Case Report - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 8
Hyperviscosity Syndrome in Undifferentiated Connective Tissue Disease: A Case Report
- *Corresponding Author:
- Sara Sharif
Department of Internal Medicine, State University of New York: Downstate -Health Science University, Brooklyn, NY, USA
E-mail: sara.sharif@downstate.edu
Abstract
Hyperviscosity Syndrome (HVS) is an oncologic emergency characterized by a triad of bleeding, visual disturbances and neurological deficits. Given its potentially fatal complications, the initial response in HVS is symptomatic treatment with a focus on decreasing blood viscosity to limit its serious clinical consequences. After symptomatic management with apheresis, the underlying cause of HVS must then be addressed.
In this report, we present a case of a 45-year-old woman with extensive rheumatologic history admitted with syncope following several months of decreased oral intake. The patient had clinical signs of HVS and an M spike with elevated gamma globulins. She was treated with pulse dose steroids following plasma exchange and continued on a prednisone taper along with biologic therapy. This case is unique in that a patient with undifferentiated connective tissue disease, not previously documented, presented with hyper-viscosity syndrome secondary to increased IgG gamma globulins with a monoclonal spike, a presentation, to our knowledge, not yet described in the literature. The treatment regimen designed for this case was plasmapheresis with pulse dose steroids followed by rituximab-based therapy for induction with Mycophenolate mofetil and Hydroxychloroquine for maintenance therapy. While the use of rituximab has been associated with a clinically significant IgM flare, which would theoretically lead to worsening of hyperviscosity, in this case rituximab showed clinical effectiveness in the treatment of HVS.
Keywords
sjogren’s disease • sjogren’s syndrome • hyperviscosity syndrome • undifferentiated connective tissue disease
Abbreviations: WBC: White Blood Cell Count; Hbg: Hemoglobin; MCV: Mean Corpuscle Volume; PLT: Platelets; aPTT: Activated Partial Thromboplastin Time; PT: Prothrombin Time; INR: International Normalized Ratio; F VII: Coagulation Factor Seven; ESR: Erythrocyte Sedimentation Rate; FLC: Free Light Chain; Ig: Immunoglobulin; M spike: Monoclonal Spike; Serum prot elp: Serum Protein Electrophoresis; % ALBSPE-albumin ; %ALPHA1SPE- alfa1 protein; % ALPHA2SPE-alfa 2 protein; %BETASPE-beta protein; %GAMMASPE- percent of Gamma Globulin; %M SPIKE: percent Monoclonal Spike; Albumin/globulin SPE-Albumin to Globulin Ratio ; Anti cardiolipin IgM Ab: Anti Cardiolipin Immunoglobin M Antibody; Cardiolipin Ab: Cardiolipin Antibody; Na: Sodium; K: Potassium; Cl: Chloride; BUN: Blood Urea Nitrogen; Cr: Creatinine; ALT: Alanine Aminotransferase; AST: Aspartate Transaminase; ALK PHOS: Alkaline Phosphatase; CRP: C-Reactive Protein; Anti DS DNA antibody: anti- Double Stranded Deoxyribonucleic Acid Antibody; FV Leiden: Factor Five Leiden; Prot S : Protein S; RNP ab: Ribonucleoprotien Antibody; Anti-CCP AB: Anti-Cyclic Citrullinated Peptide Antibody; ACE: Angiotensin Converting Enzyme; Anti ANA AB: Anti Antinuclear Antibody; Anti centromere AB: Anticentromere Antibody; Anti SSA: Anti-Sjogrens-Syndrome Related Antigen A; Anti SSB: Anti- Sjögren’s Syndrome Type B Antibody; SM AB: Smith AB; Anti Mitochondrial AB: Antimitochondrial Antibody; C-ANCA: Cytoplasmic Antineutrophil Cytoplasmic Antibody; P-ANCA: Perinuclear Anti- Neutrophil Cytoplasmic Antibody; Anti Scl 70 AB: Anti-Topoisomerase I Antibody
Introduction
Hyperviscosity Syndrome (HVS) is a pathological condition characterized by increased blood viscosity resulting in a clinical triad of mucosal or skin bleeding, visual disturbances and neurological defects [1, 2]. An increase in plasma proteins along with the high molecular weight of proteins yields an increase in plasma viscosity (3-5). HVS is often the manifestation of a condition called Waldenstrom macroglobulinemia, where there is an excess of IgM monoclonal antibodies (2). Other commonly associated conditions include Type I and Type II cryoglobulinemia, multiple myeloma, Sjogrens syndrome, HIV infection, IgG4 disease, polycythemia vera, and chronic lymphocytic leukemia (4).
