Review Article - Journal of Experimental Stroke & Translational Medicine (2010) Volume 3, Issue 1
Fundamentals of clinical trial design
- *Corresponding Author:
- Scott R. Evans, Ph.D.
Department of Statistics, Harvard University, 651 Huntington Avenue
FBX 513, Boston, MA, 02115
Phone: 614.432.2998
Fax: 617.432.3163
Email: evans@sdac.harvard.edu
Abstract
Most errors in clinical trials are a result of poor planning. Fancy statistical methods cannot rescue design flaws. Thus careful planning with clear foresight is crucial. Issues in trial conduct and analyses should be anticipated during trial design and thoughtfully addressed. Fundamental clinical trial design issues are discussed.
Keywords
p-value; confidence intervals; intent-to-treat; missing data; multiplicity; subgroup analyses; causa-tion
Introduction
The objective of clinical trials is to establish the effect of an intervention. Treatment effects are efficiently isolated by controlling for bias and confounding and by minimizing variation. Key features of clinical trials that are used to meet this objective are randomiza-tion (possibly with stratification), adherence to intent-to-treat (ITT) principles, blinding, prospective evalua-tion, and use of a control group. Compared to other types of study designs (e.g., case-control studies, cohort studies, case reports), randomized trials have high validity but are more difficult and expensive to conduct.
Design Issues
There are many issues that must be considered when designing clinical trials. Fundamental issues including clearly defining the research question, mi-nimizing variation, randomization and stratification, blinding, placebos/shams, selection of a control group, selection of the target population, the selection of endpoints, sample size, and planning for interim analyses will be discussed and common terms are defined (Table 1).
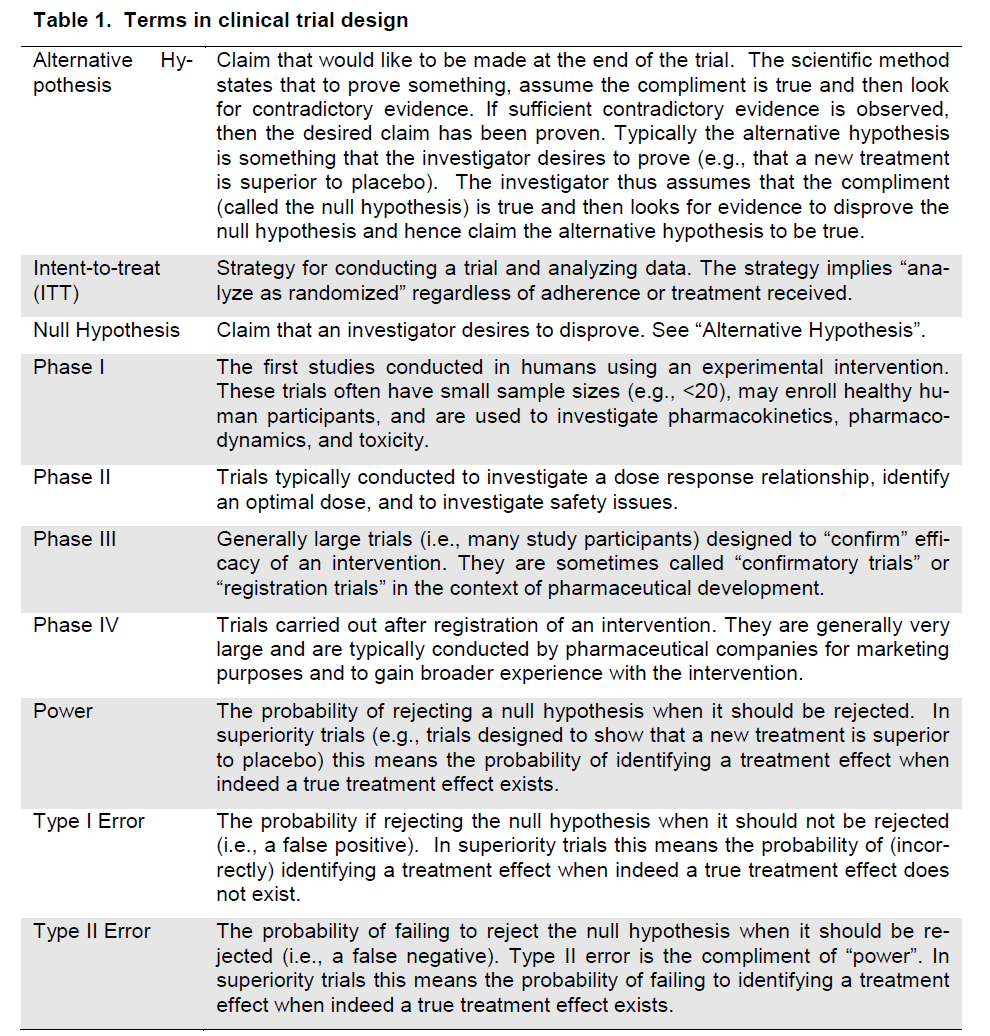
Table 1. Terms in clinical trial design
What is the question?
