Research Article - Clinical Investigation (2022) Volume 12, Issue 2
Identical Ltbp2 Mutation In Poag Patients and High Risk Individual from Different Ethnic Background
- Corresponding Author:
- Bai Lang
Department of Ophthalmology
Nanfang Hospital, Southern Medical University
Number 1838 Guangzhou North Avenue
Guangzhou 510515, Guangdong Province, China
E-mail: bailangsfy@126.com
Received: 07-February-2022, Manuscript No. fmci-22-56864; Editor assigned: 09-February-2022, PreQC No. fmci-22-56864 (PQ); Reviewed: 23-February-2022, QC No. fmci-22-56864 (Q); Revised: 28-Feb-2022, Manuscript No. fmci-22-56864 (R); Published: 07-March-2022, DOI: 10.37532/2041-6792.2022.12(2).36-47
Abstract
Background: Glaucoma refers to a group of diseases characterized by atrophy of the optic nerve and visual field defects. Pathological increase of intraocular pressure is the main risk factor. Once diagnosed, glaucoma often requires immediate treatment. The purpose of treatment is the effective and timely control of elevated intraocular pressure, in order to prevent further damage to the optic nerve. However, the current various treatment measures mainly consist in reducing the intra-ocular pressure level.
Genetic factors seem to play an undeniable role in glaucoma pathogenesis; Kass and Becker were among the first to clarify the possible association between family history and glaucoma. Their initial investigations on hereditary aspects of glaucoma led to the discovery of a relationship defying simple genetic analysis. A genetic basis for glaucoma has been established through epidemiological studies, reports of large affected families, Genome-Wide Association Studies (GWAS), and animal models of glaucoma. However, the inheritance pattern of this disorder seems to be multifactorial resulting from the interaction of one or more genes and/or environmental stimuli. To date, there have multiple genetic loci and genes have been linked to POAG.
Mutations in several genes are reported to cause POAG, these genes account for less than 10% of cases worldwide. How these genes cause or influence the likelihood of developing POAG is of major interest. Genetic background (including genetic and ethnic differences) plays an important role in the pathogenesis of glaucoma, therefore, the genetic study of glaucoma pedigrees is of great significance. Targeted screening and collection of targeted genetic data for high-risk groups can provide solid evidence for clinical treatment, save patients a lot of time and money, and provide reasonable advice to undiagnosed family members in order to facilitate early detection, early diagnosis and early treatment of the condition to avoid advanced damage to the visual field.
Objectives: This study was conducted in order to investigate the role played by genetic factors in glaucoma pathogenesis through mutation screening in 3 POAG affected patients from a different ethnic background and one high-risk individual.
Methods: 1. Review and summary of relevant literature.
1. Three POAG patients from a different ethnic background and possessing both a positive family history of the condition, one high-risk individual as well as two matching ethnic background unrelated control subjects were selected for this study from the Ophthalmology Department of Nanfang Hospital. Pedigrees were drawn after inquiry of detailed family history, information on relatives, medical records and family reports. Full body characteristics (height, weight, age, sex, blood pressure, etc.) from all participants were recorded; before proceeding to conduct a complete ophthalmic examination including visual acuity, slit lamp, tonometry, goniometer, fundus (ophthalmoscopy), perimetry (visual field) and Optical Coherence Tomography (OCT).
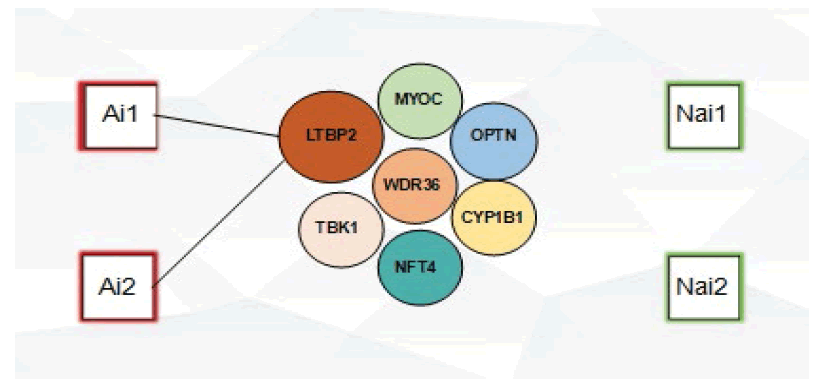
2. Peripheral blood ( 10 ml ) was then collected from and later sent to BGI to conduct a wholeexome sequencing, with special attention given to the following glaucoma-associated genes MYOC, OPTN, WDR36, TBK1, NFT4, CYP1B1, LTBP2.
Results: No MYOC, OPTN, WDR36, TBK1, NFT4, LTBP2 and CYP1B1 mutations were detected in our study, however, the presence of LTBP2 variants was observed in the case group, comprising of 6 variants observed in subject Ai1 while 3 variants were observed in subject Ai2. The rs2196861 and rs5809669 variants are harboured by both Ai1 and Ai2. No genetic mutations were observed in the control group.
Conclusion: Glaucoma is a complex disease because genetic and environmental factors both play important roles in its pathogenesis. Our study detected novel LTBP2 gene mutations in 2 POAG patients from different ethnic backgrounds and possessing both a positive family history. The LTBP2 gene is associated with PCG, but very few studies support its role in POAG occurrence. Further investigations are needed consisting in screening large POAG families for LTBP2 mutations in order to better quantify their implication to the condition.
Keywords
Glaucoma • Optic nerve • Intra-Ocular Pressure (IOP) • Aqueous humour • Single Nucleotide Polymorphism (SNP) • Long non-coding RNA
Introduction
Glaucoma (pronunciation: glaw-ko’me) is an umbrella term used for a heterogeneous group of ocular disorders with multi-factorial etiology, often associated with elevated intraocular pressure and characterized by the progressive excavation of the optic disc, optic atrophy, and gradual loss of the visual field caused by the slow death of retinal ganglion cells and their axons. Most often glaucoma is an asymptomatic disease, especially at its beginning [1]. It is painless, and peripheral visual field damage could well go unnoticed. Also, glaucoma can evolve for several years before being diagnosed with a simple routine examination with the ophthalmologist [2]. Glaucoma is not a term commonly used to solely designate elevated intraocular pressure with no detectable impact on the optic nerve, which still needs to be properly monitored because a large number of elevated IOP may yet evolve into glaucoma.