HVS is considered an oncologic emergency due to the tendency of thromboembolic events and therefore time to therapy is crucial. The standard of care for these patients is symptomatic treatment with therapeutic plasmapheresis to rapidly eliminate pathologic proteins and decrease the risk for adverse outcomes (2, 4, 6, 7). Once the patient’s symptomatic manifestations are addressed with plasmapheresis, the underlying cause of HVS must be addressed to treat the underlying disorder. Here we present a unique presentation of HVS in a patient with undifferentiated connective tissue disease.
Case Presentation
A 45-year-old woman with past medical history of Rheumatoid Arthritis (RA) with Systemic Lupus Erythematosus (SLE) overlap presented with dizziness, syncopal episodes, and months of decreased oral intake due to progressive odynophagia and dysphagia.
As per the patient’s history she initially presented 4 years prior with symptoms at another institution, where she was hospitalized with acute respiratory failure. She was found to have positive Rheumatoid Factor (RF), anti- Cyclic Citrullinated Peptide (anti-CCP), Antinuclear Antibodies (ANA) and anti-Sjogrens-Syndrome Related Antibody (anti-SSA). Clinically, she had active livedo reticularis lesions, organomegaly (hepatomegaly and splenomegaly) and increased serum IgG levels with monoclonal bands. During this admission she was diagnosed with RA/SLE overlap syndrome and treated with low dose prednisone, with improvement in her symptoms. She was followed in rheumatology clinic for about 6 m and then was lost to follow up as she relocated for work.
Over the course of the following two and a half years she developed progressive fatigue and eventually became unable to work. She developed progressive dysphagia to both solids and liquids, worsening odynophagia caused decreased oral intake resulting in significant weight loss (28lbs in 4 months). The patient could only tolerate liquids and foods with soft consistency. Her fatigue became pronounced, and she was only able to ambulate about 10 feet before becoming symptomatic. Additionally, she reported almost daily blood loss in the form of mild nosebleeds and bleeding of her gums associated with frequent pre-syncopal and syncopal events without loss of consciousness. A syncopal event with head trauma prompted a visit to the hospital and resulted in admission for further evaluation.