The design of every clinical trial starts with a primary clinical research question. Clarity and understanding of the research question can require much delibera-tion often entailing a transition from a vague concept (e.g., “to see if the drug works” or “to look at the neu-ro-biology of the drug”) to a particular hypothesis that can be tested or a quantity that can be estimated us-ing specific data collection instruments with a particu-lar duration of therapy. Secondary research ques-tions may also be of interest but the trial design usually is constructed to address the primary re-search question.
There are two strategies for framing the research question. The most common is hypothesis testing where researchers construct a null hypothesis (often “no effect” or “no difference”) that is assumed to be true and evidence is sought to disprove it. An alter-native hypothesis (the statement that is desired to be claimed) is also constructed (often the presence of an effect or difference between groups). Evidence is sought to support the alternative hypothesis. The second strategy is estimation. For example a trial might be designed to estimate the difference in re-sponse rates between two therapies with appropriate precision. Appropriate precision might be measured by the width of a confidence interval of the difference between the two response rates.
Clinical trials are classified into phases based on the objectives of the trial. Phase I trials are the first stu-dies of an intervention conducted in humans. Phase I trials have small sample sizes (e.g., <20), may enroll healthy human participants, and are used to investi-gate pharmacokinetics, pharmacodynamics, and tox-icity. Phase II trials are typically conducted to investi-gate a dose response relationship, identify an optimal dose, and to investigate safety issues. Phase III trials are generally large trials (i.e., many study participants) designed to “confirm” efficacy of an intervention. They are sometimes called “confirmatory trials” or “registration trials” in the context of pharmaceutical development. Phase IV trials are conducted after reg-istration of an intervention. They are generally very large and are typically conducted by pharmaceutical companies for marketing purposes and to gain broader experience with the intervention.
Although clinical trials are conducted prospectively, one can think of them as being designed retrospec-tively. That is, there is a vision of the scientific claim (i.e., answer to the research question) that a project team would like to make at the end of the trial. In order to make that claim, appropriate analyses must be conducted in order to justify the claim. In order to conduct the appropriate analyses, specific data must be collected in a manner suitable to conduct the ana-lyses. In order to collect these necessary data, a thorough plan for data collection must be developed. This sequential retrospective strategy continues until a trial design has been constructed to address the research question.
Once the research question is well understood and associated hypotheses have been constructed then the project team must evaluate the characteristics of the disease, the therapies, the target population, and the measurement instruments. Each disease and therapy will have its own challenges. Neurologic data has many challenging characteristics. First, some neurologic outcomes can be subject to lots of variation (e.g., cognitive outcomes). Second, some neuro-logic outcomes are subjective in nature (e.g., pain, fatigue, anxiety, depression). Thirdly, some neuro-logic outcomes lack a gold standard definition or di-agnosis (e.g., neuropathy, dementia). Forth, neuro-logic outcomes can be high dimensional (e.g., neuro-imaging outcomes or genomic information, that can-not be captured using a single numeric score). Fifth,composite outcomes are common (e.g., cognitive measures, instruments assessing depression or qual-ity of life). Consider a trial to evaluate treatments for pain. Researchers should consider the subjective and transient nature of pain, the heterogeneity of pain expression, the placebo effect often encountered in pain trials, and the likely use of concomitant and res-cue medications. Design must be customized to ad-dress these challenges. The goal of design is to construct the most efficient design within research constraints that will address the research question while considering these characteristics.
Minimizing variation
The larger the variation, the more difficult it is to iden-tify treatment effects. Thus minimizing variation is a fundamental element of clinical trial design. Minimiz-ing variation can be accomplished in several ways. One important method for reducing variation is to construct consistent and uniform endpoint definitions. Ideally endpoints could be measured objectively (e.g., via a laboratory test) however many endpoints are based on subjective evaluation. For example, the diagnosis of neuropathy or dementia may be an end-point. However these diagnoses are partly subjective. Variation in these diagnoses can be minimized with clear definitions and consistent evaluations.