It is now known that the level of intraocular pressure likely to cause glaucoma is unique to each individual, which makes the diagnosis of the disease difficult at the early stages. It is difficult to provide accurate data on the frequency of glaucoma. The numbers depend on the definition of glaucoma taken into account, the type of the ethnic group being studied and also the average age of the population. Glaucoma is currently considered to be the leading cause of irreversible blindness worldwide, even though there is an evident variation in its subtypes and risks among different races and countries [3-5].
The number of people affected with Glaucoma (POAG and PACG combined) worldwide was estimated to be 60.5 million in 2010, Open-Angle Glaucoma (OAG) patients represented 3/4 of the total number of glaucoma patients, whereas women roughly comprised nearly half of the total number of OAG patients, 70% of Angle-Closure Glaucoma (ACG). This number will increase significantly with future population growth and increasing life expectancy and it has been estimated that by the year 2020, approximately 79.6 million people will be affected including 3 million in the USA and 21 million in China half of whom ignore the fact that they have developed the disease [6-11]. According to recent studies, It has been estimated that glaucoma causes blindness in close to 10% of those affected by the disease [12]. The importance of glaucoma blindness is compounded by the fact that the damage is irreversible, and may progress from “definitional” blindness (visual acuity below 3/60 or severely constricted visual fields) to loss of light perception. Bilateral blindness, set to be present in more than 4.5 million OAG patients and 3.9 million ACG patients by the year 2010, is expected to witness a remarkable rise in 2020 and is set to reach 5.9 million and 5.3 million people respectively. The number of people affected with glaucoma worldwide is expected to reach 111.8 million by 2040 with Africa and Asia being more heavily affected [13]. Chinese PACG patients account for nearly half of the total number of PACG cases worldwide [7].
The association of family history and open-angle glaucoma has been the centre of interest of researchers for years. Kass and Becker were among the first to clarify the possible association between family history and glaucoma. Their initial investigations on hereditary aspects of glaucoma led to the discovery of a relationship defying simple genetic analysis. Despite the fact that anyone can be affected by glaucoma, people who have a family history are at an even greater risk. It has been demonstrated that positive family history, whether present at birth or acquired later in life may be a major risk factor for the presence and severity of both POAG and PACG, with siblings of affected individuals showing a greater risk of developing the disease [14-17]. Nearly half of all primary OAG patients possess a positive family history, and not only are their first degree relatives (parents, siblings or children ) said to comparatively have a 9-fold increased risk of developing glaucoma, but patients with positive family history also tend to develop the disease at a younger age than those without such a history [18-20].
Objective
This study was conducted in order to investigate the role played by genetic factors in glaucoma pathogenesis through mutations screening in 2 POAG affected patients from a different ethnic background and possessing both a positive family history.
Methodology
Study Protocol and Ethical Consideration
The study was conducted with the approval of the ethics board of Southern Medical University, Nanfang Hospital. Written informed consents were obtained from all participants before the studies and the study conformed to the Declaration of Helsinki [20].
Inclusion and Exclusion Criteria
To be included in the study, subjects had to be open-angle glaucoma affected patients with at least one direct relative also affected by the disease. Conscious with full civil capacity, presenting no other serious systemic diseases, such as hypertension, poor diabetes control, and able to clearly understand and agree with the purpose and process of the clinical study.
Patients with a previous history of severe eye diseases, secondary glaucoma induced conditions such as elevated intraocular pressure, visual field defects, and changes in the fundus caused by other diseases as well as craniocerebral disease are excluded.
Criteria for diagnosis of POAG were the presence of at least two of the following criteria: an IOP greater than 21 mmHg in at least one eye or inter-eye IOP asymmetry exceeding 8 mmHg; characteristic glaucomatous optic nerve head or Retinal Nerve Fiber Layer (RNFL) changes (e.g., vertical cupping, neural rim thinning or loss, RNFL dropout); and visual field defects not attributable to other causes. None of the patients with diagnosed POAG had other ocular anomalies, and all presented with an open anterior chamber angle in the affected eyes.
Study Subjects and Recruitment
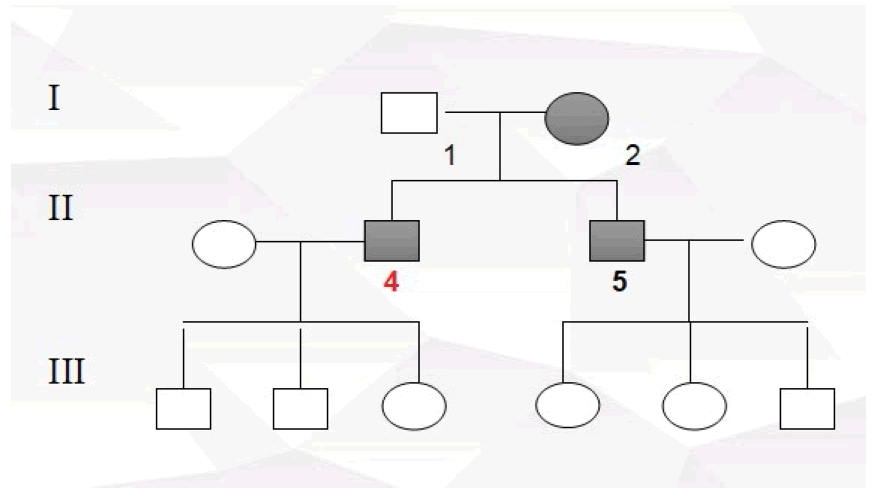
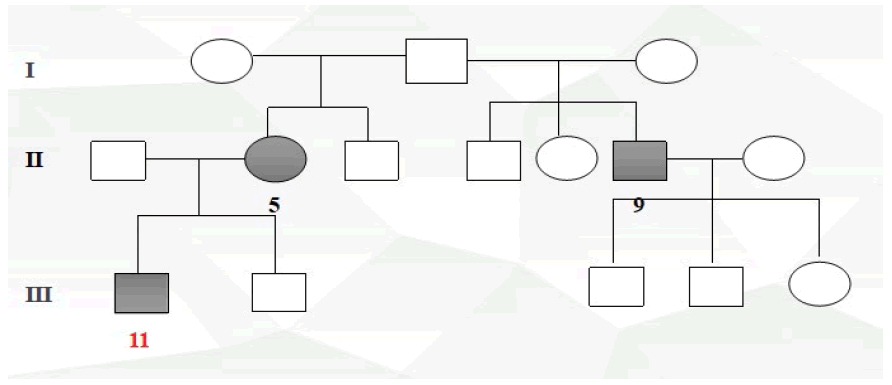
Participants were recruited from the Ophthalmology Department of Nanfang Hospital between March 2017 to March 2018. For the purpose of our study, we designed a case-control study. The cases were ethnically matched with the controls. The cases for the study were made up of glaucoma affected individuals possessing positive family history (Ai1, Ai2), the controls (Nai1, Nai2) were normal healthy subjects with no family history of glaucoma and without self-reported eyes disorders. Sample information is shown in Table 1.
