Research Paper - Imaging in Medicine (2017) Volume 9, Issue 5
Intraoperative fluorescence-imaging of nerves - a contribution to navigation surgery
Akio Sugitachi*, Koki Otsuka, Fumitaka Endo, Toshimoto Kimura, Takeshi Takahara, Yuji Akiyama, Takeshi Iwaya, Hiroyuki Nitta, Keisuke Koeda, Masaru Mizuno and Akira SasakiDepartment of Surgery, Iwate Medical University, Morioka, Japan
- *Corresponding Author:
- Akio Sugitachi
Department of Surgery
Iwate Medical University Morioka, Japan
E-mail: akiosugi@iwate-med.ac.jp
Abstract
Nerve-preservation technique plays an important role in surgery. Intraoperative nerve injuries often cause permanent neurogenic untoward events such as functional disorders, palsy, pain and numbness. To avoid such accidents, we devised an intraoperative fluorescence-imaging of peripheral nerves as a contribution to navigation surgery. Myelin-targeting fluorescent agents; namely, amphotericin B and fluorescinisothyanate, were used as markers of nerves in this study since myelin was a main component of Schwann sheath in peripheral nerve. In in vitro, cultured Schwann cells were served as model of peripheral nerves. After treating Schwann cells with each fluorescent agent, we observed the fluorescence emitted in a dark room. Then, we carried out in vivo studies with rats following the results of in vitro data and assessed the clinical significance of our newly devised method. We could clearly observe specific florescence the Schwann cells radiated in in vitro and discriminated peripheral nerve fibres from surrounding tissues in in vivo. These results strongly suggested a clinical usefulness of intraoperative visualization of peripheral nerve fibres. Our novel method would promote intraoperative nerve- preservation.
Keywords
fluorescence-imaging ▪ navigation surgery ▪ nerve-preservation ▪ myelin ▪ schwann sheath ▪ amphotericin B ▪ fluorescein isothyanate
Introduction
Current methods for intraoperative identification of nerves mainly depend on skill and wide experiences of the surgeons and otherwise, Electro-Myographic (EMG) monitoring, while both are imperfect in accuracy. Aside from the surgeons’ abilities, the EMG instrument sometimes dislocates its right spot when patient is changed the posture during operation, and besides EMG monitoring is helpless for detecting sensory fibres. Therefore, we contrived a novel intra- operative visualizing manner of peripheral nerves. Peripheral nerves contain considerable amount of galactolipids and glycoproteins, which have chemical affinity for polyene antibiotics such as amphotericin B (AmB) [1,2] and/or fluorescein isothiocyanate (FITC) [3]. So, AmB and FITC were used as fluorescent agents, since they were low-toxic and clinically available.
The paper described basic theory and a knack for our intraoperative fluorescent visualization of peripheral nerves together with a novel concept relating to nerve-preserving surgery.
Materials and Methods
Materials and reagents used were as follows. Schwann Cells (SC), Schwann Cell Medium (SCM): ScienCell Research Laboratories, Inc., COSMO BIO Co., Ltd., Tokyo, Japan.
Fluorescent agents
Amphotericin B (AmB), fluorescein isothiocyanate (FITC): Sigma-Aldrich Corporate, Sigma-Aldrich, St. Louis, Mo 63103, USA.
Albumin from bovine serum
Wako Pure Medical Co. Ltd., Osaka, Japan.
Hyaluronic acid (HA): KAKEN PHARM., Co. Ltd. Tokyo, Japan.
Exciting light source
Blue light (BL); SALARFORCE, HK: Hong Kong, ultra violet beam (UV); BIO-RAD Laboratories, USA.
Illumination photometer
LUX HiTESTER; HIOKI E.E. CORPORATION, Ueda, Nagano, Japan.
Experimental animals
Male SD rats, 300g body wt.; CLEA Japan, Inc., Tokyo, Japan.
Other reagents used were analytical grade.
In in vitro, we cultured SC to reach confluent conditions then, 5 × 105 cell count of SC were suspended in 6 mL of SCM and seeded in a 12.5 cm2 plastic flask. Each system was continued incubation in the SCM including AmB or FITC at 37°C. Concentration of the AmB and FITC added were 1 mg/10 mL SCM, respectively. After 15-30 min incubation, each system was excited with BL-beams or UV ray. The intensity of the BL was 100-300 lx and that of the UV was 70-120 lx in a darkroom. The fluorescence emitted from each specimen was microscopically observed in the dark room. In addition, we added hyaluronic acid (HA) into AmB and FITC in an attempt to sustain their nerve-marking potential. Such prepared AmB/ HA and FITC/HA agents were also tested the fluorescent efficacy of nerve-indicators in in vitro.
