Review Article - Interventional Cardiology (2011) Volume 3, Issue 2
Imaging to improve the results of cardiac resynchronization therapy
- Corresponding Author:
- Josef Kautzner
Department of Cardiology, Institute for Clinical & Experimental Medicine
Vídeňská 1958/9, 140 21 Prague 4, Czech Republic
Tel: + 420 261 365 009
Fax: + 420 261 362 985
E-mail: josef.kautzner@medicon.cz
Abstract
Keywords
cardiac imaging, cardiac resynchronization therapy, CT angiography, echocardiography, MRI, single-photon emission computed tomography
Cardiac resynchronization therapy (CRT) has evolved in the last decade into a routine treatment for advanced heart failure in patients with wide QRS complex who do not respond to drug therapy [1,2]. Despite the proven beneficial effects, several problems still exist. The most important is the proportion of nonresponders to this therapy, which varies between 25 and 35% [1]. As there is great variability of definitions of a response to CRT in the published studies [3], this figure may even be an underestimate. The nonresponder rate is generally lower in studies using functional clinical end points (e.g., New York Heart Association class, and 6‑min walk test, among others) compared with studies that used objective parameters of left ventricular (LV) remodeling. Thus, in the latter studies, nonresponse rate reached 40–50% [4]. Therefore, research interest is mainly focused on the improvement of response rates.
The situation is quite complex, since the response rate is predominantly influenced by the following factors: first, the presence and quantification of mechanical dyssynchrony; second, difficulty to implant LV leads in the lateral wall region due to anatomical constraints; third, uncertainty about the best positioning of the LV lead; and fourth, the amount of nonviable myocardium. Other factors include severity of heart failure, degree of remodeling, and setup of atrioventricular delay, among others. All may act individually or can interact in various combinations. The resulting response to CRT influences the prognosis of the patient. The aim of this review is to discuss whether novel imaging tools may overcome at least some of these problems.
Today, a variety of imaging modalities are available in cardiovascular medicine. These include all modes of echocardiography, computed Tomography angiography, rotational angiography, MRI, including delayed enhancement and single-photon emission computed tomography (SPECT). In addition, image integration could be considered that combines different images with real-time fluoroscopy.
Mechanical dyssynchrony
So far, the indication for CRT is based on ECG criteria with QRS width ≥120 ms. Reliable measure of mechanical dyssynchrony is expected to improve selection of appropriate candidates [2]. In this respect, a myriad of echocardiographic techniques have been suggested to accomplish this task [5]. Their detailed analysis is beyond the scope of this review.
They include conventional blood-derived Doppler parameters, such as the difference of pre-ejection periods of aortic and pulmonary outflow, delay between septal and posterior wall motion assessed by M-mode echocardiography, and various techniques of Tissue Doppler Imaging [5]. Despite the fact that many single-center studies have demonstrated reasonable sensitivity and specificity, clinical utility of these parameters remains questionable. Predictors of Response to Cardiac Resynchronization Therapy (PROSPECT) was the first randomized multicenter trial that tested the hypothesis on more appropriate selection of CRT candidates based on 14 echocardiographic parameters of dyssynchrony [6]. However, the study did not confirm this and demonstrated that none of these parameters had sufficient predictive value to replace routine selection criteria for CRT. The most important finding was with regards to the limited reproducibility of these parameters. Accordingly, it was concluded that [6]:
“Despite promising preliminary data from prior single-centre studies, echocardiographic measures of dyssynchrony aimed at improving patient selection criteria for CRT do not appear to have a clinically relevant impact on improving response rates when studied in a multicenter setting such as PROSPECT. Thus, at present, the echocardiographic parameters assessing dyssynchrony do not have enough predictive value to be recommended as selection criteria for CRT beyond current indications.”
The hope is that novel technologies, such as 2D strain and 3D echocardiography [7,8], will have superior reproducibility, with higher accuracy to predict response to CRT. Speckletracking echocardiography is a more recent approach that allows for strain imaging to assess dyssynchrony (Figure 1) [9–11]. Four different types of speckle-tracking approaches have been described, including radial strain (myocardial thickening) and circumferential strain (myocardial shortening), assessed from short-axis views; and transverse and longitudinal strains, assessed from apical views.
Figure 1. An example of speckle-tracking radial strain from a patient with heart failure and left bundle branch block. Dyssynchrony is shown as a time difference (arrows) between time to peak strain in the anterior septum (yellow and cyan) and posterior wall (green and purple) peak strain curves.