| Sample ID | Ai1 | Ai2 | Nai1 | Nai2 | |
|---|---|---|---|---|---|
| Gender | M | M | F | F | |
| Age at diagnosis | 51 | 26 | |||
| Present Age (years) | 53 | 27 | 26 | 23 | |
| Ethnicity | Asian | African | Asian | African | |
| Disease | POAG | JOAG | Refractive errors | Refractive errors | |
| Affected eye | Bilateral | Bilateral | |||
| IOP at diagnosis | OD: 48 mmHg OS: 46 mmHg | OD: >50 mmHg | |||
| OS: 42 mmHg | |||||
| Present IOP | OD: 20 mmHg OS: 21 mmHg | OD:13 mmHg | |||
| OS:14 mmHg | |||||
| Medical | Levobunolol, Brinzolamide, | Levobunolol, Bimatoprost | |||
| Treatment | Surgical | Bilateral Trabeculectomy | None | ||
| POAG Family History | Positive | Positive | Negative | Negative | |
| Affected relatives | Brother, Mother | Mother, Uncle | None | None | |
| Others condition | Diabetes (DM) | None | None | None | |
Table 1: Sample Information.
Two POAG affected individuals from a different ethnic background, both possessing positive family history of the condition who met our criteria as well as two unrelated control subjects sharing the same ethnic background with the recruited affected individuals were selected and enrolled for this study.
Medical Examinations and Data Collection
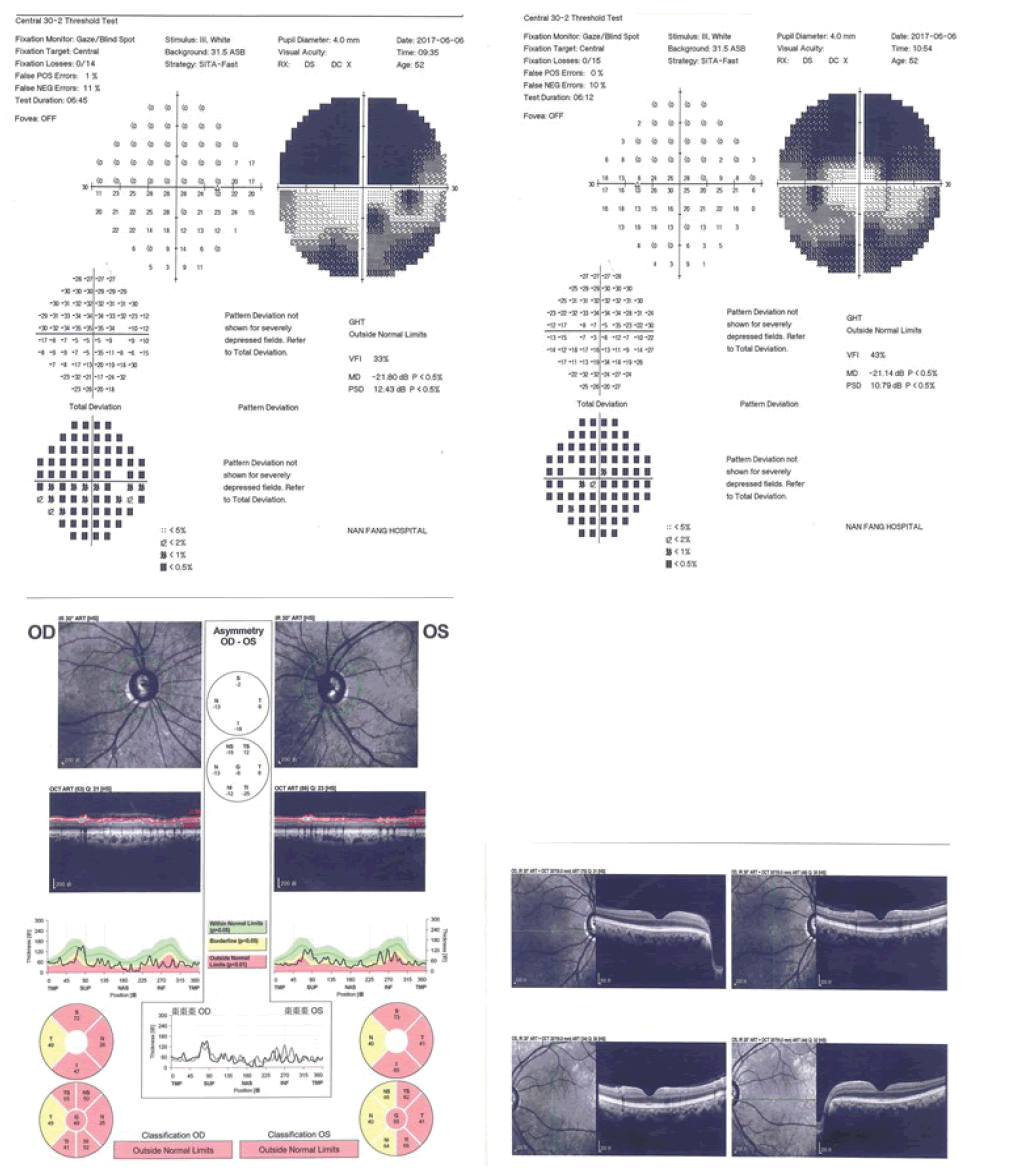
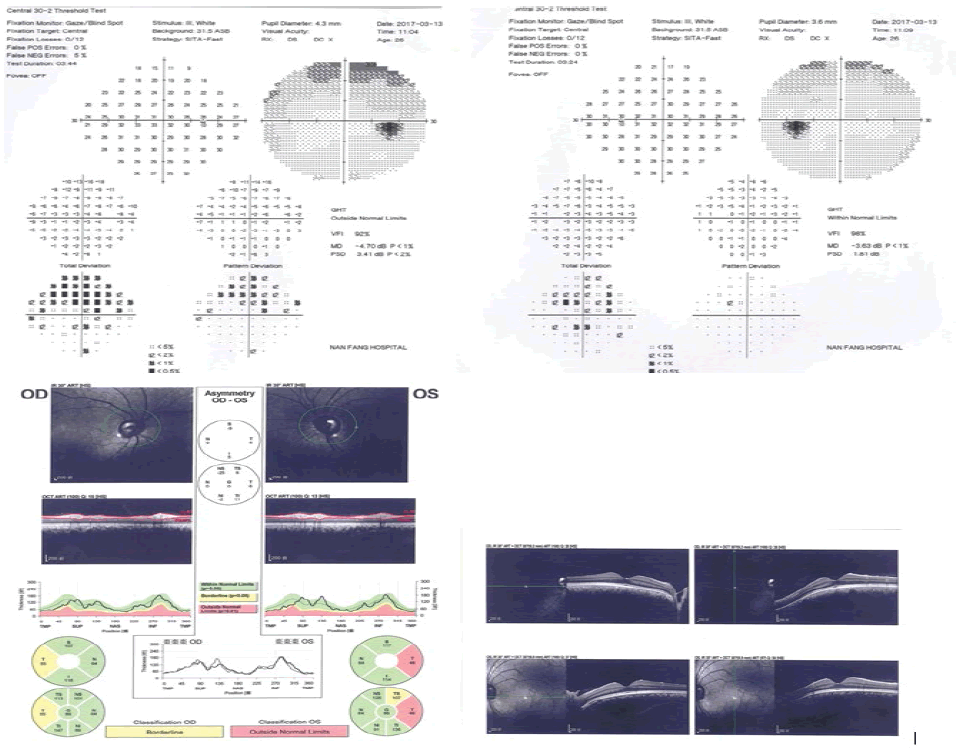
Pedigrees were drawn after inquiry of detailed family history, information on relatives, medical records, family and self-report (Figure 1 to Figure 4).