The same procedures were carried out in eight cases. Albumin solution (40 mg/mL physiological saline solution) was used for AmB and FITC as the control. Then, we individually administered the 4 different types of nervecontrast agents; namely, AmB, FITC, AmB/HA and FITC/HA, to the model rats. We exposed the sciatic nerves of the rats under inhalation anesthesia in order to topically inject each agent along the sciatic nerves. Such treated portions were temporarily covered with the surrounding muscle layers to prevent dispersion of the liquid agents. Fifteen minutes later, we reopened the wound to straightly observe the treated nerves applying an exciting light in the dark room. The intensity of the exciting BL was 100-300 lx. Distribution of the nerves was photogenically traced along the fluorescence. Physiological saline solution was topically given for the controls. We periodically observed general conditions of the rats and recorded each body weight to check side effects with the treatment. Seven days after the treatment, we harvested the nerve fibres together with the surrounding tissues to histologically investigate loco-regional healing effects with the fluorescent agents. Histological studies on the rats were limitedly carried out in the cases treated with AmB, AmB/ HA, HA and physiological saline solution. These studies were operated using 12 SD rats under the same protocol. All these animal experiments were carried out following the ethical rule of our institute.
Results
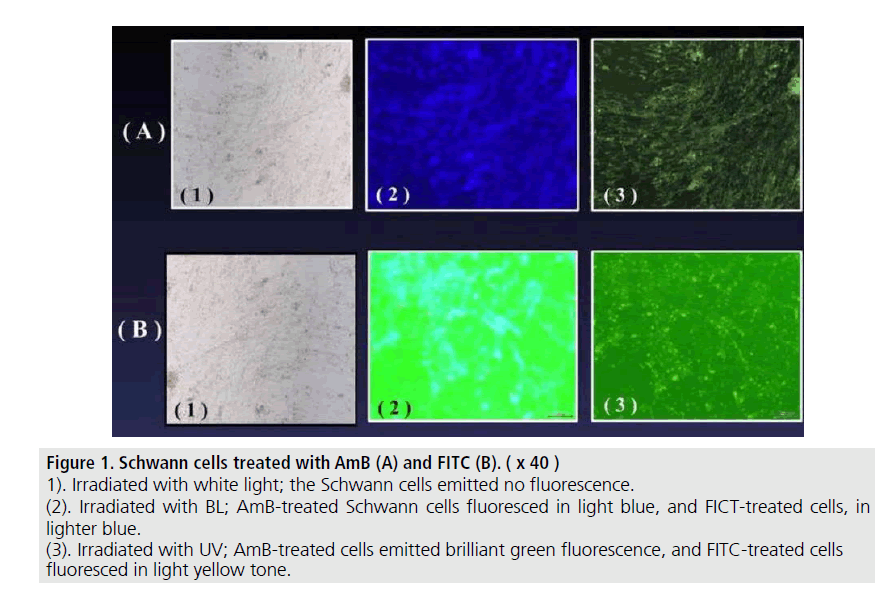
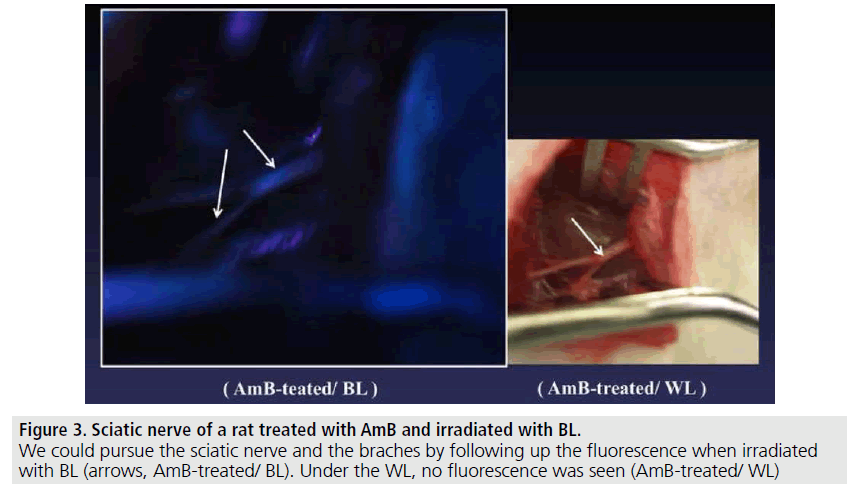
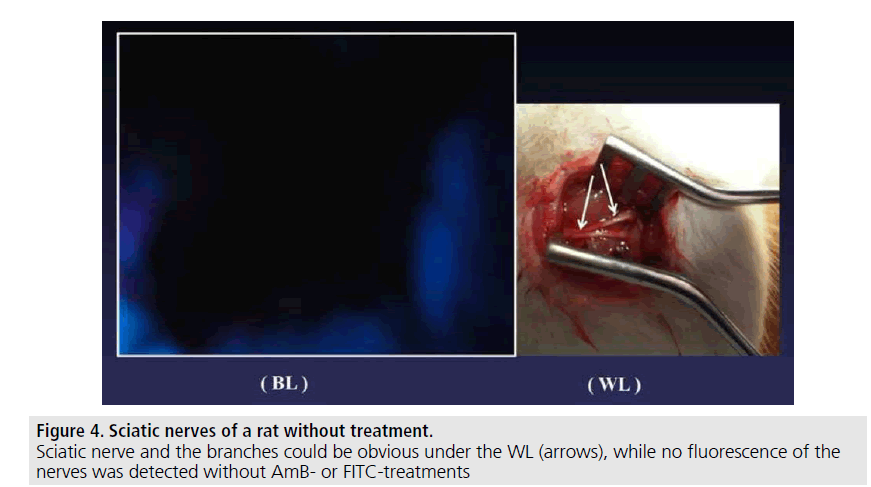