A common design feature is the use of central labs for quantitating laboratory parameters to eliminate between-lab variation or the use of central evaluators to eliminate between-evaluator variation. For exam-ple, the AIDS Clinical Trials Group (ACTG) uses a central laboratory to quantitate HIV-1 RNA viral load on all of its studies while trials using imaging modali-ties for diagnose stroke might consider using a cen-tral imaging laboratory to quantitate all images.
Variation can also be reduced with standardization of the manner in which study participants are treated and evaluated via training. For example, in studies that involve imaging, it is very important to have an imaging protocol that standardizes the manner in which images are collected to reduce added variation due to inconsistent patient positioning. Training mod-ules can be developed to instruct site personnel on the appropriate administration of evaluations. For example extensive training on the administration of neuropsychological exams was conducted in the In-ternational Neurological HIV Study (ACTG A5199) and a training module was developed to instruct sites on the proper administration of the NeuroScreen that is employed in the Adult Longitudinally Linked Ran-domized Treatment (ALLRT) trials (ACTG A5001).
Randomization and stratification
Randomization is a powerful tool that helps control for bias in clinical trials. It essentially eliminates the bias associated with treatment selection. Although randomization cannot ensure between-treatment bal-ance with respect to all participant characteristics, it does ensure the expectation of balance. Importantly randomization ensures this expectation of balance for all factors even if the factors are unknown or unmea-sured. This expectation of balance that randomiza-tion provides combined with the ITT principle, pro-vides the foundation for statistical inference.
Trials commonly employ stratified randomization to ensure that treatment groups are balanced with re-spect to confounding variables. In stratified randomi-zation, separate randomization schedules are pre-pared for each stratum. For example, gender is a po-tential confounder for estimating the effects of inter-ventions to treat or prevent stroke (e.g., a between-group imbalance with respect to gender could distort the estimate of the intervention effect). Thus trials investigating the effects of such interventions might employ stratified randomization based on gender. For example, two randomization schedules may be utilized; one for males and another for females. Stra-tified randomization ensures that the number of male participants in each treatment group is similar and that the number of female participants in each treat-ment group is similar. Stratification has a few limita-tions. First, stratification can only be utilized for known and measurable confounders. Secondly, al-though one can stratify on multiple variables, one has to be wary of over-stratification (i.e., too many strata for the given sample size). The sample size must be large enough to enroll several participants for each treatment from each stratum.
Blinding
Blinding is a fundamental tool in clinical trial design and a powerful method for preventing and reducing bias. Blinding refers to keeping study participants, investigators, or assessors unaware of the assigned intervention so that this knowledge will not affect their behavior, noting that a change in behavior can be subtle, unnoticeable, and unintentional. When study participants are blinded, they may be less likely to have biased psychological or physical responses to intervention, less likely to use adjunct intervention, less likely to drop out of the study, and more likely to adhere to the intervention. Blinding of study partici-pants is particularly important for patient reported outcomes (e.g., pain) since knowledge of treatment assignment could affect their response. When trial investigators are blinded, they may be less likely to transfer inclinations to study participants, less likely to differentially apply adjunctive therapy, adjust a dose, withdraw study participants, or encourage partici-pants to continue participation. When assessors are blinded, they may be less likely to have biases affect their outcome assessments. In a placebo controlled trial for an intervention for multiple sclerosis, an eval-uation was performed by both blinded and unblinded neurologists. A benefit of the intervention was sug-gested when using the assessments from neurolo-gists that were not blinded, but not when using the assessments from the blinded neurologists. In this case, the blinded assessment is thought to be more objective.
Clinical trialists often use the terms “single-blind” to indicate blinding of study participants, “double-blind” to indicate blinding of study participants and investi-gators, and “triple-blind” to indicate blinding of partici-pants, investigators, and the sponsor and assessors. Trials without blinding are often referred to as “open-label”.