Full body characteristics (height, weight, age, sex, blood pressure, etc.) from all participants were recorded; before taking part in a complete ophthalmic examination including visual acuity, slit lamp, tonometry, goniometer, fundus (ophthalmoscopy), perimetry (visual field) and Optical Coherence Tomography (OCT) conducted by an experienced glaucoma specialist.
Sample Collection
Peripheral blood (10 ml) was then collected from all participants, Ethylene Diamine Tetra Acetic Acid (EDTA) was added as an anticoagulant and stored at -80°C in order to minimize freeze-thaw cycles and prevent DNA degradation. Blood samples were later sent to BGI. After genomic DNA extraction, genetic and clinical information was then recorded and established. The polymerase Chain Reaction (PCR) technique was used in order to amplify fragments for data analysis and the results were compared with the corresponding reference sequence in the database (WES). Special attention was given to the following glaucoma-associated genes MYOC, OPTN, WDR36, TBK1, NFT4, CYP1B1, LTBP2, and single nucleotide polymorphism analysis was later selectively performed on all exons and bases of the LTBP2 gene.
Results
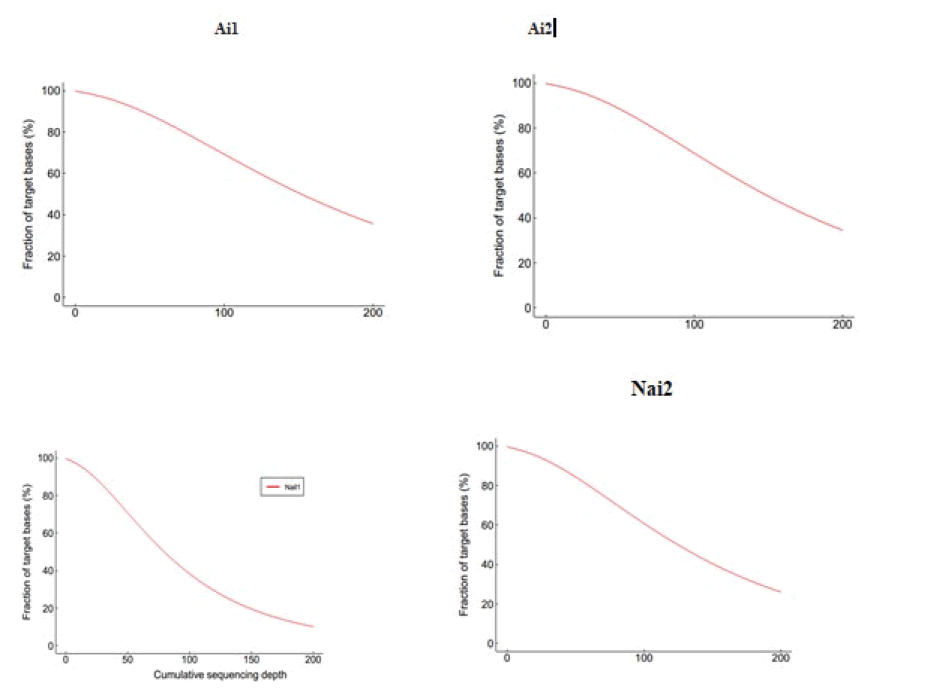
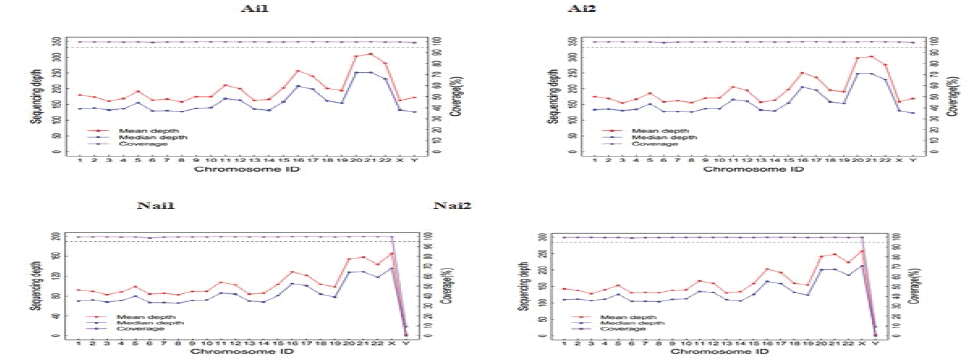
An evenness of exome capture sequencing for each sample was observed (Figure 5). Each one of these samples displays the required average depth for further proceedings, and figure 6 shows the Sequencing depth over chromosomes.