Moreover, alternative imaging techniques (e.g., MRI and gated SPECT imaging) may also prove useful in the assessment of LV dyssynchrony and prediction of response to CRT [12,13]. For instance, MRI tissue tagging by evaluating the grid-distortion throughout the cardiac cycle allows accurate analysis of diastolic strain and 3D cardiac motion (rotation, radial contraction and translation) with high temporal resolution of approximately 40 ms [14]. Another example is phase analysis of electrocardiogram-gated SPECT myocardial perfusion imaging to assess LV mechanical dyssynchrony [15]. The superior reproducibility of phase analysis to echocardiography is a promising advantage that may improve prediction of CRT response [16,17]. The first clinical studies demonstrated that clinical response to CRT is related to the presence of LV dyssynchrony. Its quantification (histogram bandwidth and phase standard deviation) appears to be useful to predict response to CRT [18].
LV lead implant
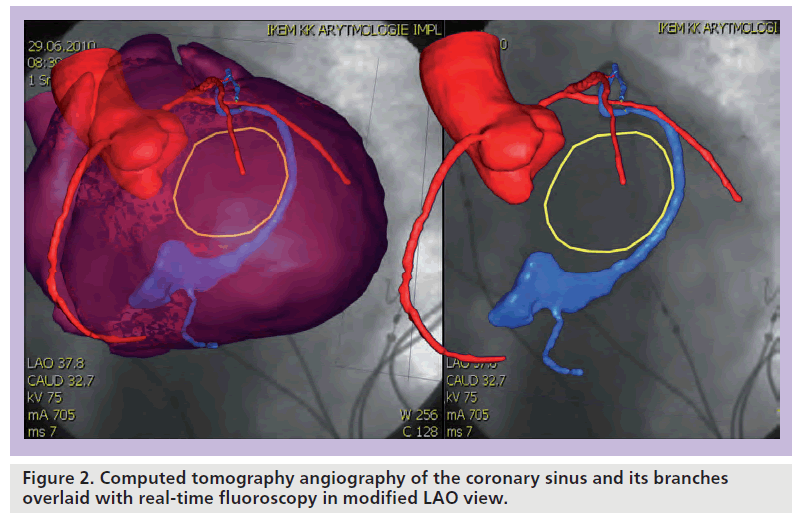
Despite a significant improvement in technical tools for LV lead delivery, some failures to implant the lead do occur. As a reference, we may review success rates of LV lead implant in several multicenter trials on CRT [19–21]. In the CARE-HF trial, which was was terminated in 2003, the implant success rate reached 86% on the first attempt and 95% in total (390 out of 404 attempts) [19]. The Companion trial, conducted between 2000 and 2002, reported successful implants in 539 out of 617 attempts (87%) in the pacemaker group and 541 of 595 implantable cardioverter-defibrillator implants (91%) [20]. The most recent Multicenter Automatic Defibrillator Implantation Trial (MADIT) CRT Trial (2004–2008) published a rate of successful implants of 92.5% (1007 out of 1089 cases) [21]. In our institution, the failure to implant LV lead to a reasonable position varies in recent years by approximately 8–11%. Data from Cleveland Clinic (OH, USA) [22] revealed that the most frequent cause of failure to implant the LV lead was inability to cannulate the ostium of the coronary sinus (49%), followed by anatomical anomalies (24%) or phrenic nerve stimulation (17%). The question is whether imaging can help to improve the implant success rate. Obviously, one can employ intracardiac echocardiography to display the ostium of the coronary sinus and navigate introduction of the delivery sheath. Although feasible, it would substantially increase the cost of the procedure. Thus, the more acceptable option is the use of preacquired 3D CT (or MR) images overlayed with real-time fluoroscopy during the implant (Figure 2). All major manufactures offer software to support this overlay, which may help to navigate to the ostium of the coronary sinus. Auricchio et al. demonstrated that this approach has favorable accuracy in depicting the course of coronary veins [23]. Similarly, rotational angiography could be used to provide a roadmap for the LV lead placement [24,25].
Figure 2. Computed tomography angiography of the coronary sinus and its branches overlaid with real-time fluoroscopy in modified LAO view.
