Research Article - Interventional Cardiology (2021)
FFRCT-guided revascularization of silent coronary ischemia compared to best medical therapy following lower-extremity revascularization
- Corresponding Author:
- Gustavs Latkovskis
Latvian Centre of Cardiology,
Pauls Stradins Clinical University Hospital,
13 Pilsonu Street,
Riga,
LV1002,
Latvia,
E-mail: gustavs.latkovskis@gmail.com
Received date: June 23, 2021 Accepted date: July 07, 2021 Published date: July 14, 2021
Abstract
Objectives: The aim of this study was to determine whether selective coronary revascularization of Peripheral Artery Disease (PAD) patients with silent coronary ischemia can improve survival following lower-extremity revascularization compared to patients with no cardiac symptoms receiving best medical therapy alone.
Methods: Matched cohort analysis of PAD patients with no cardiac history or symptoms with (a) pre-operative CT-derived fractional flow reserve (FFRCT) evaluation to detect silent coronary ischemia and selective post-operative coronary revascularization (FFRCT-Guided) or (b) standard pre-operative cardiac evaluation with monitored post-operative medical therapy in the VOYAGER PAD trial (Medical Therapy). The status of silent ischemia in Medical Therapy was unknown. Study endpoints included death, Myocardial Infarction (MI) and death or MI.
Results: Among 78 FFRCT-Guided patients, 53 (68%) had silent coronary ischemia (FFRCT ≤ 0.80) of which 29 (55%) had post-operative coronary revascularization. Among 79 Medical Therapy patients none had elective coronary revascularization. During a median follow-up of 30 months, compared to Medical Therapy, FFRCT-Guided patients had fewer deaths (5.1% vs. 22.8%; adjusted Hazard Ratio (HR): 0.292; 95% Confidence Interval (CI) 0.086-0.997; p=0.049), fewer MIs (3.8% vs. 15.2%; HR: 0.233; 95% CI 0.058-0.936; p=0.040) and fewer deaths or MI (7.7% vs. 26.6%, HR 0.323, 95% CI 0.115-0.909, p=0.032).
Conclusion: Coronary revascularization of PAD patients with silent ischemia in addition to medical therapy was associated with fewer deaths and MIs following lower-extremity revascularization compared to PAD patients with no coronary symptoms receiving best medical therapy alone.
Keywords
Peripheral artery disease • Long-term survival • Coronary CT-derived fractional flow reserve •Coronary revascularization • Best medical therapy
Introduction
Peripheral Artery Disease (PAD) is a manifestation of systemic atherosclerosis and PAD patients are at high risk for adverse cardiovascular events and premature death [1]. One year mortality following lower-extremity revascularization is 10%-20% and 5 year mortality exceeds 50% [2-5]. While it is well known that most PAD patients have angiographic evidence of significant coronary stenosis [6], CAD is often unrecognized due to patients’ inability to walk and absence of chest pain symptoms.
Current guidelines recommend no pre-operative stress testing of patients without cardiac symptoms prior to revascularization surgery, since coronary revascularization has not been shown to improve long-term survival, and highlight the importance of limiting the progression of atherosclerosis with multidisciplinary vascular team management of patients, control of risk factors and intensive evidence-based medical therapy [7]. While medical therapy has been shown to be effective in CAD patients, best medical therapy has not resulted in reduction of the high mortality rates of PAD patients following lower-extremity revascularization [1,2,4,8]. This has been attributed to poor compliance with medical therapy and a paucity of controlled trial data specific to PAD patients [9]. Controlled trials focused on medical treatment of PAD patients include many patients with known and treated CAD with up to 63% having had prior coronary revascularization [10-12]. Mortality rates in these trials are considerably lower (3% per year) than for most PAD patients following lower-extremity revascularization [2-5]. The recent VOYAGER PAD trial [13] included patients with known CAD and demonstrated a benefit of rivaroxaban over placebo in patients receiving best medical therapy following lower-extremity revascularization. However, the benefit was primarily driven by a reduction in adverse limb events with no reduction in Myocardial Infarction (MI) or all-cause mortality [13]. The continuing high mortality of PAD patients treated with best medical therapy contrasts sharply with the low mortality rate of patients with CAD who are often treated with coronary revascularization [14].