Figure 5: Evenness of exome capture sequencing for each sample (Cumulative distribution map of sequencing depth in capture area).
Figure 6: Sequencing depth over chromosomes.
The above picture is based on the statistics of the sequencing depth of each chromosome in each sample to check whether the sequencing depth of each chromosome is qualified, which is equivalent to small quality control.
The reason for the inconsistency between the experiment and the control was that the experimental group are all male, so there was sequencing depth at the Y chromosome, meanwhile, the control group members are all female and since females had no Y chromosome and that a small proportion of the X chromosome in women was associated with the Y chromosome homologous, therefore in the control group chromosome Y also presents the depth of sequencing. Table 2, detected LTBP2 variants.
No MYOC, OPTN, WDR36, TBK1, NFT4, LTBP2 and CYP1B1 mutations were detected in our study, however, the presence of novel LTBP2 variants was observed in the case group, comprising of 6 variants observed in subject Ai1 while 3 variants were observed in subject Ai2 (Figure 7). The rs2196861 and rs5809669 variants are harboured by both Ai1 and Ai2. No genetic mutations were observed in the control group.
Discussion
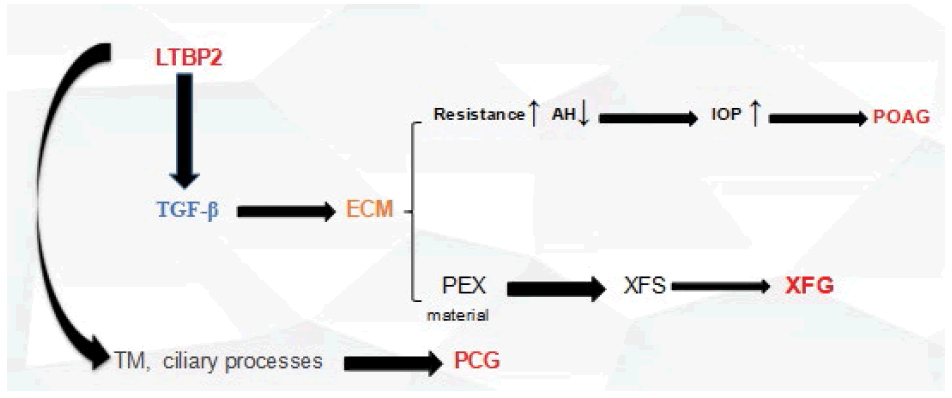
LTBP2 is a large gene with 36 exons which encodes latent transforming growth factor (TGF)-beta binding protein 2, a matrix protein containing 1821 amino acids and a member of a protein superfamily composed of fibrillins and LTBP proteins; it interacts with fibrillin containing microfibrils [21-23] LTBP2’s exact function is still not well understood, however many studies support its role in tissue repair processes, cell adhesion, structural stability of ciliary zonules and growth and development of iris [24-27]. LTBP2’s function is also suspected to be related to those of microfibrils and elastin fibres due to its presence in elastic tissues and its association with microfibrils containing fibrillin. In addition to structural roles, it may affect TGF-β activities. Mutations causing loss of function in the gene encoding LTBP2 are linked with the development of primary congenital glaucoma [28]. The meaning behind the LTBP proteins named is related to their ability to bind latent forms of TGF-βs. Four LTBP family members (LTBP1- LTBP4) have been annotated based on their structural features. TGF-βs refers to a potent multifunctional family of cytokines capable of modulating various physiological and cellular processes [29] and are also present extracellularly as latent complexes waiting to be activated by appropriate signals [30]. Despite the fact that no evidence of the direct binding process between TGF-βs and fibrillin, however, it has been suggested that some LTBP proteins do possess not only the ability to bind both molecules but also act as mediators between them [31,32].
Its role as a causative PCG causative gene was established in 2009 [33-36]. LTBP2 expressions were shown to be present in parts of the human eye including the trabecular meshwork and ciliary processes, elasticity properties changes happening in those areas may affect the outflow of the aqueous humour, whether directly or indirectly and provoke PCG [23]. Additionally, TGF-β functions can also be affected and altered by LTBP2. TGF-β has multiple important cellular functions such as modulation of extracellular matrix formation [28].
Previously identified LTBP2 mutation including the p.Ser472fsX3 deletion( observed in an Iranian family), result in truncation of the LTBP2 protein within its amino-terminal and are expected to affect not only the structure and function but might also interfere with the binding of both fibrillin 1 and fibulin 5. Nevertheless, The p.Tyr1793fsX55 deletion, previously associated with PCG occurrence in one studied family is suspected to affect LTBP2 carboxyl-terminal thus suggesting that a fibulin 5 binding interference alone might be deleterious [23].