However, there are some other techniques of the coronary sinus cannulation that do not require sophisticated imaging. Our experience suggests that one can cannulate the ostium of the coronary sinus in a very reproducible way using a simple diagnostic catheter inside the delivery sheath [26]. The pattern of intracardiac electrograms from the distal bipole of the catheter allows for easy and rapid orientation about the position of the catheter relative to the ostium. Further introduction of the catheter deeper into the coronary sinus provides a support for the delivery sheath. This can be introduced inside the coronary sinus by sliding over the catheter shaft. This strategy enables standardization of the procedure and shortens fluoroscopic times.
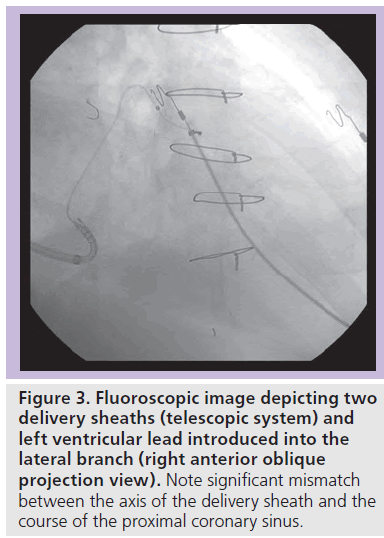
Cannulation of the coronary sinus ostium does not necessarily lead to successful deep delivery of the sheath into the coronary sinus and implantation of the LV lead. Analysis of failures to successfully implant the LV lead demonstrated that, besides operator’s experience, the main contributing factor is the size of the left ventricle [27] or the left atrium [28]. Our explanation for this that enlargement of the left-sided cardiac chambers changes the position of the heart and distorts the plane of the coronary sinus relative to the superior vena cava and the right atrium. This results in enormous angulation between the ostium and the course of the coronary sinus, which prevents deeper introduction of the delivery sheath (Figure 3). Then, it remains mainly the operator ‘s experience that helps to overcome this problem using various strategies such as ‘over-the-wire’ techniques and additional catheters or introducers. Therefore, image integration may help less experienced implants to perform successful implantation of the LV lead. On the other hand, it may prove to be an excellent training tool to speed up implanting skills.
Optimum LV pacing site
There is ongoing discussion about the most appropriate LV pacing site. Experimental studies suggested that optimum pacing site could be the major part of the lateral wall region of the left ventricle [29]. Analysis from the recently published MADIT CRT trial demonstrated that the clinical effect of CRT in less advanced heart failure is similar, whether the LV lead is implanted anteriorly, in the lateral wall or posteriorly. The only position that resulted in no improvement was the apical area of the left ventricle [101]. Nevertheless, other clinical studies provided variable results. Although the left lateral wall is considered the best region by many, some studies demonstrated individual variation in each region and emphasized the need for individualized assessment using various tools [30,31]. There are even studies showing that the optimum pacing site may vary significantly within a radius of few centimeters [30]. Some hemodynamic data suggest that the pacing site is a primary determinant of the hemodynamic response to LV pacing, at least in patients with nonischemic dilated cardiomyopathy. Pacing at the best LV site was associated acutely with fewer nonresponders [30,31]. Preliminary data suggest that positioning of the LV pacing lead outside of the site of latest mechanical activation may result in poor response to CRT [31].
Among imaging techniques, novel tissue Doppler techniques, such as speckle-tracking or triplane tissue synchronization imaging, appear to be the most promising [32,33] to assess the region of maximum mechanical dyssynchrony and help to guide the lead positioning. For example, in a preliminary study [33], 21 consecutive heart failure patients scheduled for CRT implantation were prospectively enrolled to undergo 64-slice CT to visualize the venous system, contrast venography during device implantation, and tissue synchronization imaging before and after implantation. In 12 of the 21 patients, a reasonable match was observed between the area of latest mechanical activation and LV lead position. These patients demonstrated a significant decrease in LV dyssynchrony with an acute reduction in LV endsystolic volume and an improvement in LV ejection fraction. Patients with a mismatch between the area of latest activation and LV lead position remained dyssynchronous without improvement in LV function. Therefore, such strategies may help in planning the implant procedure. Whether such an approach will result in longterm improvement of outcome remains to be confirmed in future studies.