The Schwann cells cultured with SCM including the AmB or FITC fluoresced in green or blue-tone (FIGURE 1). We could observe the emission within 15-30 min. after adding each agent into the SC-culture systems. The AmB/ HA and FITC/HA systems presented similar brightness of fluorescence-imaging to that of AmB and FITC systems. No fluorescence was observed without AmB or FITC (FIGURE 2). The albumin solution induced no fluorescence in the same systems. In in vivo, rat nerve models also glimmered in blue-tone fluorescence when each agent was topically given. We could specifically distinguish the nerve fibres from the surrounding tissues to follow up the peripheral branches (FIGURE 3) while, no fluorescence was seen without AmB or FITC-treatment (FIGURE 4). In vivo fluorescence of the nerves could be observed for 8h after the administration of AmB and FITC. In the cases of treating with AmB/HA and FITC/HA, emission of the fluorescence similarly remained for 8 h.
Figure 1.Schwann cells treated with AmB (A) and FITC (B).
(1). Irradiated with white light; the Schwann cells emitted no fluorescence.
(2). Irradiated with BL; amb-treated Schwann cells fluoresced in light blue, and FICT-treated cells, in lighter blue.
(3). Irradiated with UV; AmB-treated cells emitted brilliant green fluorescence, and FITC-treated cells fluoresced in light yellow tone
Figure 2.Schwann cells treated without AmB.
(1). Irradiated with white light; the Schwann cells emitted no fluorescence, while the arrangement of the cells could be observed.
(2). Irradiated with BL and
(3): irradiated with UV; the cells emitted no fluorescence when excited with BL or UV.
In the cases of treating with FITC, no fluorescence cloud be also induced
Figure 3.Sciatic nerve of a rat treated with AmB and irradiated with BL.