Fibrillin 1 mutations have been associated with Marfan syndrome [37-39], characterized by cardiovascular, skeletal and finally, optic defects comprised of keratectasia, ectopia lentis and glaucoma. Additionally, patients affected with diseases accompanied by secondary glaucomas such as lens dislocation, spherophakia, microspherophakia, megalocornea and Weil-Marchesani syndrome also harbour mutations in this gene [27,28,40-44].
After LTBP2’s role in PCG was established, and reports of another previously PCG related gene (CYP1B1) being linked to POAG, Jelodari-Mamaghani and his colleagues did proceed to an LTBP2 mutation screening among POAG affected individuals [43-45]. Referring to the facts that the LTBP2 protein is a component of PEX material, the occurring of extracellular affection in pseudoexfoliation syndrome and the high percentage of PEX patients developing glaucoma years later, pseudoexfoliation patients were also later screened for possible LTBP2 mutations [43]. These studies identified several sequence variations that appeared to contribute to the disease status of both groups of patients, albeit the variations did not evidence mendelian inheritance and they revealed incomplete penetrance. Microscopic studies showed that the extracellular matrix of mutation harbouring patients was disrupted [43].
Another study highlighted the possible implication of LTBP2 in PACG development by performing mutation screening of the gene in affected individuals [23].
Although there is a clear difference in the etiology of various forms of glaucoma, however, it is understood that their common features reflect partial overlap in the etiologies. Evidence for a possible role of LTBP2 in the occurrence of PACG was observed when a group of researchers trying to study a primary congenital glaucoma family discovered angle-closure glaucoma and pupillary block affected individual was harbouring a Glu229Lys causing mutation in CYP1B1 and also a p. Arg299* causing mutation in LTBP2 [46]. Further studies on Marfan syndrome affected families led to the identification of one angle-closure glaucoma and mitral valve prolapse affected family member who did exhibit the p. Arg548* mutation in LTBP2 [28].
Here, we report the presence of a combined total of 9 LTBP2 mutations in POAG patients from different ethnic backgrounds and possessing both a positive family history. However, most of the detected variants were all novel variants meaning they were not reported by any of the previous studies and besides creating an amino-acid substitution and affecting protein-coding processes, lead to an intron variant consequence, namely provoking the occurring of a transcript variant within an intron. The resulting variants may be able to affect non-coding genes, but according to the results of Variant Effect Predictor (VEP), it is difficult to estimate exact impacts. The fact that there are two mutations (rs2196861 and rs5809669) observed in both patients despite their ethnic background difference argues in favour of their pathogenicity. The mechanisms by which LTBP2 might contribute to glaucoma pathogenesis is still unknown. The microfibril hypothesis is based on genetic and biological evidence and is consistent with many key features of glaucoma that have been studied extensively. Genetic support for the microfibril hypothesis includes the involvement of LTBP2 [23,37]. Microfibril defects could contribute to glaucoma through alterations in biomechanical properties of tissue and/or through effects on signalling through transforming growth factor-beta (TGF-β). Microfibrils are the major reservoir of latent TGF-β and are central to TGF-β localization and signalling (Localization and activation of TGF-β are controlled by microfibrils [47-51]. Defective microfibrils could provide a mechanism for the elevation of TGF-β2 in the aqueous humour of glaucoma patients. If glaucoma shares mechanisms with other diseases caused by defective microfibrils, therapeutic interventions to inhibit chronic activation of TGF-β signalling used in those diseases may be applied to glaucoma.
TGF-β may play a central role in glaucoma pathogenesis in general, and in particular, may cause decreased aqueous humour outflow through the TM. Since the initial discovery in 1994 by Tripathi [52] that the aqueous humour of glaucoma patients contains elevated levels of TGFβ2, evidence has accumulated supporting elevated TGF-β2 in the aqueous humour [53-58] and additionally, the ability of TGF-β2 to increase IOP, as recently reviewed by Fuchshofer and Tamm [59]. Perfusion of human anterior segments with TGF-β2 results in the decreased facility of outflow of AH and accumulation of matrix material in the TM [60,61]. In vivo, overexpression of TGF-β in mouse eyes by transgenic or viral expression results in elevated IOP [62-64]. A plausible mechanism by which increased TGF-β2 elevates IOP is by altering ECM turnover in the TM resulting in reduced aqueous humour outflow facility [65,66]. Figure 8 shows the process of LTBP2 to PCG.
In conclusion, our study detected novel LTBP2 gene mutations in 2 POAG patients from a different ethnic backgrounds. The LTBP2 gene is associated with PCG, but very few studies support its role in POAG occurrence. This finding not only emphasize the importance of the ECM in the development of glaucoma, but it somewhat hints at the contribution of the LTBP2 gene in POAG occurrence. This study might provide reasonable advice to unrecognized family members for early detection, early diagnosis, and early treatment to avoid irreversible damage to vision.
Conclusion
Glaucoma is a complex disease because genetic and environmental factors both play important roles in its pathogenesis. Although, great advances have since been witnessed in the past decade concerning the genetic and environmental factors likely to cause or to contribute to causing glaucoma with the discovery of multiple glaucoma associated genes, genetic loci and susceptible gene variants, the genetic basis of glaucoma is still not completely understood. The LTBP2 gene is associated with PCG, but very few studies support its role in POAG occurrence. Investigating gene mutations among 2 POAG affected individuals from a different ethnic backgrounds and possessing both a positive family history, led to the detection of:
1. 9 novel LTBP2 sequence variation in open-angle glaucoma patients
2. Similar LTBP2 sequence variation (rs2196861 and rs5809669) in open-angle glaucoma patients from a different ethnic background
3. LTBP2 sequence variation (rs2196861 and rs5809669) in glaucoma patients with positive family history
Since most glaucomas are symptomless at early stages, we stress that this finding creates a paradigm for future LTBP2 studies as it might be of great importance and may prove to be useful in the early determination, early diagnosis and early treatment of asymptomatic cases. However, further investigations are needed consisting in screening large POAG families for LTBP2 mutations in order to better quantify their implication to the condition and ultimately contribute to the development of novel therapeutic strategies to help slow the progression of the disease, reduce further damage to the optic nerve and subsequently avoid blindness, and retain the quality of life.
