (× 10^3) | 4.7 (4.0-5.3)§¥ | 4.4 (3.8-4.9)¥ | 4.1 (3.4-5.0) | 3.9 (3.4-4.7) | 3.7 (3.3-4.4) | <0.001 |
| Lymphocytes (× 10^3) | 1.40 (1.24-1.6)#§¥ | 1.61 (1.29-1.78)¥ | 1.72 (1.37-1.99) | 1.67 (1.35-1.98) | 1.8 (1.56-2.02) | <0.001 |
| PLR | 221.5 (193.1-269.3)#§¥ | 192.0 (169.2-232.2)#¥ | 161.6 (140.3-211.1) | 173.8 (146.3-213.6) | 162.8 (138.3-187.3) | <0.001 |
| NLR | 3.36 (3.03-3.68)*#§¥ | 2.78 (2.6-3.18)#§¥ | 2.44 (2.32-2.71)¥ | 2.31 (2.13-2.78)¥ | 2.14 (1.93-2.35) | <0.001 |
| Metabolic syndrome components | ||||||
| Obesity | 50.0 (100.0)#§¥ | 50.0 (100.0)#§¥ | 19 (38.0) | 22 (44.0 %) | 20.0 (40.0) | <0.001 |
| BMI (kg/m^2) | 33.2 (31.5-37.6)#§¥ | 32.5 (30.8-34.9)#§¥ | 26.9 (23.7-28.0) | 27.1 (25.0-28.7) | 26.1 (23.8-27.7) | <0.001 |
| Waist circumference (cm) | 94.0 (89.0-98.0)#§¥ | 92.5 (88.0-97.3)#§¥ | 77.5 (73.8-81.0) | 79.0 (74.8-82.0) | 78.0 (75.8-81.3) | <0.001 |
| Increased blood pressure | 20 (40.0 %)#§¥ | 21 (42.0)#§¥ | - | - | - | <0.001 |
| SBP (mmHg) | 130.0 (120.0-140.0)#¥ | 130.0 (120.0-140.0)#¥ | 120.0 (110.0-120.0)§ | 120.0 (120.0-130.0) | 120.0 (120.0-130.0) | <0.001 |
| DBP (mmHg) | 82.0 (80.0-90.0)#¥ | 80.0 (80.0-90.0)#¥ | 80.0 (70.0-80.0) | 80.0 (80.0-90.0) | 80.0 (80.0-80.0) | <0.001 |
| Hyperglycemia | 46 (92.0)#§¥ | 43 (86.0)#§¥ | 8 (16.0) | 9 (18.0) | 11 (22.0) | <0.001 |
| FBG (mg/dl) | 111.0 (105.0-118.0)#§¥ | 115.0 (109.5-123.8)#§¥ | 82.5 (79.8-96.0) | 83.5 (80.0-88.0) | 81.5 (78.8-88.0) | <0.001 |
| Dyslipidemia | 37 (74.0)#§¥ | 42 (84.0)#§¥ | 5 (10.0) | 12 (24.0) | 17 (34.0) | <0.001 |
| Triglycerides (mg/dl) | 178.0 (151.0-206.3)#§¥ | 153.0 (142.0-185.0)#§¥ | 120.0 (108.8-132.0) | 103.0 (69.3-124.0) | 118.5 (99.5-129.0) | <0.001 |
| HDL (mg/dl) | 50.0 (42.8-60.0)* | 45.0 (39.5-49.0)#§¥ | 36.0 (35.0-40.0) | 32.0 (30.0-35.8) | 35.0 (31.0-39.0) | <0.001 |
| Treatment received | ||||||
| DMARDs | - | 20 (40.0) | - | 18 (36.0) | - | 0.57 |
| DMARDs + NSAIDs | - | 27 (54.0) | - | 26 (52.0) | - | |
| DMARDs + etanercept | - | 3 (6.0) | - | 6 (12.0) | - | |
Data expressed as mean ± standard deviation (SD), median and interquartile range (IQR) or number and percent.