We could pursue the sciatic nerve and the braches by following up the fluorescence when irradiated with BL (arrows, AmB-treated/ BL). Under the WL, no fluorescence was seen (AmB-treated/ WL)
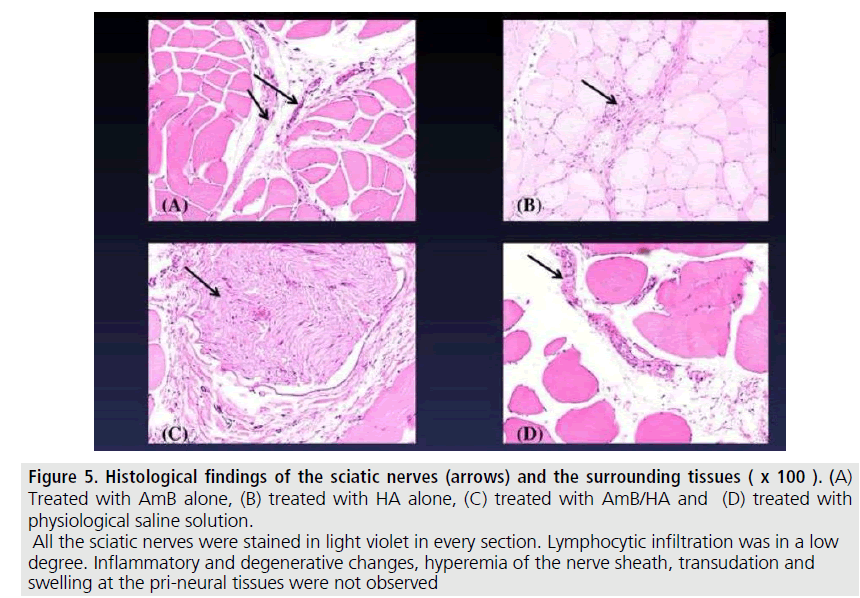
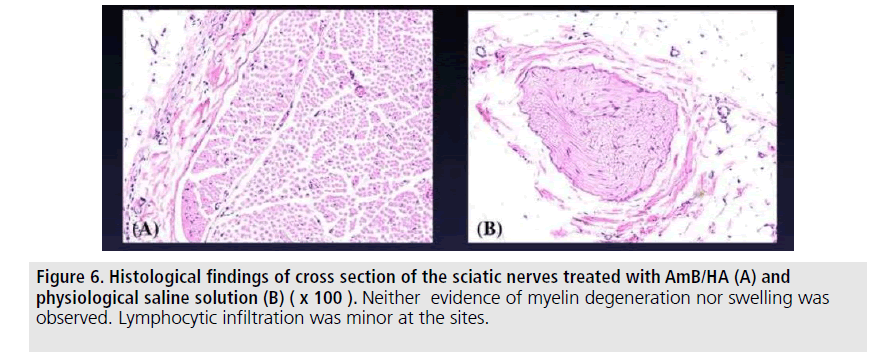
Histological findings of these treated nerves were as follows. Neither serious inflammatory signs nor peri-neural adhesion were perceived. No evidence of degenerative changes in the myelin sheath and topical muscular changes were found in such treated sites. Proliferation of mesothelial cells at the site was not observed (FIGURES 5 and 6). No quantitative analysis was attained in these four different groups.
Figure 5.Histological findings of the sciatic nerves (arrows) and the surrounding tissues.
Treated with AmB alone, (B) treated with HA alone, (C) treated with AmB/HA and (D) treated with physiological saline solution. All the sciatic nerves were stained in light violet in every section. Lymphocytic infiltration was in a low degree. Inflammatory and degenerative changes, hyperemia of the nerve sheath, transudation and swelling at the pri-neural tissues were not observed
Discussion
We could observe fluorescence which the nerve models emitted by staining Schwann sheath with AmB or FITC. In the cases of using AmB/HA and FITC/HA, we could also observe similar brightness of fluorescence. In in vivo studies, we could clearly discriminate fluorescence of the peripheral nerve fibres treated with AmB or FITC from the surrounding tissues. These results strongly suggested that our novel method warranted further studies for nerveimaging as navigation surgery to be clinically useful in intra-operative nerve-preservation. So far as we have experienced, the AmB seemed to be superior to FITC as a nerve-indicator; for the AmB more clearly presented the nerve fibres than the FITC treating. As the excitement (λex) of AmB is reported to be in shorter range than 400 nm (ultraviolet spectrum) and the emission (λem) to be 450-525 nm, the stokes shift [4] is calculated to be 50-125 nm. While, the λex of FITC is 494 nm and the λem is 521 nm, respectively. Therefore, the range of stokes shift of FITC is smaller than 30 nm. We considered that since difference of the range of stokes shift had a significant influence upon the clearness of fluorescent emission, we could catch clear fluorescent images with the AmB than with the FITC.
Our in vivo histological findings revealed that the extent of inflammatory signs and perineural adhesion was minor in both the AmB/ HA-treated and the AmB-treated specimens. In this study, we could not clearly find out the significance of HA. While we inferred that HA might be responsible for nerve adhesion prevention to protect the nerve fibers from adhesions to the surrounding tissues [5,6] and moreover, the HA might effectively function as vehicle of the fluorescent agent to maintain the sustained release of their imaging potential owing to its proper viscosity. We therefore assumed that the HA played an important role in both enhancing anti-adhesion in the process of wound healing and sustained release of the fluorescent agents. The efficacy with our novel nerve-preserving systems would owe to the collaboration of AmB, FITC and HA.