Successful blinding is not trivial. In a placebo-controlled trial, a placebo must be created to look, smell, and taste just like the intervention. For exam-ple a concern for a trial evaluating the effects of mi-nocycline on cognitive function may be that minocyc-line can cause a change in skin pigmentation, thus unblinding the intervention. Blinding can be challeng-ing or impractical in many trials. For example surgical trials often cannot be double-blind for ethical reasons. The effects of the intervention may also be a threat to the blind. For example, an injection site reaction of swelling or itching may indicate an active intervention rather than a sham injection. Researchers could then consider using a sham injection that induces a similar reaction.
In late phase clinical trials, it is common to compare two active interventions. These interventions may have different treatment schedules (e.g., dosing fre-quencies), may be administered via different routes (e.g., oral vs. intravenously), or may look, taste, or smell different. A typical way to blind such a study is the “double-dummy” approach that utilizes two place-bos, one for each intervention. This is often easier than trying to make the two interventions look like each other. Participants are then randomized to re-ceive one active treatment and one placebo (but are blinded). The downside of this approach is that the treatment schedules become more complicated (i.e., each participant must adhere to two regimens).
When blinding is implemented in a clinical trial, a plan for assessing the effectiveness of the blinding may be arranged. This usually requires two blinding ques-tionnaires, one completed by the trial participant and the other completed by the local investigator or per-son that conducts the evaluation of the trial partici-pant. Having “double-blind” in the title of a trial does not imply that blinding was successful. Reviews of blinded trials suggest that many trials experience is-sues that jeopardize the blind. For example in a study assessing zinc for the treatment of the common cold(Prasad et al 2000) the blinding failed because the taste and aftertaste of zinc was distinctive. Crea-tive designs can be utilized to help maintain the blind. For example, OHARA and the ACTG are developing a study to evaluate the use of gentian violet (GV) for the treatment of oral candidiasis. GV has staining potential which could jeopardize the blind when the assessors conduct oral examinations after treatment. A staining cough drop could be given to study partici-pants prior to evaluation to help maintain the blind.
Unplanned unblinding should only be undertaken to protect participant safety (i.e., if the treatment as-signment is critical for making immediate therapeutic decisions).
Blinding has been poorly reported in the literature. Researchers should explicitly state whether a study was blinded, who was blinded, how blinding was achieved, the reasons for any unplanned unblinding, and state the results of an evaluation of the success of the blinding.
Placebos/Shams
A placebo can be defined as an inert pill, injection, or other sham intervention that masks as an active in-tervention in an effort to maintain blinding of treat-ment assignment. It is termed the “sugar pill” and does not contain an active ingredient for treating the underlying disease or syndrome but is used in clinical trials as a control to account for the natural history of disease and for psychological effects. One disadvan-tage to the use of placebos is that sometimes they can be costly to obtain.
Although the placebo pill or injection has no activity for the disease being treated, it can provide impres-sive treatment effects. This is especially true when the endpoint is subjective (e.g., pain, depression, an-xiety, or other patient reported outcomes). Evans et.al. (Evans et al 2007) reported a significant im-provement in pain in the placebo arm of a trial inves-tigating an intervention for the treatment of painful HIV-associated peripheral neuropathy.
There can be many logistic and ethical concerns in clinical trials where neither a placebo, nor a sham control can be applied. The inability to use placebos is common in the development of devices. For exam-ple, surgical trials rarely have a sham/placebo.
Selection of a control group
The selection of a control group is a critical decision in clinical trial design. The control group provides da-ta about what would have happened to participants if they were not treated or had received a different in-tervention. Without a control group, researchers would be unable to discriminate the effects caused by the investigational intervention from effects due to the natural history of the disease, patient or clinician ex-pectations, or the effects of other interventions.
There are three primary types of control groups: 1) historical controls, 2) placebo/sham controls and 3) active controls. The selection of a control group de-pends on the research question of interest. If it is desirable to show any effect, then placebo-controls are the most credible and should be considered as a first option. However placebo controls may not be ethical in some cases and thus active controls may be utilized. If it is desirable to show noninferiority or superiority to other active interventions then active controls may be utilized.
Historical controls are obtained from studies that have already been conducted and are often pub-lished in the medical literature. The data for such controls is external to the trial being designed and will be compared with data collected in the trial being de-signed. The advantage of using historical controls is that the current trial will require fewer participants and thus use of historical controls provides an attractive option from a cost and efficiency perspective. The drawback of trials that utilize historical controls is that they are non-randomized studies (i.e., the compari-son of newly enrolled trial participants to the historical controls is a non-randomized comparison) and thus subject to considerable bias, requiring additional as-sumptions when making group comparison (although note that the historical controls themselves may have been drawn from randomized trials). Historical con-trols are rarely used in clinical trials for drug devel-opment due to the concerns for bias. However, when historical data are very reliable, well documented and other disease and treatment conditions have not changed since the historical trial was conducted, then they can be considered. Historical controls have be-come common in device trials when placebo-controls are not a viable option. Historical controls can be helpful in interpreting the results from trials for which placebo controls are not ethical (e.g., oncology trials).