*significant results versus G II, # significant results versus G III, § significant results versus G IV, ¥: significant results versus G V. BMI: Body Mass Index; CRP: C-Reactive Protein; DAS: Disease Activity Score; DBP: Diastolic Blood Pressure; DMARDs: Disease- Modifying Antirheumatic Drugs; ESR: Erythrocyte Sedimentation Rate; FBG: Fasting Blood Glucose; HAQ: Health Assessment Questionnaire; HDL: High-Density Lipoprotein; MetS: Metabolic Syndrome; NLR: Neutrophil Lymphocyte Ratio; NSAIDs: Non- Steroidal Anti-Inflammatory Drugs; PLR: Platelets Lymphocyte Ratio; RA: Rheumatoid Arthritis; SBP: Systolic Blood Pressure; VAS: Visual Analog Scale; WBCs: White Blood Cells
Table 1. Comparison between the studied groups regarding the clinical data.
| G I Treatment-naïve |
G II Treatment-experienced |
G III Treatment-naïve |
G IV Treatment-experienced |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PLR | NLR | PLR | NLR | PLR | NLR | PLR | NLR | |||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Clinical findings | ||||||||||||||||
| Age | -0.2 | 0.16 | 0.02 | 0.88 | 0.35 | 0.012 | 0.32 | 0.22 | 0.18 | 0.21 | 0.13 | 0.37 | 0.05 | 0.73 | 0.19 | 0.19 |
| Disease duration | -0.32 | 0.025 | -0.1 | 0.48 | 0.09 | 0.52 | -0.004 | 0.98 | -0.003 | 0.98 | 0.14 | 0.34 | 0.12 | 0.42 | -0.02 | 0.88 |
| Tender point count | 0.06 | 0.69 | -0.25 | 0.08 | 0.31 | 0.028 | 0.12 | 0.42 | 0.04 | 0.81 | 0.02 | 0.89 | 0.1 | 0.5 | -0.04 | 0.78 |
| Swollen point count | -0.13 | 0.39 | -0.1 | 0.49 | 0.24 | 0.91 | 0.1 | 0.51 | -0.06 | 0.67 | 0.21 | 0.14 | -0.11 | 0.43 | 0.02 | 0.89 |
| VAS | -0.01 | 0.94 | 0.07 | 0.63 | 0.35 | 0.012 | 0.19 | 0.19 | -0.03 | 0.82 | 0.14 | 0.34 | 0.09 | 0.54 | 0.11 | 0.44 |
| DAS-28 | 0.84 | <0.001 | 0.32 | 0.024 | 0.65 | <0.001 | 0.3 | 0.036 | 0.38 | 0.007 | 0.34 | 0.017 | 0.52 | <0.001 | 0.29 | 0.042 |
| HAQ | -0.22 | 0.13 | -0.27 | 0.057 | 0.06 | 0.7 | -0.03 | 0.83 | 0.01 | 0.92 | 0.06 | 0.69 | -0.04 | 0.78 | 0.15 | 0.32 |
| Laboratory data | ||||||||||||||||
| ESR | -0.13 | 0.37 | 0.07 | 0.61 | 0.32 | 0.25 | 0.22 | 0.13 | -0.02 | 0.88 | 0.027 | 0.85 | 0.14 | 0.35 | 0.16 | 0.27 |
| CRP | -0.005 | 0.97 | -0.17 | 0.24 | 0.24 | 0.09 | 0.11 | 0.44 | -0.04 | 0.76 | 0.06 | 0.7 | 0.07 | 0.61 | -0.001 | 0.99 |
| Hemoglobin | 0.17 | 0.25 | 0.17 | 0.23 | 0.04 | 0.81 | 0.29 | 0.039 | 0.14 | 0.32 | 0.02 | 0.87 | 0.13 | 0.36 | -0.03 | 0.83 |
| WBCs | -0.68 | <0.001 | 0.15 | 0.31 | -0.75 | <0.001 | -0.17 | 0.25 | -0.83 | <0.001 | -0.12 | 0.41 | -0.84 | <0.001 | -0.18 | 0.22 |
| Platelets | 0.43 | 0.002 | -0.02 | 0.89 | 0.22 | 0.13 | -0.05 | 0.75 | 0.45 | 0.001 | 0.15 | 0.29 | 0.16 | 0.27 | -0.22 | 0.12 |
| Neutrophils | -0.63 | <0.001 | 0.27 | 0.055 | -0.72 | <0.001 | -0.08 | 0.59 | -0.81 | <0.001 | -0.03 | 0.83 | -0.76 | <0.001 | -0.04 | 0.79 |