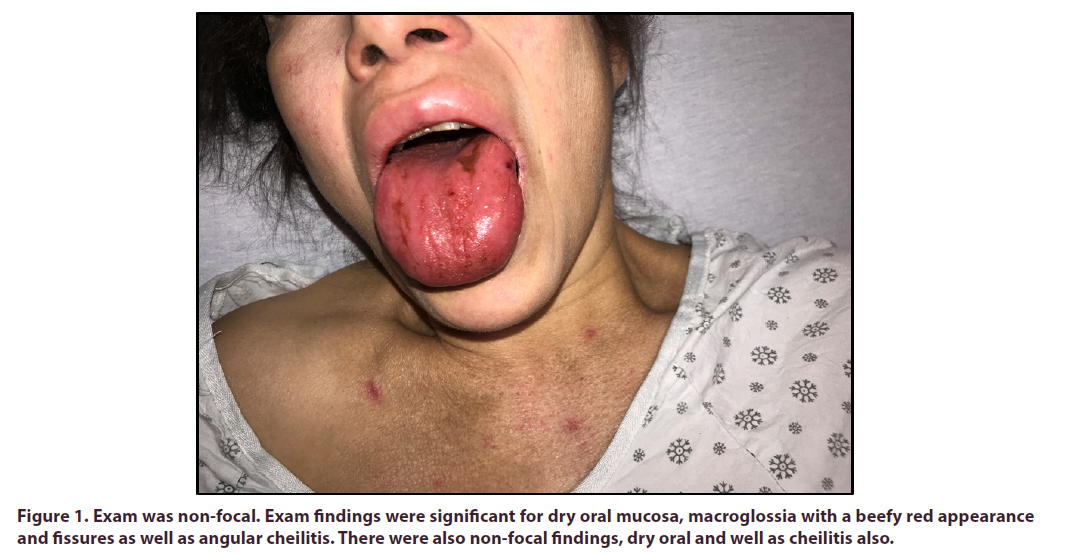
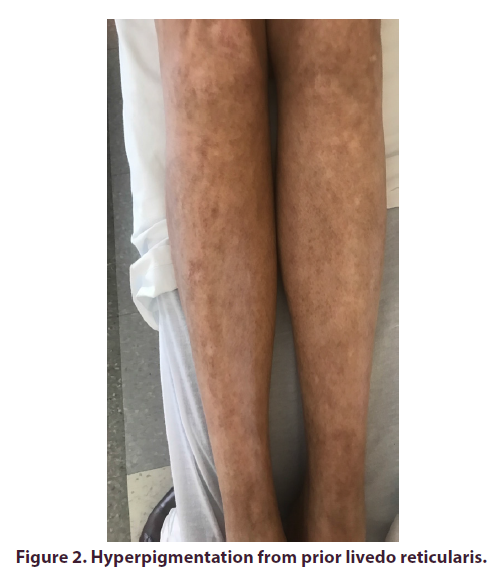
At presentation, the patient was hemodynamically stable. She weighed 75 kg and her BMI was 27 kg/m. Cardiac and respiratory exams were within normal limits and the neurologic exam was non-focal. Exam findings were significant for dry oral mucosa, macroglossia with a beefy red appearance and fissures as well as angular cheilitis. There were also telangiectasias on her tongue, face and chest (Figure 1). She also had tenderness to palpation and swelling of her submandibular and parotid glands bilaterally. The abdominal exam was significant for hepatomegaly and tenderness over the liver. Lower extremities had hyperpigmented lesions from past livedo reticularis (Figure 2).
Initial lab work showed pancytopenia with leukocytes of 4.9 K/mcL (4.7-10.3) and lymphocytic predominance, hemoglobin of 7.3 g/dL (12.0-15.8), Mean Corpuscular Volume (MCV) of 85.0 (78.9-96.2 fL) and platelets of 91 K/mcL (133-402) (Table 1). Complete comprehensive studies were inconclusive due to the samples clotting in the lab. Repeated specimens were sent with results as follows: potassium low at 3.4 (3.5-5.0mmol/L), total protein elevated at 13 (6.0-8.0 g/dL) Aspartate Aminotransferase (AST) elevated at 100 (5-40 U/L) and Alanine Transaminase (ALT) normal at 30 (10-41 U/L). Coagulation studies revealed a Partial Thromboplastin Time (aPTT) of 41.2 (29.3-35.7 seconds), Prothrombin Time (PT) of 17.2 (10.1-12.4 seconds) and International Normalized Ratio (INR) of 1.5 (0.9-1.1 ratio). D-Dimer elevated at 6328 (0-250 ng/mL), HIV negative, hepatitis panel negative. Fibrinogen level resulted as low at 284 (322-496 mg/dL), haptoglobin within normal limits at 80 (34-200 mg/dL).
