Case Report - Interventional Cardiology (2018) Volume 10, Issue 5
Mechanical circulatory support for post-acute myocardial infarction with refractory cardiogenic shock: A decade of lessons
- Corresponding Author:
- Francesco Nappi epartment of Cardiothoracic Surgery, Centre Cardiologique du Nord St Denis, Paris, France E-mail: Francesconappi2@gmail.com
Received date: August 25, 2018 Accepted date: September 21, 2018 Published date: September 28, 2018
Abstract
Introduction: There are 0.9 catheterization labs per 100,000 inhabitants in Scotland for percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI), which are much less accessible to patients in remote and rural areas. An uncommon but sinister sequalae following AMI is cardiogenic shock (CS) that is refractory to inotropic support. CS complicates 5-15% of AMIs occurring in ST-segment Elevation Myocardial Infarctions (STEMIs). Outcomes of CS are poor with mortalities of up to 90% reported in the literature in the absence of experienced care. We report our experience as the tertiary referral centre in Scotland for MCS and heart transplantation over 8 years. Methodology: A retrospective review of prospectively collected data was undertaken on all patients registered to the MCS service. The database was interrogated for patient demographics, type of mechanical circulatory support and duration of MCS support, PCI-outcomes and survival to 30-days. A time-to-event analysis was performed using patient survival as the primary outcome measure. Results: Twenty-three patients (16M:7F) were included. The median age of the patients was 50 years (45-56 years). VA-ECMO was the initial MCS of choice in 17(73.9%) patients with BIVAD for 4(17.4%) patients and LVAD for 2(8.7%) patients. 30-day mortality was 21.8% in this cohort, however survival to discharge was 52.2%. Eleven (47.8%) patients recovered without the need for any further support, however only 9 (81.8%) patients in this subgroup survived to discharge. Three (13%) patients received a durable LVAD. In this subgroup, one patient was transplanted whereas two patients died due to complications while on support. The median length of in-hospital MCS support was 4 days. Median in-hospital stay was 27 days. Long-term follow up of up to 8 years demonstrates a high mortality beyond 30-days up to the first 6-months post MCS support. Conclusion: MCS usage in these patients carries a high mortality in the early post-implantation period. However, there is a significant benefit to patients who survive the initial bridging period to recovery or destination therapy
Introduction
In the preceding decades, Scotland has drastically reduced the mortality from coronary heart disease (72% reduction in 2009 compared to 1950) [1]. Despite this, post-MI mortality remains among the highest in Western Europe [1], branding Scotland as the ‘sick man of Europe’ [2]. The inequality in Scottish morbidity and mortality has resulted in an overall increase in health inequalities across the United Kingdom [3]. Ischaemic heart disease is associated with a higher level of disability-adjusted life years (DALY) than any other condition in Scotland, mirroring not just the UK, but also DALY in the Global Burden of Disease Survey [4].
There are 0.9 catheterization labs per 100,000 inhabitants in Scotland for percutaneous coronary intervention (PCI) for acute myocardial infarction (AMI) [5], which are much less accessible to patients in remote and rural areas. An uncommon but sinister sequalae following AMI is cardiogenic shock (CS) that is refractory to inotropic support. CS complicates 5-15% of AMIs occurring in ST-segment Elevation Myocardial Infarctions (STEMIs) [6-8]. Outcomes of CS are poor with mortalities of up to 90% reported in the literature in the absence of experienced care [9].