| Lymphocytes | -0.84 | <0.001 | -0.32 | 0.026 | -0.89 | <0.001 | -0.62 | <0.001 | -0.91 | <0.001 | -0.44 | 0.001 | -0.94 | <0.001 | -0.66 | <0.001 |
| Metabolic syndrome components | ||||||||||||||||
| BMI | -0.08 | 0.59 | 0.21 | 0.15 | 0.19 | 0.19 | 0.33 | 0.018 | 0.32 | 0.024 | 0.24 | 0.09 | 0.03 | 0.85 | -0.14 | 0.32 |
| Waist circumference | -0.01 | 0.92 | -0.06 | 0.68 | -0.05 | 0.73 | 0.15 | 0.31 | 0.06 | 0.7 | -0.18 | 0.54 | -0.08 | 0.58 | -0.11 | 0.46 |
| SBP | -0.29 | 0.045 | 0.06 | 0.66 | 0.19 | 0.18 | 0.14 | 0.32 | 0.16 | 0.28 | -0.05 | 0.71 | -0.06 | 0.69 | -0.19 | 0.18 |
| DBP | -0.24 | 0.097 | -0.08 | 0.61 | 0.15 | 0.3 | 0.05 | 0.71 | 0.12 | 0.4 | -0.06 | 0.68 | -0.08 | 0.56 | -0.26 | 0.07 |
| FBG | -0.03 | 0.85 | -0.07 | 0.61 | -0.05 | 0.76 | 0.39 | 0.006 | 0.1 | 0.48 | 0.04 | 0.78 | -0.02 | 0.88 | -0.02 | 0.89 |
| Triglycerides | 0.17 | 0.24 | 0.1 | 0.5 | -0.12 | 0.41 | 0.45 | 0.001 | -0.14 | 0.35 | -0.1 | 0.48 | 0.12 | 0.41 | 0.02 | 0.89 |
| HDL | 0.08 | 0.6 | -0.09 | 0.54 | -0.16 | 0.26 | -0.4 | 0.001 | 0.03 | 0.85 | 0.14 | 0.33 | -0.2 | 0.15 | 0.05 | 0.71 |
Table 2. Correlation between PLR, NLR and clinical and laboratory data in the studied patients groups.
Logistic regression analysis identified presence of MetS [OR (95% CI): 19.9 (5.47-72.7), p< 0.001], increased PLR [OR (95% CI): 0.97 (0.95-0.98), p<0.001] and increased NLR [OR (95% CI): 0.06 (0.017-0.23), p<0.001] as significant predictors of high DAS28 in treatment-naïve RA patients. However, in multivariate analysis, only MetS [OR (95%CI): 8.66 (1.34-56.1), p=0.024] and increased PLR (OR (95%CI): 0.98 (0.96- 0.99), p=0.002) remained significant (Table 3). In treatment-experienced patients, MetS [OR (95% CI: 2.71 (1.04-7.04), p=0.041] and increased PLR [OR (95% CI: 0.98 (0.97-0.99), p<0.001] were significant predictors of high DAS28 in univariate analysis. Only increased PLR remained significant in multivariate analysis [OR (95% CI: 0.98 (0.96-0.99), p=0.001] (Table 4).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age | 1.0 (0.96-1.06) | 0.80 | - | - |
| Sex | 1.27 (0.42-3.8) | 0.68 | - | - |
| Disease duration | 1.04 (0.82-1.32) | 0.72 | - | - |
| MetS | 19.9 (5.47-72.7) | <0.001 | 8.66 (1.34-56.1) | 0.024 |
| PLR | 0.97 (0.95-0.98) | <0.001 | 0.98 (0.96-0.99) | 0.002 |
| NLR | 0.06 (0.017-0.23) | <0.001 | 1.02 (0.17-6.0) | 0.98 |
Table 3. Predictors of high disease activity in treatment-naïve patients.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95 % CI) | P | OR (95 % CI) | P | |
| Age | 0.99 (0.95-1.05) | 0.89 | - | - |
| Sex | 1.99 (0.53-7.5) | 0.31 | - | - |
| Disease duration | 1.01 (0.99-1.03) | 0.079 | 1.0 (0.99-1.02) | 0.36 |
| MetS | 2.71 (1.04-7.04) | 0.041 | 2.8 (0.77-10.44) | 0.12 |
| PLR | 0.98 (0.97-0.99) | < 0.001 | 0.98 (0.96-0.99) | 0.001 |
| NLR | 0.43 (0.17-1.11) | 0.08 | 3.08 (0.5-19.1) | 0.23 |
Table 4. Predictors of high disease activity in treatment-experienced patients.