| Labs On Presentation | Reference Range | ||
|---|---|---|---|
| Complete Blood count | WBC | 4.9 | 3.8-10.5 K/mcL |
| Hbg | 7.3 | 11.5-15.5 g/dL | |
| MCV | 85 | 80-100 fL | |
| PLT | 91 | 133-402 K/mcL | |
| Coagulation studies | aPTT | 41.2 | 29.3-35.7 sec |
| PT | 17.2 | 9.4-12.5 sec | |
| INR | 1.5 | 0.8-1.2 ratio | |
| F VII | 56 | 60-125 % | |
| Fibrinogen | 284 | 322 -496 mg/dL | |
| Fibrin split products | <5 | <5ug/mL | |
| ESR | 130 | 0 -15 mm/hr | |
| Immunoglobulins | Bence Jones concentration | absent | |
| Kappa Lambda FLC ratio | 2.43 | 0.26 -1.65 ratio | |
| Ig A | 850 | 84-499 mg/dL | |
| Ig G | 7240 | 610-1660 mg/dL | |
| Ig M | 170 | 35-242 mg/dL | |
| Ig Kappa FLC | 25 | 0.33-1.94 mg/dL | |
| Ig Lambda FLC | 10.3 | 0.5-2.63 | |
| Alpha-1 globulin fraction | 0.4 | 0.1-0.4 g/dL | |
| Alpha-2 globulin fraction | 0.8 | 0.5-1.0 g/dL | |
| Gamma Globulin Fraction | 7.1 | 0.6-1.6 g/dL | |
| M spike | 6.1 | 0.0 -0.0 g/dL | |
| % M spike | 48.1 | % | |
| Protein Electrophoresis | Total protein | 12.6 | 6.0 -8.3 g/dL |
| Albumin SPE | 3 | 3.6-5.5 g/dL | |
| Alpha-1 globulin fraction | 0.4 | 0.1-0.4 g/dL | |
| Alpha- 2 globulin fraction | 0.8 | 0.5-1.0 g/dL | |
| Beta globulin fraction | 1.3 | 0.5-1.0 g/dL | |
| Gamma globulin fraction | 7.1 | 0.6-1.6 g/dL | |
| M spike | 6.1 | 0.0 -0.0 g/dL | |
| Serum protein help interpretation | Gamma migrating paraprotein identified | ||
| % ALBSPE | 23.8 | % | |
| %ALPHA1SPE | 3.2 | % | |
| %ALPHA2SPE | 6.2 | % | |
| %BETASPE | 10.1 | % | |
| %GAMMASPE | 56.7 | % | |
| %M SPIKE | 48.1 | % | |
| Albumin/globulin SPE | 0.3 | Ratio | |
| Serologies | Anti cardiolipin IgM Ab | 6.7 | 0.0-12.5 MPL |
| Cardiolipin Ab | Negative | ||
| Lupus Anticoagulant | Negative | ||
Table 1. Initial lab work showed pancytopenia with leukocytes of 4.9 K/mcL (4.7-10.3) and lymphocytic predominance, hemoglobin of 7.3 g/dL (12.0-15.8), Mean Corpuscular Volume (MCV) of 85.0 (78.9-96.2 fL) and platelets of 91 K/mcL (133-402).
The patient’s syncopal work-up included a CT head without any evidence of acute ischemic processes. An Esophagogastroduodenoscopy (EGD) was performed due to odynophagia and dysphagia, which showed no evidence of obstruction, however the patient did have hypertensive gastropathy and Grade I esophageal varices. A right upper quadrant sonogram showed an echogenic liver suggestive of fatty infiltrates versus parenchymal disease.
Rheumatology and hematology consult services recommended to repeat antibody serologies and to initiate a Plasma Exchange (PLEX) of 1.5 plasma volumes with 5% albumin replacement fluid, as this patient’s presentation was likely a result of HVS based on patient’s history of present illness, imaging and labs.
After one round of PLEX therapy was completed, repeat lab work was performed still showing pancytopenia, but with normalization of liver function tests and of INR. Urine protein electrophoresis was within normal limits, with no Bence Jones protein noted. Serum electrophoresis showed an M spike with gamma globulins of 3.4 g/dL.