Parallel to echocardiographic techniques, MRI is another tool to evaluate and locate mechanical dyssynchrony, either by tagging or by velocity-encoded imaging [34]. It allows quantitative strain analysis based on 3D circumferential and longitudinal myocardial activation data and has very high spatial and temporal resolution, and high reproducibility. This appears to be the advantage against echocardiography. Advances in the rapid analysis of tagged magnetic resonance images such as harmonic phase and strain-encoded imaging, and the design of novel global indexes of cardiac dyssynchrony may provide a more comprehensive method for selecting maximum dyssynchrony region and optimize location of the pacing site. The harmonic phase method measures the motion from tagged MR images by filtering certain regions in the frequency domain of the images called harmonic peaks [35,36]. The technique called strain-encoded imaging is derived from a standard myocardial tagging sequence that tags the tissue at end-diastole with a sinusoidal tag pattern designed to modulate the longitudinal magnetization orthogonal to the imaging plane. Deformations of tissue during systole will change the local frequency of the pattern in proportion to the through-plane strain component. The distribution of regional contraction (circumferential shortening in long-axis views or longitudinal compression in short-axis views) is then displayed as contrast in the images. Velocityencoded MRI, when applied for myocardial wall motion measurement [37,38], potentially allows direct myocardial wall motion measurement similar to tissue Doppler imaging (i.e., comparing velocity graphs obtained in different parts of the myocardial wall during systole).
The third method potentially suitable for localization of the latest activation site within LV is phase analysis of ECG-gated SPECT myocardial perfusion imaging [39,40]. Ypenburg et al. evaluated echocardiographic and clinical outcome 6 months after CRT in a relatively large cohort of patients with ischemic or dilated cardiomyopathy [39]. A total of 153 (60%) patients had an LV lead positioned at or adjacent to the site of latest activation and these subjects presented with signs of reverse remodeling whereas the rest of the study group did not. They had also lower mortality. In the study by Boogers et al. [40], the patients in whom the LV lead was positioned in the latest activated region had a significant response to CRT compared with patients with a discordant LV lead position (79 vs 26%; p < 0.01). In addition to LV dyssynchrony, nuclear imaging also provides information on viability and scar.
Some authors express their scepticism at using mechanical dyssynchrony imaging to guide the lead implant, especially in patients with narrow QRS complexes. The argument is that identification of dyssynchrony regions may not mean that pacing in these sites will better correct mechanical dyssynchrony. Some studies demonstrated that such regions do not necessarily correspond with the late activated regions and, thus, may not be correctable by pacing [41,42].
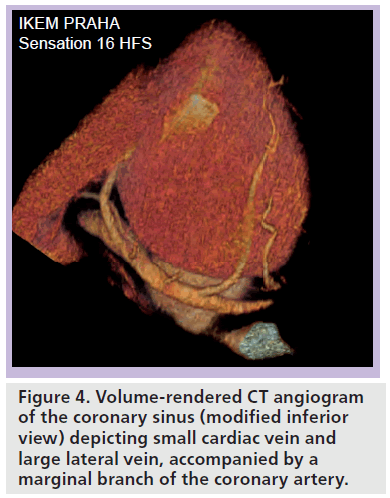
However, before the aforementioned techniques will prove effective in selecting the optimum pacing site, the majority of routine implants are performed with the goal to place the lead in the lateral and/or posterolateral or anterolateral vein, depending on the individual anatomy. In this situation, preprocedural assessment of the anatomy of the coronary venous tree may prove to be very helpful in planning the implant. In particular, CT angiography is able to evaluate all branches of the coronary sinus and great cardiac veins in detail (Figure 4) [43,44]. MRI is another option that allows evaluation of cardiac venous anatomy without radiation exposure [45,46]. All these imaging techniques permit identification of potential anatomic factors that may pose difficulties in cannulating and advancing the LV pacing lead, such as valves at the ostium of the ventricular veins or absence of the lateral vein. In patients without suitable posterolateral veins, in order to allow successful implantation of the LV pacing lead, a surgical implantation (via minithoracotomy or videoassisted) at the latest activated region may be preferred. Importantly, in some studies, the venous anatomy was strongly related to the presence of prior myocardial infarction; patients with previous myocardial infarction had left marginal veins significantly less frequently [44].
Assessment of myocardial viability
One imaging modality that permits noninvasive assessment of viability is nuclear perfusion imaging [13]. Some studies using SPECT in patients with an ischemic cardiomyopathy and poor systolic function found that nonviable tissue in the inferior or lateral wall was more frequently present in patients with a QRS ≥120 ms than in patients with a QRS <120 ms (29 vs 7%; p < 0.01) [47]. Another study evaluated the presence of scar tissue with gated SPECT using 99mTc-tetrofosmin before CRT implantation. Patients without scar tissue in the region of the LV lead placement significantly improved in functional class, quality of life, 6-min walk test, LV volumes and ejection fraction, whereas no improvement was observed in patients with scar tissue [48].