Initial management of CS consists of identifying incidental complications e.g. acute left ventricular rupture or mitral regurgitation, assessing haemodynamics, and optimising the reperfusion in the culprit coronary artery. Clinical trials of therapeutic interventions have not led to changes in practice. The results of the Intra-Aortic Balloon Pump in Cardiogenic shock II (IABP-SHOCK II) trial highlighted the lack of survival benefit from the routine use of IABP therapy for this condition [10]. The only available option for patients with refractory, life-threatening illness would be the institution of mechanical circulatory support (MCS), involving either Extracorporeal Membranous Oxygenation (ECMO) or Ventricular Assist Devices (VADs). MCS can potentially improve survival, however, evidence is lacking. EURO-SHOCK (ID754946-2), which is a clinical trial funded by the EU-Horizons 2020 7th Framework programme, will address this gap. EURO-SHOCK is a multicentre, randomized, controlled trial of management involving ECMO vs. standard care without ECMO in patient with cardiogenic shock post-MI. Given the current gap in knowledge, we studied the outcomes following use of MCS for treatment of cardiogenic shock post-AMI in Scotland during an 8-year period.
Methodology
Patients
All patients who were referred to the MCS service in the Golden Jubilee National Hospital from January 2009 - August 2017 following primary PCI-treated ST-segment elevation MI (STEMI) with refractory cardiogenic shock were included in this study.
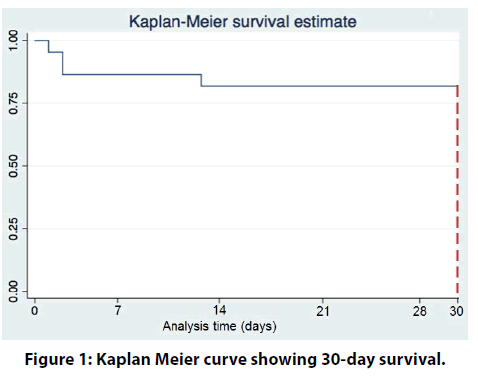
A retrospective review of prospectively collected data was undertaken on all patients registered to the MCS service. The database was interrogated for patient demographics, type of mechanical circulatory support (Veno-arterial Extracorporeal Membranous Oxygenation/Ventricular Assist Device) and duration of MCS support, PCI-outcomes and survival to 30- days. A time-to-event analysis was performed using patient survival as the primary outcome measure. Kaplan-Meier curves were used to graphically display data of 30-day survival. Student’s t-tests and Mann- Whitney U tests were used to analyse data for 30-day survival for continuous data with Fisher’s exact test used for categorical data. The study was registered with the Clinical Governance Department.
Results
Twenty-three patients (16 M: 7 F) were included (Table 1). The median age of the patients was 50 years (45-56 years). VA-ECMO was the initial MCS of choice in 17 (73.9%) patients with BIVAD for 4 (17.4%) patients and LVAD for 2 (8.7%) patients. 30 day mortality was 21.8% in this cohort, however survival to discharge was 52.2% (Table 2).
| Details | Total (n=23) |
Survivors (n=18) | Non-Survivor (n=5) |
|---|---|---|---|
| Age (years) | 50 (11) | 50 (9.3) | 56 (16) |
| Male gender (%) | 65 | 72 | 60 |
| BMI (kg/m2) | 28.2±3.3 | 28.7±2.7 | 27.2±4.1 |
| Hypertension (%) | 13 (3/23) | 6 (1/18) | 40 (3/5) |
| Smoker (%) | 35 (8/23) | 38 (7/18) | 20 (1/5) |
| Diabetes Mellitus (%) | 4 (1/23) | 0 | 20 (1/5) |
| Blood Group A (%) | 48 (11/23) | 44 (8/18) | 60 (3/5) |
Table 1. Preoperative demographics of survivors and non-survivors
| Details | Total (n=23) |
Survivors (n=18) | Non-Survivor (n=5) | p-value |
|---|---|---|---|---|
| Post PCI MAP mmHg | 47.9±8.1 | 48.1±8.7 | 47.0±5.4 | 0.755 |
| Creatinine μmol/L | 200.7±109.2 | 198.4±87.9 | 201±120 | 0.951 |