An active control is an active intervention that has often shown effectiveness to treat the disease under study. Often an active control is selected because it is the standard of care (SOC) treatment for the dis-ease under study. Active controls are selected for use in noninferiority trials. Active controls and place-bo controls can be used simultaneously and provide useful data. For example, if the new intervention was unable to show superiority to placebo, but an active control group was able to demonstrate superiority to placebo, then this may be evidence that the new in-tervention is not effective. However, if the active con-trol with established efficacy did not demonstrate su-periority to placebo, then it is possible the trial was flawed or may have been underpowered because of the placebo response or variability being unexpected high.
Selection of a population and entry criteria
In selecting a population to enroll into a trial, re-searchers must consider the target use of the inter-vention since it will be desirable to generalize the re-sults of the trial to the target population. However researchers also select entry criteria to help ensure a high quality trial and to address the specific objec-tives of the trial.
The selection of a population can depend on the trial phase since different phases have different objec-tives. Early phase trials tend to select populations that are more homogenous since it is easier to re-duce response variation and thus isolate effects. Lat-er phase trials tend to target more heterogeneous populations since it is desirable to have the results of such trials to be generalizable to the population in which the intervention will be utilized in practice. It is often desirable for this targeted patient population to be as large as possible to maximize the impact of the intervention. Thus phase III trials tend to have more relaxed entry criteria that are representative (both in demographics and underlying disease status) to the patient population for which the intervention is tar-geted to treat.
When constructing entry criteria, the safety of the study participant is paramount. Researcher should consider the appropriateness of recruiting partici-pants with various conditions into the trial. The ability to accrue study participants can also affect the selec-tion of entry criteria. Although strict entry criteria may be scientifically desirable in some cases, studies with strict entry criteria may be difficult to accrue particu-larly when the disease is rare or alternative interven-tions or trials are available. Entry criteria may need to be relaxed so that enrollment can be completed within a reasonable time frame.
Researchers should also consider restricting entry criteria to reduce variation and potential for bias. Par-ticipants that enroll with confounding indications that could influence treatment outcome could be excluded to reduce potential bias. For example, in a trial eva-luating interventions for HIV-associated painful neu-ropathy, conditions that may confound an evaluation of neuropathy such as diabetes or a B12 deficiency may be considered exclusionary.
Selection of endpoints
The selection of endpoints in a clinical trial is ex-tremely important and requires a marriage of clinical relevance with statistical reasoning. The motivation for every clinical trial begins with a scientific question. The primary objective of the trial is to address the scientific question by collecting appropriate data. The selection of the primary endpoint is made to address the primary objective of the trial. The primary end-point should be clinically relevant, interpretable, sen-sitive to the effects of intervention, practical and af-fordable to measure, and ideally can be measured in an unbiased manner.
Endpoints can generally be categorized by their scale of measurement. The three most common types of endpoints in clinical trials are continuous endpoints (e.g., pain on a visual analogue scale), categorical (including binary, e.g., response vs. no response) endpoints, and event-time endpoints (e.g., time to death). The scale of the primary endpoint impacts the analyses, trial power, and thus costs.
In many situations, more than one efficacy endpoints are used to address the primary objective. This creates a multiplicity issue since multiple tests will be conducted. Decisions regarding how the statistical error rates (e.g., Type I error) will be controlled should be described in the protocol and in the statis-tical analysis plan.
Endpoints can be classified as being objective or subjective. Objective endpoints are those that can be measured without prejudice or favor. Death is an objective endpoint in trials of stroke. Subjective end-points are more susceptible to individual interpreta-tion. For example, neuropathy trials employ pain as a subjective endpoint. Other examples of subjective endpoints include depression, anxiety, or sleep quali-ty. Objective endpoints are generally preferred to subjective endpoints since they are less subject to bias.