Immunofixation showed a monoclonal IgG kappa band and the beta 2 microglobulin was elevated to 6.7 (0.8- 2.2 mg/L). Repeated serologies were significant for RF 526 (<=14), Anti-ANA 1:1280 (Negative 1:40), anti-centromere positive (Negative 1:40) and other studies were negative as follows: anti-Sjögren’s syndrome type B antibody (anti- SSB) negative, Anti-SSA negative, Smooth Muscle antibody negative (1:20 Negative), anti-mitochondrial antibody negative, anti-smith antibody negative, anti-Ribonucleoprotien (anti-RNP) antibody negative, anti-topoisomerase I (anti- Scl-70) antibody negative, and cryoglobulin negative. Complement levels, both C3 and C4, were reduced. Parotid and submandibular gland ultrasound performed at bedside with evidence of grossly heterogenous tissue consistent clinically with Sjogren’s syndrome. The serologies along with the patient’s clinical presentation are diagnostic for Undifferentiated Connective Tissue Disease (UCTD) with clinical signs of overlap syndrome of Sjogren’s and positive RF and anti-CCP antibodies with no peripheral arthropathy.
After PLEX the patient received a bone marrow biopsy, which showed no evidence of multiple myeloma or other plasma cell dyscrasias. The patient was treated with pulse dose steroids between PLEX sessions, 1 gr of Solu-Medrol for 3 days and continued a prednisone taper with plans to initiate biologic therapy. She was also initiated on B-complex vitamins and multivitamins due to months of decreased oral intake. Steroid therapy was followed by induction therapy with Rituximab. With this regimen, symptoms improved, the patient no longer exhibited signs of hyperviscosity and was able to tolerate a diet. She was discharged in stable condition for outpatient follow up with Rheumatology. When she was seen in the outpatient clinic her labs continued to show improvement (Table 2) and clinically, signs of HVS were no longer evident. Additionally, the patient reported significant improvement in oral intake. She was maintained in remission on mycophenolate mofetil 1500 mg two times a day and hydroxychloroquine 200 mg two times a day and is stable now 18 months after the initial presentation. Hypergammaglobulinemia and M-spike resolved.
| Before | After | Follow up ( 3 days later) | Follow up ( 1 month later) | Follow up (6 months later) | Reference Range | ||
|---|---|---|---|---|---|---|---|
| PLEX | PLEX | ||||||
| Comprehensive metabolic panel | Na | 136 | 142 | 136 | 141 | 141 | 135 -145 mmol/L |
| K | 3.7 | 3.9 | 3.5 | 4 | 3.9 | 3.5 -5.3 mmol/L | |
| Cl | 108 | 107 | 105 | 106 | 104 | 96 -108 mmol/L | |
| BUN | Unable to perform | 7 | 10 | 15 | 11 | 7-23 mg/dL | |
| Cr | Unable to perform | 0.68 | 0.51 | 0.64 | 0.69 | 0.5-1.3 mg/dL | |
| ALT | Unable to perform | 29 | 28 | 84 | 23 | 10-45 U/L | |