| PCI - MCS initiation time (hours) | 7 (18.5) | 8 (19.5) | 4 (2) | 0.370 |
| CPR in Cath Lab % (n) | 48 (11/23) | 39 (7/18) | 80 (4/5) | 0.155 |
| IABP in Cath Lab % (n) | 91 (21/23) | 94 (17/18) | 80 (4/5) | 0.395 |
| Bilirubin mg/dL | 11.5 (11) | 11 (11) | 16 (10) | 0.551 |
| AST u/L | 463 (457.5) | 383 (396.5) | 825 (1298) | 0.052 |
| ALT u/L | 174 (234) | 164 (201.5) | 258 (487) | 0.126 |
| HsTnI ng/L | 18057(11241) | 18057(12422) | 20211(12708) | 0.559 |
| Pulmonary oedema at presentation % (n) | 78 (18/23) | 72(13/18) | 100 (5/5) | 0.545 |
| Culprit Vessel | ||||
| Isolated LAD % (n) | 39 (9/23) | 39 (7/18) | 40 (2/5) | 0.999 |
| Isolated RCA % (n) | 26 (6/23) | 33 (6/18) | 0 | 0.272 |
| Isolated LCx % (n) | 4 (1/23) | 0 (0/18) | 20 (1/5) | 0.217 |
| Isolated LMS% (n) | 9 (2/23) | 11 (2/18) | 0 | 0.999 |
| >1 vessel involvement % (n) | 22 (5/23) | 17 (3/18) | 40 (2/5) | 0.291 |
Table 2: PCI demographics of survivors’ vs non-survivors
Eleven (47.8%) patients recovered without the need for any further support, however only 9 (81.8%) patients in this subgroup survived to discharge. Three (13%) patients received a durable LVAD. In this subgroup, one to complications while on support (VAD thrombus, Disseminated Intravascular Coagulation).
The median length of in-hospital MCS support was 4 days (4-43 days). Median in-hospital stay was 27 days (9-41 days).
The 30-day mortality data of survivors vs. nonsurvivors are as follows.
The median length of in-hospital MCS support was 4 days (4-43 days). Median in-hospital stay was 27 days (9-41 days).
Death on explant of MCS
Eleven (47.8%) patients recovered without the need for any further support, however only 9 (81.8%) of patients in this subgroup survived to discharge (Table 3).
| Details | Total (n=23) |
Survivors (n=18) | Non-Survivor (n=5) | p-value |
|---|---|---|---|---|
| ECMO | 74 (17/23) | 72 (13/18) | 80 (4/5) | 0.999 |
| VAD | 26 (6/23) | 28 (5/18) | 20 (1/5) | 0.999 |
| Post MCS Lactate | 6.64±3.64 | 6.07±3.22 | 8.60±5.03 | 0.339 |
| PaO2/FiO2 | 0.402±0.135 | 0.394±0.133 | 0.444±0.169 | 0.675 |
| Post MCS MAP | 64.83±6.76 | 64.44±7.59 | 66.20±3.90 | 0.494 |
| Inotrope Score | 25.0±18.3 | 18.1±10.2 | 50.0±22.1 | 0.035 |
| Platelet | 211.4±81.2 | 216.9±87.1 | 176.2±61.6 | 0.267 |
| CRRT post-MCS | 11/23 | 11/18 | 0/5 | 0.037 |
| Sequential Organ Failure Assessment Score (SOFA) | 8.00±2.35 | 8.22±2.51 | 7.20±1.64 | 0.307 |
Table 3: Post-operative details of survivors vs non-survivors
Three (13%) of patients received a durable LVAD. In this subgroup, one patient was transplanted whereas two patients died due to complications while on support (VAD thrombus, intracerebral haemorrhage) (Table 4).
| Complications | Total (n=23) |
Survivors (n=18) | Non-Survivor (n=5) | p-value |
|---|---|---|---|---|
| Pump Thrombus % (n) | 8 (2) | 6 (1) | 20(1) | 0.395 |
| Bleeding % (n) | 13 (3) | 11 (2) | 20(1) | 0.539 |
| ICH % (n) | 13 (3) | 11 (2) | 20(1) | 0.539 |
| TIA/Stroke % (n) | 13 (3) | 17 (3) | 0 | 0.999 |
| Distal Limb Amputation % (n) | 8 (2) | 11 (2) | 0 | 0.999 |
| Ischaemic colitis % (n) | 4 (1) | 0 | 20(1) | 0.999 |
| Malignant Arrhythmia % (n) | 8 (2) | 11 (2) | 0 | 0.999 |
| Aspiration pneumonia | 4 (1) | 0 | 20(1) | 0.250 |
Table 4: Post-operative complications of survivors vs non-survivors
Death post-explant of MCS were caused by malignant arrhythmia (n=2).