Composite endpoints
An intervention can have effects on several important endpoints. Composite endpoints combine a number of endpoints into a single measure. The CHARISMA (Bhatt et al 2006), MATCH (Diener et al 2004), and CAPRIE (Committee 1996) studies of clopidogrel for the prevention of vascular ischemic events use com-binations of MI, stroke, death, and re-hospitalization as components of composite endpoints. The advan-tages of composite endpoints are that they may result in a more completed characterization of intervention effects as there may be interest in a variety of out-comes. Composite endpoints may also result in high-er power and resulting smaller sample sizes in event-driven trials since more events will be observed (as-suming that the effect size is unchanged). Composite endpoints may also reduce the bias due to competing risks and informative censoring. This is because one event can censor other events and if data were only analyzed on a single component then informative censoring can occur. Composite endpoints may also help avoid the multiplicity issue of evaluating many endpoints individually.
Composite endpoints have several limitations. Firstly, significance of the composite does not necessarily imply significance of the components nor does signi-ficance of the components necessarily imply signific-ance of the composite. For example one intervention could be better on one component but worse on another and thus result in a non-significant composite. Another concern with composite endpoints is that the interpretation can be challenging particularly when the relative importance of the components differs and the intervention effects on the components also differ. For example, how do we interpret a study in which the overall event rate in one arm is lower but the types of events occurring in that arm are more se-rious? Higher event rates and larger effects for less important components could lead to a misinterpreta-tion of intervention impact. It is also possible that intervention effects for different components can go in different directions. Power can be reduced if there is little effect on some of the components (i.e., the intervention effect is diluted with the inclusion of these components).
When designing trials with composite endpoints, it is advisable to consider including events that are more severe (e.g., death) than the events of interest as part of the definition of the composite to avoid the bias induced by informative censoring. It is also ad-visable to collect data and evaluate each of the com-ponents as secondary analyses. This means that study participants should continue to be followed for other components after experiencing a component event. When utilizing a composite endpoint, there are several considerations including: (i) whether the components are of similar importance, (ii) whether the components occur with similar frequency, and (iii) whether the treatment effect is similar across the components.
Surrogate Endpoints
In the treatment of some diseases, it may take a very long time to observe the definitive endpoint (e.g., death). A surrogate endpoint is a measure that is predictive of the clinical event but takes a shorter time to observe. The definitive endpoint often meas-ures clinical benefit whereas the surrogate endpoint tracks the progress or extent of disease. Surrogate endpoints could also be used when the clinical end-point is too expensive or difficult to measure, or not ethical to measure.
An example of a surrogate endpoint is blood pressure for hemorrhagic stroke.
Surrogate markers must be validated. Ideally evalua-tion of the surrogate endpoint would result in the same conclusions if the definitive endpoint had been used. The criteria for a surrogate marker are: (1) the marker is predictive of the clinical event, and (2) the intervention effect on the clinical outcome manifests itself entirely through its effect on the marker. It is important to note that significant correlation does not necessarily imply that a marker will be an acceptable surrogate.
Preventing missing data and encouraging adhe-rence to protocol
Missing data is one of the biggest threats to the inte-grity of a clinical trial. Missing data can create biased estimates of treatment effects. Thus it is important when designing a trial to consider methods that can prevent missing data. Researchers can prevent miss-ing data by designing simple clinical trials (e.g., de-signing protocols that are easy to adhere to; having easy instructions; having patient visits and evalua-tions that are not too burdensome; having short, clear case report forms that are easy to complete, etc.) and adhering to the ITT principle (i.e., following all pa-tients after randomization for the scheduled duration of follow-up regardless of treatment status, etc.).
Similarly it is important to consider adherence to pro-tocol (e.g., treatment adherence) in order address the biological aspect of treatment comparisons. Envision a trial comparing two treatments in which the trial par-ticipants in both groups do not adhere to the as-signed intervention. Then when evaluating the trial endpoints, the two interventions will appear to have similar effects regardless of any differences in the biological effects of the two interventions. Note how-ever that the fact that trial participants in neither in-tervention arm adhere to therapy may indicate that the two interventions do not differ with respect to the strategy of applying the intervention (i.e., making a decision to treat a patient). Researchers need to be careful about influencing participant adherence since the goal of the trial may be to evaluate the strategy of how the interventions will work in practice (which may not include incentives to motivate patients similar to that used in the trial).