| AST | Unable to perform | 75 | 91 | 86 | 52 | 10-40 U/L | |
| ALK PHOS | 109 | 227 | 110 | 168 | 200 | 40-120 U/L | |
| Complement C3 | 65 | 72 | 133 | 80-160 mg/dL | |||
| Complement C4 | 3 | 9 | 17 | 16-48 mg/dL | |||
| ESR | 67 | 22 | 0-20 mm/hr | ||||
| CRP | 0.89 | 2.74 | <10 mg/L | ||||
| Complete Blood count | WBC | 3.3 | 4.37 | 3.23 | 2.56 | 4.8 | 3.8 - 10.5 K/mcL |
| Hbg | 7.4 | 10.6 | 9.3 | 9.4 | 11.8 | 11.5 -15.5 g/dL | |
| PLT | 72 | 69 | 46 | 44 | 136 | 150 -400 K/ mcL | |
| Coagulation studies | aPTT | 29 | 27.7 | 28.1 | |||
| PT | 18 | 11.9 | 12.5 | 9.4 -12.5 sec | |||
| INR | 1.6 | 1.2 | 1.2 | 0.8 -1.2 ratio | |||
| Protein Elp | Total Protein | 8.3 | 6.7 | 7 | 6.0 -8.3 g/dL | ||
| Alpha-1 globulin fraction | 0.3 | 0.3 | 0.3 | 0.1-0.4. g/dL | |||
| Alpha-2 globulin fraction | 0.6 | 0.5 | 0.6 | 0.5-1.0 g/dL | |||
| Beta globulin fraction | 0.8 | 0.8 | 1 | 0.5-1.0 g/dL | |||
| Gamma globulin fraction | 3.4 | 1.4 | 1.1 | 0.6-1.6 g/dL | |||
| M spike | 2.4 | 0.7 | 0.7 | 0.0-0.0 g/dL | |||
| Ig A | 340 | 300 | 245 | 0.8-3.0 g/L | |||
| Ig G | 3360 | 1278 | 1216 | 6-16.0 g/L | |||
| Ig M | 127 | 80 | 45 | 0.4-2.5 g/L | |||
| Kappa Light chain | 26.38 | 2.93 | 1.89 | 3.3-19 mg/dL | |||
| Lambda light chain | 10.11 | 2.63 | 1.84 | 5.71-26.3 mg/ dL | |||
| Immunofixation | Ig G Kappa band identified | Ig G Kappa band identified | No monoclonal bands identified | None detected | |||
| %ALBSPE | 39 | 55.4 | 57.3 | % | |||
| %ALPHA1SPE | 3.1 | 4 | 3.9 | % | |||
| %ALPHA2SPE | 6.8 | 7.7 | 8.8 | % | |||
| %BETASPE | 9.6 | 11.5 | 13.8 | % | |||
| %GAMMASPE | 41.5 | 21.5 | 16.2 | % | |||
| %M SPIKE | 29 | 9.9 | % | ||||
| Alb/Globulin SPE | 0.6 | 1.2 | 1.3 | Ratio | |||
| Serologies AA | Anti DS DNA antibody | 806 | 67 | 47 | 36 | <100 negative | |
| FV Leiden | Normal | ||||||
| Prot S | 19 | 61-131 % | |||||
| RNP ab | Negative | <0.1 negative | |||||
| >= 0.1 positive | |||||||
| Beta 2 microglobulin | 6.7 | 0.8-2.2 mg/L | |||||
| RF | 536 | 526 | <=14 | ||||
| Anti-CCP AB | >250 | <20 u/ml | |||||
| ACE | 68 | <40nm/m | |||||
| Anti ANA AB | 0.930555556 | 1:40 negative | |||||
| Anti centromere AB | Positive | 1:40 negative | |||||
| Anti SSB | Negative | negative | |||||
| Anti SSA | Negative | negative | |||||
| SM AB | Negative | 1:20 negative | |||||
| Anti-mitochondrial AB | Negative | negative | |||||
| Anti Smith AB | Negative | negative | |||||
| Anti RNP AB | Negative | negative | |||||
| C-ANCA | Negative | negative | |||||
| P-ANCA | Negative | negative | |||||
| Anti Scl 70 AB | Negative | negative | |||||
| Cryoglobulin | Negative | negative | |||||
| Blood viscosity | 15.3 | 1.4-2.0 | |||||
Table 2. After PLEX the patient received a bone marrow biopsy, which showed no evidence of multiple myeloma or other plasma cell dyscrasias.