Post 30-day survival
Three (13%) of patients underwent heart transplantation and are well at up to 6 years postoperatively.
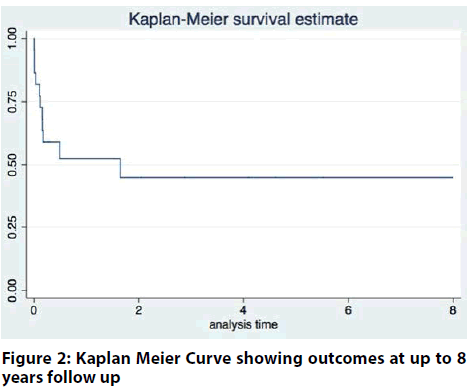
Long-term follow up of up to 8 years is depicted in the figure 1 & 2. The curve demonstrates a high mortality beyond 30-days up to the first 6-months post MCS support.
Discussion
For the first time, we have documented MCS therapy and related outcomes in a contemporary Scottish population of patients with AMI complicated by CS. More than three quarters of patients survived to 30- days. This result compares favourably to other studies investigating outcomes of patients receiving MCS therapy in AMI/CS. In the ENCOURAGE study, approximately half of the cohort survived to % 30-days [11]. One other North American study reported a 30- day mortality rate of 41% [12]. Most other publications report a 30-day survival rate of 23-76% survival rate in this specific patient cohort [13-15].
Among the survivors, 16.7% subsequently underwent heart transplantation.
There are challenges to comparisons of outcomes in post AMI-CS patients between studies, not least because of the heterogeneity in patient populations and practice. Comparing outcomes of existing studies however is complicated by the variability of the cohorts as CS comprises a wide spectrum of clinical and haemodynamic instability. There is substantial heterogeneity with presentations of the patients with several factors being predictors of poor outcome in larger studies. A literature review revealed older age [6,16], signs of end-organ hypo-perfusion [17], involvement of the LAD artery, severity of disease (triple vessel disease) and renal failure (identified by elevated creatinine) [16,18]. Our cohort was limited in size. Nonetheless, our findings indicate 30-day mortality results are similar to or potentially better than prior cohort studies.
We concentrated on the presenting pathology (AMI with CS) and not the device (VAD vs ECMO) as we felt most patients would receive a strategy that was either escalated or de-escalated based on recovery. Patients who were improving for example were stepped down from ECMO to a VAD (short term or long term). ECMO was the treatment of choice in most patients as in the acute phase, almost all the patients presented with acute pulmonary oedema.
Almost half (47.8%) of the patients were explanted without any further support device or transplantation. Myocardial recovery has been reported in previous publications [19-21]. Veno-arterial ECMO (VAECMO) is readily available and can be rapidly instituted percutaneously negating the need for operating theatre resources. Some limitations to ECMO have been reported in the literature. This includes inadequate left ventricular decompression as emptying depends on the native ejection function of the ventricle. Decreasing the flow rate on the ECMO circuit also reduces afterload alongside using inotropes such as dobutamine to improve contractility and decrease ejection. This may result in pulmonary hypertension, oedema and bleeding [22]. The interaction between the tubing surfaces causes activation of monocytes and release of interleukins 1 and 6 [23]. Some of the decompression can be attenuated by IABP insertion. It is associated with a smaller left ventricular dimension and a lower pulmonary artery pressure by restoring pulsatility and decreasing left ventricular afterload [24]. IABP may also reduce the mean of cerebral blood flow during myocardial stunning, and increases the mean flow during cardiac recovery [25]. Activation of clotting cascades is the predominant reason for bleeding complications. Frequent echocardiograms are done at our unit to ensure there is adequate decompression of the right and left ventricles. Another deleterious effect of VA ECMO is the neurological morbidity. Brain death has been reported in up to 21% in adults treated in ECMO centres. Up to 50% of patients have evidence of cerebral injury [25]. In our cohort, 26.1% of patients had evidence of a cerebral injury. The same deleterious effects of ECMO are also noted in VADs (about 20%) [26].