Sample size
Sample size is an important element of trial design because too large of a sample size is wasteful of re-sources but too small of a sample size could result in inconclusive results. Calculation of the sample size requires a clearly defined objective. The analyses to address the objective must then be envisioned via a hypothesis to be tested or a quantity to be estimated. The sample size is then based on the planned ana-lyses. A typical conceptual strategy based on hypo-thesis testing is as follows:
1. Formulate null and alternative hypotheses. For example, the null hypothesis might be that the response rate in the intervention and placebo arms of a trial are the same and the alternative hypothesis is that the response rate in the intervention arm is greater than the placebo arm by a certain amount (typical-ly selected as the “minimum clinically rele-vant difference”).
2. Select the Type I error rate. Type I error is the probability of incorrectly rejecting the null hypothesis when the null hypothesis is true. In the example above, a Type I error often implies that you incorrectly conclude that an intervention is effective (since the alternative hypothesis is that the response rate in the in-tervention is greater than in the placebo arm). In regulatory settings for Phase III trials, the Type I error is set at 5%. In other instances the investigator can evaluate the “cost” of a Type I error and decide upon an acceptable level of Type I error given other design con-straints. For example, when evaluating a new intervention, an investigator may con-sider using a smaller Type I error (e.g., 1%) when a safe and effective intervention al-ready exists or when the new intervention appears to be “risky”. Alternatively a larger Type I error (e.g., 10%) might be considered when a safe and effective intervention does not exist and when the new intervention ap-pears to have low risk.
3. Select the Type II error rate. Type II error is the probability of incorrectly failing to reject the null hypothesis when the null hypothesis should be rejected. The implication of a Type II error in the example above is that an effec-tive intervention is not identified as effective. The compliment of Type II error is “power”, i.e., the probability of rejecting the null hypo-thesis when it should be rejected. Type II er-ror and power are not generally regulated and thus investigators can evaluate the Type II error that is acceptable. For example, when evaluating a new intervention for a serious disease that has no effective treatment, the investigator may opt for a lower Type II error (e.g., 10%) and thus higher power (90%), but may allow Type II error to be higher (e.g., 20%) when effective alternative interventions are available. Typically Type II error is set at 10-20%.
4. Obtain estimates of quantities that may be needed (e.g., estimates of variation or a con-trol group response rate). This may require searching the literature for prior data or run-ning pilot studies.
5. Select the minimum sample size such that two conditions hold: (1) if the hull hypothesis is true then the probability of incorrectly re-jecting is no more than the selected Type I error rate, and (2) if the alternative hypothe-sis is true then the probability of incorrectly failing to reject is no more than the selected Type II error (or equivalently that the proba-bility of correctly rejecting the null hypothesis is the selected power).
The selection of quantities such as the “minimum clinically relevant difference”, Type I error, and Type II error, reflects the assumptions, limitations, and compromises of the study design, and thus require diligent consideration. Since assumptions are made when sizing the trial (e.g., via an estimate of varia-tion), evaluation of the sensitivity of the required sample size to variation in these assumptions is pru-dent as the assumptions may turn-out to be incorrect. Interim analyses can be used to evaluate the accura-cy of these assumptions and potentially make sample size adjustments should the assumptions not hold. Sample size calculations may also need to be ad-justed for the possibility of a lack of adherence or par-ticipant drop-out. In general, the following increases the required sample size: lower Type I error, lower Type II error, larger variation, and the desire to detect a smaller effect size or have greater precision.
An alternative method for calculating the sample size is to identify a primary quantity to be estimated and then estimate it with acceptable precision. For ex-ample, the quantity to be estimated may be the be-tween-group difference in the mean response. A sample size is then calculated to ensure that there is a high probability that this quantity is estimated with acceptable precision as measured by say the width of the confidence interval for the between-group differ-ence in means.
Planning for interim analyses
Interim analysis should be considered during trial de-sign since it can affect the sample size and planning of the trial. When trials are very large or long in dura-tion, when the interventions have associated serious safety concerns, or when the disease being studied is very serious, then interim data monitoring should be considered. Typically a group of independent experts (i.e., people not associated with the trial but with rele-vant expertise in the disease or treatments being stu-died, e.g., clinicians and statisticians) are recruited to form a Data Safety Monitoring Board (DSMB). The DSMB meets regularly to review data from the trial to ensure participant safety and efficacy, that trial objec-tives can be met, to assess trial design assumptions, and assess the overall risk-benefit of the intervention. The project team typically remains blinded to these data if applicable. The DSMB then makes recom-mendations to the trial sponsor regarding whether the trial should continue as planned or whether modifica-tions to the trial design are needed.