Discussion
The present study aimed to assess and compare how the coexistence of MetS and RA can affect disease activity in treatment-naïve and treatment-experienced patients. Also, we sought to investigate if this coexistence is associated with the altered inflammatory conditions as expressed by the emerging hematological markers of inflammation PLR and NLR.
In our study, treatment-naïve and treatment-experienced RA patients with MetS had significantly higher disease activity when compared with their corresponding counterparts without MetS. Moreover, we identified MetS and an independent predictor of high disease activity in treatment-naïve and treatment-experienced RA patients. The association betweendisease activity in RA patients and MetS was previously reported [7,18- 22]. However, other authors opposed this finding [23-25]. Of note, these studies recruited a substantial percentage of treated patients and didn’t distinguish the effect of MetS on disease activity between treatment-naïve and treatment-experienced patients.
In our study, levels of the hematological markers of inflammation PLR and NLR showed diverse results in the studied groups. Both markers were significantly elevated in treatment-naïve patients with MetS in comparison to the corresponding group without MetS. Elevation of these markers in association with MetS was reported by other studies [26,27]. The increased inflammatory burden in RA patients with associated MetS is attributed to the additional inflammatory and immune mediators released by adipose tissues in those patients [8]. Also, increased PLR and NLR were noted in RA patients [28].
However, in treatment-experienced patients, no significant differences were noted between RA patients with MetS and RA patients without. This may be explained by the anti-inflammatory effects of RA treatment that alleviate the added inflammatory burden of MetS in those patients. This explanation is supported by the weaker correlation between PLR and NLR and DAS28 in treatment-experienced patients. Our conclusions are supported by the study ofToms et al. [29] who noted that methotrexate use was associated with a lower risk of MetS in RA patients. Also,Dao et al. [19] identified less methotrexate use as a predictor of the presence of MetS in women with RA. Moreover, the studies of Bilecik et al. [30] and Abourazzak et al. [31] noted that the prevalence of MetS in patients with RA using methotrexate was significantly lower than without RA.
Conclusion
Metabolic syndrome is associated with elevated hematological markers of inflammation and disease activity in treatment-naïve RA patients. Both PLR and NLR are risk factors for RA activity in treatment-naïve patients with PLR being more strongly correlated with disease activity.
Acknowledgments
None
Funding
None
Conflict of interest
Authors declare that there is no conflict of interest.
Author contributions
Conception and design: AG; Data analysis, acquisition, and interpretation: HA, AA; Manuscript writing: AG; Critical revision: HA, AA; Agreement: All authors; Accountability: AG
References
- Aletaha D, Kerschbaumer A. Rheumatoid arthritis. Z. Rheumatol.76(1), 8-14 (2017).
- Fazal SA, Khan M, Nishi SE et al. A Clinical Update and Global Economic Burden of Rheumatoid Arthritis. Endocr. Metab. Immune. Disord. Drug. Targets. 18(2), 98-109 (2018).
- Bullock J, Rizvi SAA, Saleh AM et al. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 27(6), 501-507 (2018).
- Kerekes G, Nurmohamed MT, Gonzalez-Gay MA et al. Rheumatoid arthritis and metabolic syndrome. Nat. Rev. Rheumatol.10(11), 691-696 (2014).
- Hallajzadeh J, Safiri S, Mansournia MA et al. Metabolic syndrome and its components among rheumatoid arthritis patients: A comprehensive updated systematic review and meta-analysis. PLoS One. 12(3), e0170361 (2017).
- Pandey PK, Swami A, Biswas TK et al. Prevalence of Metabolic Syndrome in Treatment Naive Rheumatoid Arthritis and Correlation with Disease Parameters. Arch. Rheumatol. 32(1), 46-52 (2017).
- Gomes KWP, Luz AJP, Felipe MRB et al. Prevalence of metabolic syndrome in rheumatoid arthritis patients from Northeastern Brazil: Association with disease activity. Mod. Rheumatol. 28(2), 258-263 (2018).
- Ferraccioli G, Gremese E. Adiposity, joint and systemic inflammation: the additional risk of having a metabolic syndrome in rheumatoid arthritis. Swiss. Med. Wkly. 141, w13211 (2011).
- Calabresi E, Petrelli F, Bonifacio AF et al .One year in review 2018: pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 36(2), 175-184 (2018).
- Reddy P, Lent-Schochet D, Ramakrishnan N et al. Metabolic syndrome is an inflammatory disorder: A conspiracy between adipose tissue and phagocytes. Clin. Chim. Acta. 496, 35-44 (2019).