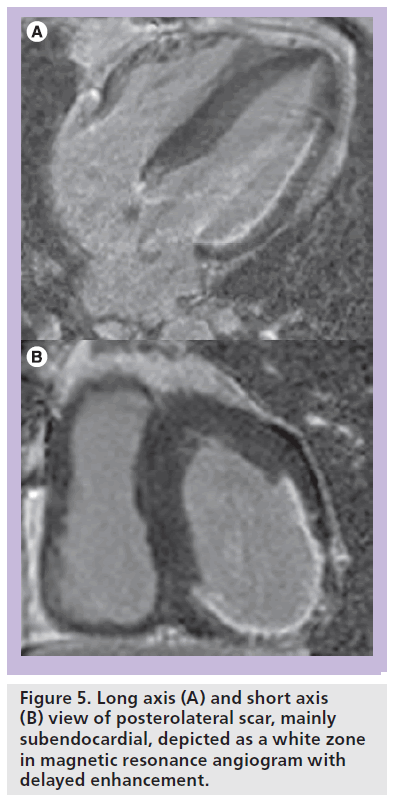
The more promising noninvasive imaging modality to evaluate myocardial scar is cardiac MRI. In addition to the detection of wall motion abnormalities, contrast-enhanced MRI enables the depiction of transmural and nontransmural infarctions (Figure 5) with better spatial and contrast resolution and better accuracy than scintigraphic techniques [49]. This is especially pertinent in patients with ischemic cardiomyopathy who comprise a subgroup of subjects with a significantly worse response rate to CRT. One of the first studies by Bleeker et al. studied a total of 40 coronary artery disease patients with MRI before undergoing CRT [50]. The authors documented a transmural posterolateral scar in a third of the patients. In contrast to patients without posterolateral scar tissue, these patients demonstrated a low response rate and did not demonstrate an improvement in clinical or echocardiographic parameters. In addition, parameters of LV dyssynchrony remained unchanged after CRT implantation in the presence of scar tissue. A study by White et al. evaluated the ability of delayed enhancement MRI to predict clinical response to CRT and found that the amount of total scar was significantly higher in the nonresponse compared with the response group [51]. Similarly, Ypenburg et al. studied 34 patients with an ischemic cardiomyopathy scheduled to undergo CRT [52]. Contrast-enhanced MRI was used to determine total scar burden, using a 17-segment model with a five-point hyperenhancement scale. Again, the amount of scar tissue correlated inversely with response to CRT.
Figure 5. Long axis (A) and short axis (B) view of posterolateral scar, mainly subendocardial, depicted as a white zone in magnetic resonance angiogram with delayed enhancement.
Some authors believe that patients identified by the aforementioned techniques cannot be simply considered as candidates with a very high probability of treatment failure who cannot respond to CRT. Therefore, they challenge the view that patients with ischemic cardiomyopathy and extensive scar tissue should be witheld CRT and suggest that such patients might benefit the most from invasive mapping-targeted selection of LV pacing sites and endocardial pacing [53]. Others demonstrated that the response to CRT could be independent of the presence of extensive myocardial scarring [54]. It was demonstrated that LV pacing at sites with ischemia, hibernation or nontransmural scar did not appear to modify the effect of CRT compared with viable tissue. It follows that incorporation in the standard selection criteria of algorithms to predict the response to CRT is not yet ready for clinical use.
Conclusion
Novel imaging tools have the potential to increase the proportion of responders to CRT. Sophisticated echocardiographic techniques, MRI or phase analysis of ECG-gated SPECT myocardial perfusion imaging may help to assess and quantify mechanical dyssynchrony and, thus, better selection of candidates to CRT. However, to date, there is no agreement on which technique is best suited to this task. In addition, practical feasibility for everyday use remains an issue. 3D venous anatomy assessed by CT angiography, rotational angiography and/or MRI could be integrated with online fluoroscopy and may help to improve implant rates and increase skills of the implanters. Techniques detecting mechanical dyssynchrony are studied in order to assist LV lead positioning. Evaluation of the extent and location of myocardial scars using SPECT or MRI with delayed enhancement can further improve selection of responders to CRT. At this stage, multicenter trials are needed to confirm these expectations and change clinical practice.