Myocardial ischaemia is the preceding event in CS [9,27]. It impairs myocardial contractility which in turn reduces stroke volume. An impeded cardiac index causes tissue hypoperfusion, which includes coronary hypoperfusion causing worsening myocardial ischaemia, resulting in a vicious cycle. Serum lactate, creatinine and AST are used as surrogates of organ hypoperfusion in our study. Initial compensatory vasoconstriction arises from catecholamine release to increase blood pressure but systemic inflammatory response syndrome (SIRS) mediated pathological release of vasodilatory agents results in a net reduction in cardiac index. This acts in conjunction with the reduction in left ventricular function as a result of myocardial stunning from the primary insult. There is a small window of reversibility afforded during myocardial stunning by reperfusion which is facilitated by early reperfusion [28]. Capillary leakage from SIRS causes tissue oedema and a reduction in circulating volume.
Decision making for MCS is also an important part of the discussion. Traditional ethical principles are not straightforward when applied to ECMO patients as it is often seen as the ceiling of therapy available. A survey of self-reported physicians with vast experience in VAECMO revealed majority of physicians felt physicians should have the right to discontinue management over family’s objection [29]. MCS is a costly intervention thereby complicating the decision-making process with finite resources available for clinicians in the National Health Service (NHS). In our unit, a multidisciplinary team is consulted to ensure an informed decision which takes into account all facets of care prior to initiating MCS support.
The data presented represents the first reported series of patients in Scotland with AMI complicated by CS treated with MCS. However, as it is a retrospective study with a small cohort of patients, the reproducibility of the results may vary and may not capture the European or British population as a whole. There is a selection bias in the sample as only patients who were deemed potentially salvageable were included in the study, which may comparisons with other studies difficult.
Conclusion
MCS usage in these patients carries a high mortality in the early post-implantation period. However, there is a significant benefit to patients who survive the initial bridging period to recovery or destination therapy. Further prospective studies are needed to identify predictors of long term survival.
References
- Lewsey JD, Lawson KD, Ford I, et al. A cardiovascular disease policy model that predicts life expectancy taking into account socioeconomic deprivation. Heart. 101(3): 201-8 (2015).
- McCartney G, Walsh D, Whyte B, et al. Has Scotland always been the ‘sick man’ of Europe? An observational study from 1855 to 2006. Eur J Public Health. 22(6): 756-60 (2012).
- Hanlon P, Lawder RS, Buchanan D, et al. Why is mortality higher in Scotland than in England and Wales? Decreasing influence of socioeconomic deprivation between 1981 and 2001 supports the existence of a 'Scottish Effect'. J Public Health. 27(2): 199-204 (205).
- Kassebaum NJ, Arora M, Barber RM, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet.388(10053): 1603-58 (2016).
- Hagen TP, Häkkinen U, Belicza E, et al. on behalf of the Euro Hsg. Acute Myocardial Infarction, Use of Percutaneous Coronary Intervention, and Mortality: A Comparative Effectiveness Analysis Covering Seven European Countries. Health Econ. 24: 88-101 (2015).
- Babaev A, Frederick PD, Pasta DJ, et al. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA. 294(4): 448-54 (2005).
- Goldberg RJ, Spencer FA, Gore JM, et al. Thirty-Year Trends (1975 to 2005) in the Magnitude of, Management of, and Hospital Death Rates Associated With Cardiogenic Shock in Patients With Acute Myocardial Infarction. A Population-Based Perspective. Circulation. 119(9): 1211-9 (2009).
- Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French Nationwide Registries. European Heart J. 33(20): 2535-43 (2012).
- Reynolds HR, Hochman JS. Cardiogenic Shock: Current Concepts and Improving Outcomes. Circulation. 117(5): 686-97.117 (2008).
- Thiele H, Zeymer U, Neumann F-J, et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. New Eng J Med. 367(14): 1287-96 (2012).
- Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long-term outcomes after VA-ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 42(3): 370-8 (2016).
- Truby L, Naka Y, Kalesan B, et al. Important role of mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Eur J Cardiothorac Surg. 48(2): 322-8 (2015).
- Tang GH, Malekan R, Kai M, et al. Peripheral venoarterial extracorporeal membrane oxygenation improves survival in myocardial infarction with cardiogenic shock. J Thorac Cardiovasc Surg. 145(3): e32-3 (2013).
- Kar B, Gregoric ID, Basra SS, et al. The percutaneous ventricular assist device in severe refractory cardiogenic shock. J Am Coll Cardiol. 57(6): 688-96 (2011).
- Hendry PJ, Masters RG, Mussivand TV, et al. Circulatory support for cardiogenic shock due to acute myocardial infarction: a Canadian experience. Can J Cardiol. 15(10): 1090-1104 (1999).
- Zeymer U, Vogt A, Zahn R, et al. Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); Results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausarzte (ALKK). Eur Heart J. 25(4): 322-8 (2004).
- Sleeper LA, Reynolds HR, White HD, et al. A severity scoring system for risk assessment of patients with cardiogenic shock: a report from the SHOCK Trial and Registry. Am Heart J. 160(3): 443-50 (2010).
- Klein LW, Shaw RE, Krone RJ, et al. Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 96(1): 35-41 (2005).
- Abrams D, Combes A, Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J Am Coll Cardiol. 63: 2769-2778 (2014).
- Combes A, Leprince P, Luyt CE, Bonnet N, Trouillet JL, Leger P, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Critical care medicine. 36(5): 1404-1411 (2008).
- Brechot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Critical care medicine. 41(7):1616-14626 (2013).
- Pagani FD, Lynch W, Swaniker F, et al. Extracorporeal Life Support to Left Ventricular Assist Device Bridge to Heart Transplant. A Strategy to Optimize Survival and Resource Utilization. Circulation. 100 (suppl 2):II-206-Ii-10 (1999).
- Pennington DG, Merjavy JP, Swartz MT, et al. The importance of biventricular failure in patients with postoperative cardiogenic shock. Ann Thorac Surg. 39(1):16-26 (1985).
- Petroni T, Harrois A, Amour et al. Intra-aortic balloon pump effects on macrocirculation and microcirculation in cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation*. Crit Care Med. 42(9): 2075-2082 (2004).
- Risnes I, Wagner K, Nome T, IA, et al. Cerebral outcome in adult patients treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 81(4): 1401-1406 (2016).
- Backes D, van den Bergh WM, van Duijn AL, et al. Cerebrovascular complications of left ventricular assist devices. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. Eur J Cardiothorac Surg. 42(4): 612-620 (2016).
- Thiele H, Allam B, Chatellier G, et al. Shock in acute myocardial infarction: the Cape Horn for trials. Eur Heart J. 31(15): 1828-1835 (2010).
- Kajimoto M, O'Kelly Priddy CM, Ledee DR, et al. Myocardial Reloading After Extracorpcoreal Membrane Oxygenation Alters Substrate Metabolism While Promoting Protein Synthesis. J Am Heart Assoc. 2(4): e000106 (2013).
- Meltzer EC, Ivascu NS, Stark M, et al. A Survey of Physicians' Attitudes toward Decision-Making Authority for Initiating and Withdrawing VA-ECMO: Results and Ethical Implications for Shared Decision Making. J Clin Ethics. 127(4): 281-290 (2016).