Limitations
The number of limited participants makes it difficult to quantify the extent of the possible changes caused by the detected LTBP2 mutations.
Future Research Suggestions
1. Screening large POAG families for LTBP2 mutations.
2. Cells and animals experiment to further investigate the function of LTBP2.
3. Collection and examination of TGF-β from aqueous humour of POAG patients.
4. Examination of skin tissue from patients possessing LTBP2 sequence variations.
Statements
Contributorship Statement
Involved: In conception, design (Cissé Yacouba), literature search and writing of the manuscript (Cissé Yacouba), supervision (Bai Lang), critical reading (Bai Lang, Meng Ting), approval of the final proofs of the article (Cissé Yacouba, Bai Lang, Meng Ting).
Funding
This research is supported by the National Natural Science Foundation of China (Grant No. 1714050006116) and Horizontal Topic Matching Funds of Nanfang Hospital, Southern Medical University (Grant No. 1714050006116).
Competing interest
The authors have declared that no competing interest exists.
Acknowledgement
The authors are thankful to all members of Prof. Bai Lang’s research group for their participation and critical assessments of this manuscript.
References
- Jaeger EC. A source-book of biological names and terms. Springfield (IL): Charles C Thomas. 110 (1959).
Google Scholar Crossref - Gillespie B, Musch DF, Guire KE, et al. The collaborative initial glaucoma treatment study: baseline visual field and test-retest variability. Invest Ophthalmol Vis Sci 44:2613-2620 (2003).
Google Scholar Crossref - Thylefors B, Negrel AD, Pararajasegaram R, et al. Available data on blindness (update 1994). Ophthalmic Epidemiol 2:5-39 (1995).
Google Scholar Crossref - Thylefors B, Negrel AD, Pararajasegaram R, et al. Global data on blindness. Bull World Health Organ 73: 115-121 (1995).
Google Scholar Crossref - Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ 82:844-851 (2004).
Google Scholar Crossref - Friedman DS, Wolfs RC, O'Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol 122:532-538 (2004).
Google Scholar Crossref - Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmology 90:262-267 (2006).
Google Scholar Crossref - Kendrick R. Gradual painless visual loss: glaucoma. Clin Geriatr Med 15:95-101 (1999).
Google Scholar Crossref - Topouzis F, Coleman AL, Harris A, et al. Factors associated with undiagnosed open-angle glaucoma: The Thessaloniki eye study. Am J Ophthalmol 145:327-335 (2008).
Google Scholar Crossref - Rotchford AP, Kirwan JF, Muller MA, et al. Temba glaucoma study: a population-based cross-sectional survey in urban South Africa. Ophthalmology 110:376-382 (2003).
Google Scholar Crossref - Shaikh Y, Yu F, Coleman AL. Burden of undetected and untreated glaucoma in the United States. Am J Ophthalmol 158:1121-1129 (2014).
Google Scholar Crossref - Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 80:389-393 (1996).
Google Scholar Crossref - Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121:2081-2090 (2014).
Google Scholar Crossref - Klein BE, Klein RF, Lee KE. Heritability of risk factors for primary open-angle glaucoma: the Beaver Dam Eye Study. Investigat Ophthalmol Vis Sci 45:59-62 (2004).
Google Scholar Crossref - Kong X, Chen Y, Chen X, et al. Influence of family history as a risk factor on primary angle closure and primary open angle glaucoma in a Chinese population. Ophthalmic Epidemiol 18:226-232 (2011).
Google Scholar Crossref - Yazdani S, Akbarian S, Pakravan M, et al. Prevalence of angle closure in siblings of patients with primary angle-closure glaucoma. J Glaucoma 24:149-153 (2015).
Google Scholar Crossref - Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and treatment of glaucoma: a review. JAMA 311:1901-1911 (2014).
Google Scholar Crossref - Awadalla MS, Fingert JH, Roos BE, et al. Copy number variations of TBK1 in Australian patients with primary open-angle glaucoma. Am J Ophthalmol 59:124-130 (2015).
Google Scholar Crossref - Landers J, Goldberg I, Graham S. Does a family history of glaucoma affect disease severity at the time of diagnosis? J Glaucoma 12:31-35 (2003).
Google Scholar Crossref - Konareva-Kostianeva M. Family history and some other factors in primary open angle glaucoma. Folia Med (Plovdiv) 40:78-81 (1998).
Google Scholar Crossref - Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 310:2191-2194 (2013).
Google Scholar Crossref - Haji-Seyed-Javadi R, Jelodari-Mamaghani SJ, Paylakhi SH, et al. LTBP2 mutations cause Weill-Marchesani and Weill-Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum Mutat 33:1182-1187 (2012).
Google Scholar Crossref - Narooie-Nejad M, Paylakhi SH, Shojaee S, et al. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum Mol Gen 18:3969-77 (2009).
Google Scholar Crossref - Sinha S, Nevett C, Shuttleworth CA, et al. Cellular and extracellular biology of the latent transforming growth factor-beta binding proteins. Matrix Biol 17:529-545 (1998).
Google Scholar Crossref - Hyytiainen M, Keski-Oja J. Latent TGF-beta binding protein LTBP-2 decreases fibroblast adhesion to fibronectin. J Cell Biol 163:1363-1374 (2003).
Google Scholar Crossref - Vehvilainen P, Hyytiainen M, Keski-Oja J. Latent transforming growth factor-beta-binding protein 2 is an adhesion protein for melanoma cells. J Biol Chem 278:24705-24713 (2003).
Google Scholar Crossref - Kumar A, Duvvari MR, Prabhakaran VC, et al. A homozygous mutation in LTBP2 causes isolated microspherophakia. Hum Genet 128:365-371 (2010).