Future perspective
Improvement in quantification of mechanical dyssynchrony and a better understanding of its relationship to electrical dyssynchrony is expected in the next 5–10 years to improve selection of the most appropriate candidates for CRT. Optimization of the pacing site and/or combination with other interventions such as percutaneous mitral annuloplasty may have additional value. Such a tailored approach should maximize the benefit of this therapy, possibly expanding its indications.
Financial & competing interests disclosure
This work was supported by the research grant NS/9698-2 of the Internal Grant Agency of the Ministry of Health of the Czech Republic. J Kautzner received consulting honoraria from Biosense Webster, Boston Scientif ic, Biotronik, GE Healthcare, Medtronic, Siemens and St Jude Medical. This paper was supported by the Internal Grant Agency of the Czech Ministry of Health Care (Grant MZ ?R NS/9698-2). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
▪ Novel imaging tools include all modes of echocardiography, CT angiography, rotational angiography, MRI, SPECT and image integration that combines different images with real-time fluoroscopy.
Mechanical dyssynchrony
▪ Reliable assessment of mechanical dyssynchrony is a prerequisite of more tailored selection of candidates for CRT. Techniques such as 2D strain, 3D echocardiography, MRI and phase analysis of electrocardiogram-gated SPECT myocardial perfusion imaging are the most promising imaging modalities.
Left ventricular lead implant
▪ Preacquired 3D CT (or MR) images overlaid with real-time fluoroscopy during the implant, may help to guide successful implantation of the left ventricular lead and minimize complications. A similar role may be applicable to rotational angiography.
Optimum left ventricular pacing site
▪ Special imaging techniques such as tissue Doppler, MRI or SPECT appear to be the most promising at assessing the region of maximum mechanical dyssynchrony and optimizing left ventricular lead positioning. Preprocedural CT or MR angiography helps to evaluate the coronary venous anatomy and select the appropriate implant strategy.
Assessment of myocardial viability
▪ SPECT or contrast-enhanced MRI are useful to evaluate the extent of myocardial scars, especially in patients with ischemic cardiomyopathy. Preliminary data suggest that the magnitude of scar burden is inversely proportional to degree of reverse remodeling of the left ventricle.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- McAlister FA, Ezekowitz J, Hooton N et al.: Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 297(22), 2502–2514 (2007).
- Dickstein K, Vardas PE, Auricchio A et al.: 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Eur. Heart J. 31(21), 2677–2687 (2010).
- Fornwalt BK, Sprague WW, BeDell P et al.: Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation 121(18), 1985–1991 (2010).
- Birnie DH, Tang AS: The problem of non-response to cardiac resynchronization therapy. Curr. Opin. Cardiol. 21(1), 20–26 (2006).
- Gorcsan J 3rd, Abraham T, Agler DA et al.: Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting – a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J. Am. Soc. Echocardiogr. 21(3), 191–213 (2008).
- Chung ES, Leon AR, Tavazzi L et al.: Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 117(20), 2608–2616 (2008).
- Kleijn SA, van DJ, de Cock CC, Allaart CP, van Rossum AC, Kamp O: Assessment of intraventricular mechanical dyssynchrony and prediction of response to cardiac resynchronization therapy: comparison between tissue Doppler imaging and real-time three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 22(9), 1047–1054 (2009).
- Soliman OI, Geleijnse ML, Theuns DA et al.: Usefulness of left ventricular systolic dyssynchrony by real-time three-dimensional echocardiography to predict long-term response to cardiac resynchronization therapy. Am. J. Cardiol. 103(11), 1586–1591 (2009).
- Delgado V, Ypenburg C, van Bommel RJ et al.: Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J. Am. Coll. Cardiol. 51(20), 1944–1952 (2008).
- Tanaka H, Hara H, Saba S, Gorcsan J 3rd: Prediction of response to cardiac resynchronization therapy by speckle tracking echocardiography using different software approaches. J. Am. Soc. Echocardiogr. 22(6), 677–684 (2009).
- Tanaka H, Hara H, Saba S, Gorcsan J 3rd: Usefulness of three-dimensional speckle tracking strain to quantify dyssynchrony and the site of latest mechanical activation. Am. J. Cardiol. 105(2), 235–242 (2010).
- Westenberg JJ, Lamb HJ, van der Geest RJ et al.: Assessment of left ventricular dyssynchrony in patients with conduction delay and idiopathic dilated cardiomyopathy: head-to-head comparison between tissue doppler imaging and velocity-encoded magnetic resonance imaging. J. Am. Coll. Cardiol. 47(10), 2042–2048 (2006).