Careful planning of interim analyses is prudent in trial design. Care must be taken to avoid inflation of sta-tistical error rates associated with multiple testing to avoid other biases that can arise by examining data prior to trial completion, and to maintain the trial blind.
Common Structural Designs
Many structural designs can be considered when planning a clinical trial. Common clinical trial designs include single-arm trials, placebo-controlled trials, crossover trials, factorial trials, noninferiority trials, and designs for validating a diagnostic device. The choice of the structural design depends on the specif-ic research questions of interest, characteristics of the disease and therapy, the endpoints, the availabili-ty of a control group, and on the availability of funding. Structural designs are discussed in an accompanying article in this special issue.
Summary
This manuscript summarizes and discusses funda-mental issues in clinical trial design. A clear under-standing of the research question is a most important first step in designing a clinical trial. Minimizing varia-tion in trial design will help to elucidate treatment ef-fects. Randomization helps to eliminate bias asso-ciated with treatment selection. Stratified randomiza-tion can be used to help ensure that treatment groups are balanced with respect to potentially confounding variables. Blinding participants and trial investigators helps to prevent and reduce bias. Placebos are uti-lized so that blinding can be accomplished. Control groups help to discriminate between intervention ef-fects and natural history. There are three primary types of control groups: (1) historical controls, (2) placebo/sham controls, and (3) active controls. The selection of a control group depends on the research question, ethical constraints, the feasibility of blinding, the availability of quality data, and the ability to recruit participants. The selection of entry criteria is guided by the desire to generalize the results, concerns for participant safety, and minimizing bias associated with confounding conditions. Endpoints are selected to address the objectives of the trial and should be clinically relevant, interpretable, sensitive to the ef-fects of an intervention, practical and affordable to obtain, and measured in an unbiased manner. Com-posite endpoints combine a number of component endpoints into a single measure. Surrogate end-points are measures that are predictive of a clinical event but take a shorter time to observe than the clin-ical endpoint of interest. Interim analyses should be considered for larger trials of long duration or trials of serious disease or trials that evaluate potentially harmful interventions. Sample size should be considered carefully so as not to be wasteful of resources and to ensure that a trial reaches conclusive results.
There are many issues to consider during the design of a clinical trial. Researchers should understand these issues when designing clinical trials.
Acknowledgement
The author would like to thank Dr. Justin McArthur and Dr. John Griffin for their invitation to participate as part of the ANAs Summer Course for Clinical and Translational Research in the Neurosciences. The author thanks the students and faculty in the course for their helpful feedback. This work was supported in part by Neurologic AIDS Research Consortium (NS32228) and the Statistical and Data Management Center for the AIDS Clinical Trials Group (U01 068634).
References
- Bhatt DL, Fox KAA, Hacke W, Berger liB, Black HR, Boden WE, Cacoub li, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak K-H, Mas J-L, Montalescot G, liearson TA, Steg liG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Toliol EJ (2006) Cloliidogrel and Asliirin Versus Asliirin Alone for the lirevention of Atherothrombotic Events. N Engl J Med 16:1706-17.
- CAliRIE Committee (1996) A Randomised, Blinded, Trial of Cloliidogrel Versus Asliirin in liatients at Risk of Ischaemic Events (CAliRIE) . Lancet 348:1329–39.
- Diener H, Bogousslavsky J, Brass L, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rulilirecht H, in-vestigators obotM (2004) Asliirin and Cloliidogrel Comliared with Cloliidogrel Alone after Recent Ischaemic Stroke or Rransient Ischaemic Attack in High-Risk liatients (MATCH): Randomized, Double-Blind, lilacebo-Controlled Trial. Lancet 364.
- Evans S, Simlison D, Kitch D, King A, Clifford D, Cohen B, MacArthur J (2007) A Randomized Trial Evaluating lirosalitide™ for HIV-Associated Sensory Neurolia-thies: Use of an Electronic Diary to Record Neuroliath-ic liain. liLoS ONE 2:e551.doi:10.1371/journal.lione.0000551.
- lirasad A, Fitzgerald J, Bao B, Beck F, Chandrasekar li (2000) Duration of Symlitoms and lilasma Cytokine Levels in liatients with the Common Cold Treated with Zinc Acetate. Ann Intern Med 133:245-52.