Google Scholar Crossref - Gibson MA, Hatzinikolas G, Davis EC, et al. Bovine latent transforming growth factor beta 1-binding protein 2: molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils. Mol Cell Biol 15:6932-6942 (1995).
Google Scholar Crossref - Lawrence DA. Latent-TGF-beta: an overview. Mol Cell Biochem 219:163-70 (2001).
Google Scholar Crossref - Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF beta activation. J Cell Sci 116:217-224 (2003).
Google Scholar Crossref - Hirani R, Hanssen E, Gibson MA. LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol 26:213-223 (2007).
Google Scholar Crossref - Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem 278:2750-2757 (2003).
Google Scholar Crossref - Ali M, McKibbin M, Booth A, et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 84:664-671 (2009).
Google Scholar Crossref - Narooie-Nejad M, Paylakhi SH, Shojaee S, et al. Loss of function mutations in the gene encoding latent transforming growth factor beta binding protein 2, LTBP2, cause primary congenital glaucoma. Hum Mol Genet 18:3969-3977 (2009).
Google Scholar Crossref - Oklu R, Hesketh R. The latent transforming growth factor beta binding protein (LTBP) family. Biochem J 352:601-610 (2000).
Google Scholar Crossref - Moren A, Olofsson A, Stenman G, et al. Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein. J Biol Chem 269:32469-32478 (1994).
Google Scholar Crossref - Ali M, McKibbin M, Booth A, et al. Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 84:664-671 (2009).
Google Scholar Crossref - Izquierdo NJ, Traboulsi EI, Enger C, et al. Glaucoma in the Marfan syndrome. Trans Am Ophthalmol Soc 90: 111-117 (1992).
Google Scholar Crossref - Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337-339 (1991).
Google Scholar Crossref - Nemet AY, Assia Ei, Apple DJ, et al. Current concepts of ocular manifestations in Marfan syndrome. Surv Ophthalmol 51: 561-575 (2006).
Google Scholar Crossref - Khan AO, Aldahmesh MA, Alkuraya FS. Congenital megalocornea with zonular weakness and childhood lens-related secondary glaucoma - A distinct phenotype caused by recessive LTBP2 mutations. Mol Vis 17:2570-2579 (2011).
Google Scholar Crossref - Desir J, Sznajer Y, Depasse F, et al. LTBP2 null mutations in an autosomal recessive ocular syndrome with megalocornea, spherophakia, and secondary glaucoma. Eur J Hum Genet 18:761-767 (2010).
Google Scholar Crossref - Jelodari-Mamaghani S, Haji-Seyed-Javadi R, Suri F, et al. Contribution of the latent transforming growth factor-beta binding protein 2 gene to etiology of primary open angle glaucoma and pseudoexfoliation syndrome. Mol Vis 19:333-347 (2013).
Google Scholar Crossref - Ben Yahia S, Ouechtati F, Jelliti B, et al. Clinical and genetic investigation of isolated microspherophakia in a consanguineous Tunisian family. J Hum Genet 54:550-553 (2009).
Google Scholar Crossref - Suri F, Kalhor R, Zargar SJ, et al. Screening of common CYP1B1 mutations in Iranian POAG patients using a microarray-based PrASE protocol. Mol Vis 14:2349-2356 (2008).
Google Scholar Crossref - Azmanov DN, Dimitrova S, Florez L, et al. LTBP2 and CYP1B1 mutations and associated ocular phenotypes in the Roma/Gypsy founder population. Eur J Hum Genet 19:326-333 (2011).
Google Scholar Crossref - Jensen SA, Robertson IB, Handford PA. Dissecting the fibrillin microfibril: structural insights into organization and function. Structure 20:215-225 (2012).
Google Scholar Crossref - Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res 339:71-82 (2010).
Google Scholar Crossref - Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol 21:616-22 (2009).
Google Scholar Crossref - Kielty CM, Sherratt MJ, Marson A, et al. Fibrillin microfibrils. Adv Protein Chem 70:405-36 (2005).
Google Scholar Crossref - Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-β function. J Biochem 152:321-329 (2012).
Google Scholar Crossref - Tripathi RC, Li J, Chan WF, et al. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res 59:723-727 (1994).
Google Scholar Crossref - Min SH, Lee TI, Chung YS, et al. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol 20:162-165 (2006).
Google Scholar Crossref - Yamamoto N, Itonaga K, Marunouchi T, et al. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic Res 37:29-33 (2005).
Google Scholar Crossref - Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol 25:19-22 (2004).
Google Scholar Crossref - Picht G, Welge-Luessen U, Grehn F, et al. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol 239:199-207 (2001).
Google Scholar Crossref - Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol 46:249-253 (2002).
Google Scholar Crossref - Inatani M, Tanihara H, Katsuta H, et al. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol 239:109-113 (2001).
Google Scholar Crossref - Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res 347:279-290 (2012).
Google Scholar Crossref - Gottanka J, Chan D, Eichhorn M, et al. Effects of TGF-β2 in perfused human eyes. Invest Ophthalmol Vis Sci 45:153-158 (2004).
Google Scholar Crossref - Fleenor DL, Shepard AR, Hellberg PE, et al. TGF β2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci 47:226-234 (2006).
Google Scholar Crossref - Robertson JV, Siwakoti A, West-Mays JA. Altered expression of transforming growth factor beta 1 and matrix metalloproteinase-9 results in elevated intraocular pressure in mice. Mol Vis 19:684-695 (2013).
Google Scholar Crossref - Shepard AR, Millar JC, Pang IH, et al. Adenoviral gene transfer of active human transforming growth factor-(beta) 2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci 51:2067-276 (2010).
Google Scholar Crossref - Robertson JV, Golesic E, Gauldie J, et al. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest Ophthalmol Vis Sci 51:308-318 (2010).
Google Scholar Crossref - Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res 86:543-61 (2008).
Google Scholar Crossref - Johnson M. What controls aqueous humour outflow resistance?. Exp Eye Res 82:545-557 (2006).
Google Scholar Crossref