- Henneman MM, van der Wall EE, Ypenburg C et al.: Nuclear imaging in cardiac resynchronization therapy. J. Nucl. Med. 48(12), 2001–2010 (2007).
- Fischer SE, McKinnon GC, Scheidegger MB, Prins W, Meier D, Boesiger P: True myocardial motion tracking. Magn. Reson. Med. 31(4), 401–413 (1994).
- Chen J, Garcia EV, Folks RD et al.: Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J. Nucl. Cardiol. 12, 687–695 (2005).
- Trimble MA, Velazquez EJ, Adams GL et al.: Repeatability and reproducibility of phase analysis of gated SPECT myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl. Med. Commun. 29, 374–381 (2008).
- Lin X, Xu H, Zhao X et al.: Repeatability of left ventricular dyssynchrony and function parameters in serial gated myocardial perfusion SPECT studies. J. Nucl. Cardiol. 17, 811–816 (2010).
- Henneman MM, Chen J, Dibbets P et al.: Can LV dyssynchrony as assessed with phase analysis on gated myocardial perfusion SPECT predict response to CRT? J. Nucl. Med. 48, 1104–1111 (2007).
- Cleland JG, Daubert JC, Erdmann E et al.: The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 352(15), 1539–1549 (2005).
- Bristow MR, Saxon LA, Boehmer J et al.: Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 350(21), 2140–2150 (2004).
- Moss AJ, Hall WJ, Cannom DS et al.: Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361(14), 1329–1338 (2009).
- Navia JL, Atik FA, Grimm RA et al.: Minimally invasive left ventricular epicardial lead placement: surgical techniques for heart failure resynchronization therapy. Ann. Thorac. Surg. 79(5), 1536–1544 (2005).
- Auricchio A, Sorgente A, Soubelet E et al.: Accuracy and usefulness of fusion imaging between three-dimensional coronary sinus and coronary veins computed tomographic images with projection images obtained using fluoroscopy. Europace 11(11), 1483–1490 (2009).
- Blendea D, Mansour M, Shah RV et al.: Usefulness of high-speed rotational coronary venous angiography during cardiac resynchronization therapy. Am. J. Cardiol. 100(10), 1561–1565 (2007).
- Kofune M, Watanabe I, Ashino S et al.: Three-dimensional reconstruction of the coronary sinus with rotational angiography. Circ. J. 72(6), 1020–1021 (2008).
- Kautzner J, Riedlbauchova L, Cihak R, Bytesnik J, Vancura V: Technical aspects of implantation of LV lead for cardiac resynchronization therapy in chronic heart failure. Pacing Clin. Electrophysiol. 27(6 Pt 1), 783–790 (2004).
- Bisch L, Da CA, Dauphinot V et al.: Predictive factors of difficult implantation procedure in cardiac resynchronization therapy. Europace 12(8), 1141–1148 (2010).
- Macias A, Gavira JJ, Castano S, Alegria E, Garcia-Bolao I: Left ventricular pacing site in cardiac resynchronization therapy: clinical follow-up and predictors of failed lateral implant. Eur. J. Heart Fail. 10(4), 421–427 (2008).
- Helm RH, Byrne M, Helm PA et al.: Three-dimensional mapping of optimal left ventricular pacing site for cardiac resynchronization. Circulation 115(8), 953–961 (2007).
- Delnoy PP, Ottervanger JP, Luttikhuis HO et al.: Pressure-volume loop analysis during implantation of biventricular pacemaker/ cardiac resynchronization therapy device to optimize right and left ventricular pacing sites. Eur. Heart J. 30(7), 797–804 (2009).
- Derval N, Steendijk P, Gula LJ et al.: Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J. Am. Coll. Cardiol. 55(6), 566–575 (2010).
- Becker M, Altiok E, Ocklenburg C et al.: Analysis of LV lead position in cardiac resynchronization therapy using different imaging modalities. JACC Cardiovasc. Imaging 3(5), 472–481 (2010).
- Van de Veire NR, Marsan NA, Schuijf JD et al.: Noninvasive imaging of cardiac venous anatomy with 64-slice multi-slice computed tomography and noninvasive assessment of left ventricular dyssynchrony by 3-dimensional tissue synchronization imaging in patients with heart failure scheduled for cardiac resynchronization therapy. Am. J. Cardiol. 101(7), 1023–1029 (2008).
- Lardo AC, Abraham TP, Kass DA: Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J. Am. Coll. Cardiol. 46(12), 2223–2228 (2005).