As fluorescent neuro-tract tracers, various kinds of compound substances such as carbocyanines [7]; neuro-DiO and DiI are available. They have been practically applied in basic biological uses alone, but not for clinical applications. In 2011, Gibbs-Strauss and others showed visualization of nerves with their newly synthesized agents [8]. Whitney and others also developed nerve-highlighting fluorescent agents in the same year [9]. In their animal experiments, the peripheral nerves fluoresced within 2 h after intra-venous administration of the agent and it was maintained for 8 h afterward. In 2012, Cotero et al. [10] reported intraoperative visualization of nerves using GE3111 which they newly synthesized in their own methods and evaluated its effect by intravenous administration of the agent into mice. They observed mice nerve systems in detail several h after intra-venous injection of the agent. These newly devised nerve-contrast agents have been examined in vivo by intravenous administration. Contrarily, we locoregionally placed AmB or FITC to the nerve to observe the fluorescence within 5-15 min, which was maintained for 8 h. In our study, the fluorescence could be always visible when the nerve fibres were squarely exposed in the view. No fluorescence could be seen when they were veiled with the surrounding cellular elements such as fat and/or connective tissues. In in vivo studies, we carefully exposed the sciatic nerves keeping the sheath then, gently injected our own agent along the sheath not to injure the nerve fibers under white light. Consequently, we could detect neural fluorescence in the darkroom. From these experiences, we proposed to finely expose the neural tracts as carefully as possible in order to fluorescently visualize nerve systems. Thickness of veiling tissues of the neural tracts was nearly 0 mm in our trials.
Conclusion
For the purpose of clinical application of AmB or FITC as a fluorescent marker, we would like to place the agent along the nerve fibres. The topical administration of each agent will facilitate in intraoperative nerve-preserving techniques.
Since the present study was merely carried out as an exploratory trial applying simple tools and handy equipment in the laboratory, the numbers of present experiment were so limited that we did not statistical analysis whole through the study this time.
We are now programing following studies in more precisely designed using larger animals such as experimental hogs.
Acknowledgement
The authors are grateful to Mr. Naoki Omura, Center of Medical Imaging and Informatics, Iwate Medical University, Morioka, Japan for technical assistance in the experiments.
References
- Coetzee T, Suzuki K, Popko B. New perspectives on the function of myelin galactolipids. Trends. Neurosci. 21(3), 126-130 (1998).
- Sheikh S, Sturuzu A, Kalbacher H et al. Fluorescence imaging of human cells with a novel conjugate of the antifungal nystatin. Med. Chem. 10(4), 348-354 (2014).
- Meador WJH, Yoshino JE, Bigbee JW et al. Differential proliferative responses of cultured Schwann cells to axolemma and myelin-enriched fractions. II. Morphological studies. J. Neurocytol. 14(4), 619-635 (1985).
- Albani JR. Structure and dynamics of macromolecules: Absorption and fluorescence studies. Elsevier Science, Philadelphia, USA (2004).
- Becker JM, Dayton MT, Fazio VW et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane, a prospective randomized, double-blind multicenter study. J. Am. Coll. Surg. 183(4), 297-306 (1996).
- Beck DE, Cohen Z, Study Group Steering Committee et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm® adhesion barrier in abdominopelvic surgery of the intestine. Dis. Colon. Rectum. 46(10), 1310-1319 (2003).
- Julius RL, Martin A, Michaela D et al. Carbocyanine postmortem neuronal tracing: Influence of different parameters on tracing distance and combination with immunocytochemistry. J. Histochem. Cytochem. 46, 901-910 (1998).
- Gibbs SSL, Nasr KA, Fish KM et al. Nerve-highlighting fluorescent contrast agents for image-guided surgery. Mol. Imaging. 10(2), 91-101 (2011).
- Whitney MA, Crisp JL, Nguyen LT et al; Fluorescence studies highlight peripheral nerves during surgery in mice. Nat. Biotechnol. 29(4), 352-356 (2011).
- Cotero VE, Silcovan T, Zhang R et al. Intraoperative fluorescence imaging of peripheral and central nerves through a myelin-selective contrast agent. Mol. Imaging. Biol. 14(6), 708-717 (2012).