- Zengin O, Onder ME, Kalem A et al. New inflammatory markers in early rheumatoid arthritis. Z. Rheumatol. 77(2), 144-150 (2018).
- Surendar J, Indulekha K, Mohan V et al. Association of neutrophil-lymphocyte ratio with metabolic syndrome and its components in Asian Indians (CURES-143). J. Diabetes. Complications. 30(8), 1525-1529 (2016).
- Meng G, Zhu Q, Shao J et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin. Chim. Acta. 475, 1-6 (2017).
- Kay J, Upchurch KS. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 51(Suppl 6), vi5-9 (2012).
- Anderson J, Caplan L, Yazdany J et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis. Care. Res (Hoboken). 64(5), 640-647 (2012).
- Bruce B, Fries JF. The Health Assessment Questionnaire (HAQ). Clin. Exp. Rheumatol.23(5 Suppl 39), S14-S18 (2005).
- Alberti KG, Eckel RH, Grundy SM et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120(16), 1640-1645 (2009).
- Karvounaris SA, Sidiropoulos PI, Papadakis JA et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Ann. Rheum. Dis. 66(1), 28-33 (2007).
- Dao HH, Do QT, Sakamoto J. Increased frequency of metabolic syndrome among Vietnamese women with early rheumatoid arthritis: a cross-sectional study. Arthritis. Res. Ther. 12(6), R218 (2010).
- da Cunha VR, Brenol CV, Brenol JC et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand. J. Rheumatol. 41(3), 186-191 (2012).
- Parra-Salcedo F, Contreras-Yanez I, Elias-Lopez D et al.Prevalence, incidence and characteristics of the metabolic syndrome (MetS) in a cohort of Mexican Mestizo early rheumatoid arthritis patients treated with conventional disease modifying anti-rheumatic drugs: the complex relationship between MetS and disease activity. Arthritis. Res. Ther. 17, 34 (2015).
- Tantayakom P, Koolvisoot A, Arromdee E et al. Metabolic syndrome is associated with disease activity in patients with rheumatoid arthritis. Joint. Bone. Spine. 83(5), 563-567 (2016).
- Sahebari M, Goshayeshi L, Mirfeizi Z et al. Investigation of the association between metabolic syndrome and disease activity in rheumatoid arthritis. Scientific. World. J. 11, 1195-1205 (2011).
- Karimi M, Mazloomzadeh S, Kafan S et al. The frequency of metabolic syndrome in women with rheumatoid arthritis and in controls. Int. J. Rheum. Dis. 14(3), 248-254 (2011).
- Slimani S, Abbas A, Ben Ammar A et al. Prevalence of metabolic syndrome in Algerian rheumatoid arthritis patients. Correlation with disease activity and functional status. Diabetes. Metab. Syndr.11(Suppl 1), S425-S427 (2017).
- Rkhaya SA, Bulatova N, Kasabri V et al. Increased malondialdehyde vs. reduced sirtuin 1 in relation with adiposity, atherogenicity and hematological indices in metabolic syndrome patients with and without prediabetes. Diabetes. Metab. Syndr. 12(6), 903-909 (2018).
- Horan AA, Albsoul-Younes A, Kasabri Vet al.Correlates of resistin and retinol-binding protein 4 in metabolic syndrome patients with and without prediabetes. Horm. Mol. Biol. Clin. Investig. 37(3) (2019).
- Erre GL, Paliogiannis P, Castagna F et al. Meta-analysis of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio in rheumatoid arthritis. Eur. J. Clin. Invest. 49(1), e13037 (2019).
- Toms TE, Panoulas VF, John H et al. Methotrexate therapy associates with reduced prevalence of the metabolic syndrome in rheumatoid arthritis patients over the age of 60- more than just an anti-inflammatory effect? A cross sectional study. Arthritis. Res. Ther. 11(4), R110 (2009).
- Bilecik NA, Tuna S, Samanci N et al. Prevalence of metabolic syndrome in women with rheumatoid arthritis and effective factors. Int. J. Clin. Exp. Med. 7(8), 2258-2265 (2014).
- Abourazzak FE, Mansouri S, Najdi A et al. Prevalence of metabolic syndrome in patients with rheumatoid arthritis in Morocco: a cross-sectional study of 179 cases. Clin. Rheumatol. 33(11), 1549-1555 (2014).