- Osman NF, McVeigh ER, Prince JL: Imaging heart motion using harmonic phase MRI. IEEE Trans. Med. Imaging 19(3), 186–202 (2000).
- Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP: Human heart: tagging with MR imaging – a method for noninvasive assessment of myocardial motion. Radiology 169(1), 59–63 (1988).
- Prinzen FW, Hunter WC, Wyman BT, McVeigh ER: Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J. Am. Coll. Cardiol. 33(6), 1735–1742 (1999).
- Leclercq C, Faris O, Tunin R et al.: Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation 106(14), 1760–1763 (2002).
- Ypenburg C, van Bommel RJ, Delgado V et al.: Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J. Am. Coll. Cardiol. 52, 1402–1409 (2008).
- Boogers MJ, Chen J, van Bommel RJ et al.: Optimal left ventricular lead position assessed with phase analysis on gated myocardial perfusion SPECT. Eur. J. Nucl. Med. Mol. Imaging 38(2), 230–238 (2011).
- Kass DA: An epidemic of dyssynchrony: but what does it mean? J. Am. Coll. Cardiol. 51(1), 12–17 (2008).
- Klemm HU, Krause KT, Ventura R et al.: Slow wall motion rather than electrical conduction delay underlies mechanical dyssynchrony in postinfarction patients with narrow QRS complex. J. Cardiovasc. Electrophysiol. 21(1), 70–77 (2010).
- Jongbloed MR, Lamb HJ, Bax JJ et al.: Noninvasive visualization of the cardiac venous system using multislice computed tomography. J. Am. Coll. Cardiol. 45(5), 749–753 (2005).
- Van de Veire NR, Schuijf JD, De SJ et al.: Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J. Am. Coll. Cardiol. 48(9), 1832–1838 (2006).
- Chiribiri A, Kelle S, Gotze S et al.: Visualization of the cardiac venous system using cardiac magnetic resonance. Am. J. Cardiol. 101(3), 407–412 (2008).
- Younger JF, Plein S, Crean A, Ball SG, Greenwood JP: Visualization of coronary venous anatomy by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 11, 26 (2009).
- De Winter O, Van de Veire N, Van Heuverswijn F, Van Pottelberge G, Gillebert TC, De Sutter J: Relationship between QRS duration, left ventricular volumes and prevalence of nonviability in patients with coronary artery disease and severe left ventricular dysfunction. Eur. J. Heart Fail. 8(3), 275–277 (2006).
- Ypenburg C, Schalij MJ, Bleeker GB et al.: Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur. Heart J. 28(1), 33–41 (2007).
- Kim RJ, Fieno DS, Parrish TB et al.: Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 100(19), 1992–2002 (1999).
- Bleeker GB, Kaandorp TA, Lamb HJ et al.: Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation 113(7), 969–976 (2006).
- White JA, Yee R, Yuan X et al.: Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J. Am. Coll. Cardiol. 48(10), 1953–1960 (2006).
- Ypenburg C, Roes SD, Bleeker GB et al.: Effect of total scar burden on contrastenhanced magnetic resonance imaging on response to cardiac resynchronization therapy. Am. J. Cardiol. 99(5), 657–660 (2007).
- Zaca V, Mondillo S, Gaddi R, Favilli R: Profiling cardiac resynchronization therapy patients: responders, non-responders and those who cannot respond – the good, the bad and the ugly? Int. J. Cardiovasc. Imaging 27(1), 51–57 (2011).
- Riedlbauchova L, Brunken R, Jaber WA et al.: The impact of myocardial viability on the clinical outcome of cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 20(1), 50–57 (2009).
- Singh JP: Left ventricular lead position and clinical outcome: findings from MADIT-CRT www.hrsonline.org/Sessions/PastMeetings/ upload/HRS_10_LBFlyer1Thu-v3.pdf
▪ A comprehensive review on the definition of nonresponse to cardiac resynchronization therapy (CRT) and its causes.
▪▪ Detailed review of all echocardiographic techniques used in the field of CRT.
▪▪ Landmark randomized trial on the predicitive power of selected echocardiographic parameters of dyssynchrony in selection of appropriate candidates for CRT.
▪ Technical paper on overlay of 3D computed tomography images of the coronary venous tree and real-time fluoroscopy.
▪ Study on the use of myocardial deformation imaging during left ventricular pacing to optimize the left ventricular lead position in cardiac resynchronization therapy.
▪ Interesting review of the problem of nonresponse to CRT, with some specific suggestions for how to proceed further.
▪ Website