Discussion
This case is unique in that our patient with UCTD presented with hyperviscosity syndrome secondary to increased IgG gammaglobulins with a monoclonal spike (M-spike). A presentation like this has not yet been described in the literature to our knowledge. Additionally, the treatment regimen used for this presentation was unique. Treatment of hyperviscosity syndrome included PLEX therapy every other day and pulse-dose steroids between the PLEX sessions. The plasma exchange that was done consisted of 1.5 volume of plasma exchanged for 1.5 volume of 5% albumin for five days, occurring every other day with 2 units of fresh frozen plasma to supplement coagulation factors during each session. Subsequently, induction therapy was initiated and consisted of 1 cycle of Rituximab (2 doses 2 weeks apart). With this, the patient’s symptoms of hyperviscosity completely resolved. Subsequently, the patient was placed on maintenance therapy with mycophenolate mofetil and hydroxychloroquine along with oral steroids taper and discharged with outpatient follow up.
Having a monoclonal spike of antibodies occurs when there is a dysfunction in the plasma cells producing antibodies. In this case, the patient had an excess of IgG immunoglobulins resulting in an M-spike. When an excess of a single immunoglobulin is produced in the body it can often be caused by plasma cell dyscrasias or hematologic cancers. Chemotherapy is useful in treating an M-spike in order to down regulate the excess production of IgG immunoglobulins from the plasma cells and therefore reduce serum viscosity (4, 8, 9). Using a rituximab-based therapy, however, is not usually considered first line therapy for rapid reduction of hyperviscosity as it can take months to achieve an appropriate therapy and rituximab was previously described in literature as being associated with a clinically significant IgM flare, which would lead to worsening of HVS. Having polyclonal gammaglobulinemia suggests that there is an immune response resulting in increased activation of B cells. This can also be seen in myeloproliferative disorders however the BM biopsy was negative for this. As this presentation was a polygammopathy it suggests that all lines of the B cells were activated (10). It is for that reason that rituximab, a B cell depleting biological medication, was used in this patient as an induction therapy option for remission of a patient’s primary auto-immune disease and also helped to treat HVS symptoms indirectly. A few days after the first dose of rituximab the patient started to show clinical signs of improvement and the inflammatory markers in the blood had reduced. In addition, maintenance therapy of mycophenolate and hydroxychloroquine in similar cases has not yet been described. It is our hope that this case presentation and treatment regimen may help guide treatment for other cases of HVS in the setting of underlying Connective Tissue Disorder in the future.
Conclusion
This case of HVS caused by UCTD has added to rheumatologic literature in that rituximab can be used for induction therapy and Mycophenolate and Hydroxychloroquine can be used for maintenance therapy.
References
- Mosca M, Tani C, Talarico R et al. Undifferentiated connective tissue diseases (UCTD): Simplified systemic autoimmune diseases. Autoimmunity. Reviews. 10(5), 256–258 (2011).
- Perez Rogers A, Estes M. Hyperviscosity Syndrome. In: StatPearls, Treasure Island (FL): StatPearls, (2020).
- West S. Systemic Connective Tissue Diseases. Rheumatology. Secrets. 170, (2020).
- Gertz MA. Acute hyperviscosity: Syndromes and management. Blood. 132(13), 1379–1385 (2018).
- Dumas G, Merceron S, Zafrani et al. Syndrome d’hyperviscosité plasmatique. La. Revue. De. Médecine. Interne. 36(9), 588–595 (2015).
- Datta SS, Mukherjee S, Talukder B et al. Immunoglobulin M ‘Flare’ Seen in a Case of Waldenstrom’s Macroglobulinemia: Successfully Managed by Therapeutic Plasma Exchange. Indian. J. Hematol. Blood. Transfus. 32(S1), 148–151 (2015).
- Castillo B, DasguptaA, Klein K et al. Apheresis. Transfus. Med. Pathol. 113–124 (2018).
- Kyle RA, Rajkumar SV. Multiple Myeloma. N. Engl. J. Med. 351(18), 1860–1873 (2004).
- Abbas AK, Lichtman AH, Pillai S et al. Cellular and molecular immunology (8th edn). Philadelphia, (2015).
- “Hypergammaglobulinemia.” Encyclopedia of Immunology, by Peter J. Delves and Ivan M. Roitt, Academic. Press. 1161–1166 (1998).