Guideline recommendations against coronary revascularization of PAD patients are based on randomized trial evidence from 20 years ago using coronary angiography-guided revascularization [15] rather than the current standard of Fractional Flow Reserve (FFR)- guided coronary revascularization. Contemporary randomized trials have shown reduced 5 year death or MI rates with FFRguided Percutaneous Coronary Intervention (PCI) compared to best medical therapy in patients with both symptomatic and asymptomatic CAD [16-18]. It is now possible to identify patients with ischemia-producing coronary lesions noninvasively using coronary CT-derived Fractional Flow Reserve ( FFRCT) [19]. FFRCT accurately reflects invasively measured FFR, can reliably identify patients who may benefit from coronary revascularization and can help-guide procedural planning [20-22]. This new noninvasive cardiac diagnostic modality has now been used to evaluate patients with symptomatic PAD who are at high risk for cardiac events and death [23-25]. Systematic cardiac evaluation of critical limb ischemia patients with no cardiac history or symptoms using FFRCT revealed a high prevalence of unsuspected, silent coronary ischemia [23]. This allowed risk stratification of patients and facilitated multidisciplinary vascular team management [7] including selective coronary revascularization of PAD patients with silent ischemia following recovery from peripheral revascularization surgery [23]. This new strategy was associated with fewer cardiovascular deaths, fewer MIs and improved one [24] and two-year survival [25] compared to control patients receiving standard cardiac evaluation and care.
The purpose of this study was to compare the outcome of PAD patients with FFRCT-guided coronary revascularization of silent ischemia following lower-extremity revascularization to the outcome of PAD patients receiving best medical therapy in a controlled setting following lower-extremity revascularization.
Materials and Methods
Study design
Matched cohort analysis of symptomatic PAD patients who underwent lower-extremity revascularization in consecutive prospective studies at Pauls Stradins Clinical University Hospital in Riga, Latvia. The first study was the multicenter, double-blinded VOYAGER PAD trial which randomized patients to receive rivaroxaban plus aspirin or placebo plus aspirin in addition to best medical therapy following infra-inguinal revascularization [13]. This study included patients with symptomatic CAD and prior MI. Our hospital was a top enrolling site in this worldwide study of 6564 patients, enrolling 145 patients (2.2% of the total). The second study was aimed at the same population of PAD patients but did not include patients with symptomatic CAD or prior MI since the objective was to determine the prevalence of unsuspected (silent) coronary ischemia using coronary CT-derived FFR ( FFRCT). Both studies were approved by the Pauls Stradins Clinical University Hospital Foundation Ethics Committee (020316-3E and 060319-1L), all patients signed written informed consent and details of each study have been published [13,24].
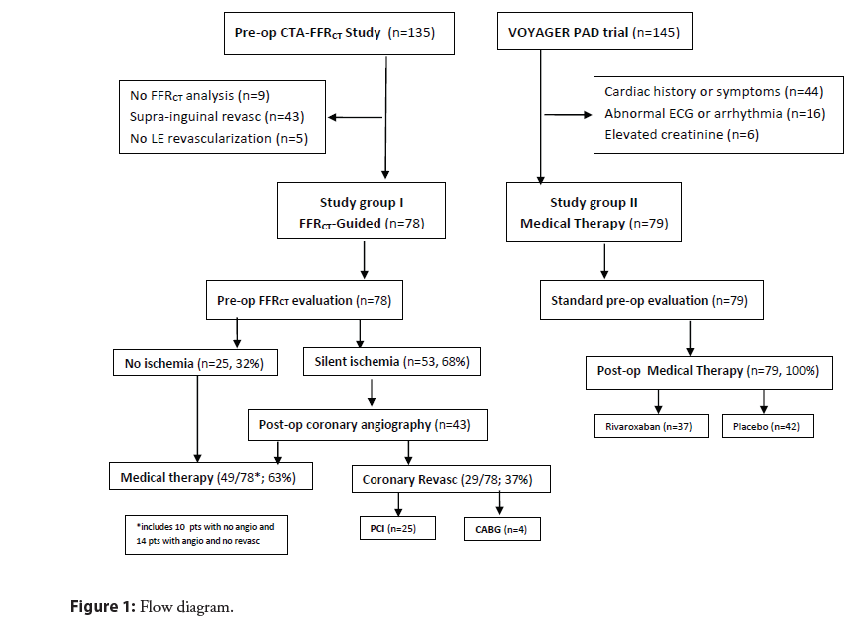
All patients enrolled in each of the two studies who met the following inclusion and exclusion criteria were selected for this comparative outcome analysis. Inclusion criteria: Age ≥ 50 years, limiting claudication or Chronic Limb-Threatening Ischemia (CLTI), infra-inguinal surgical revascularization, and pre-operative FFRCT analysis ( FFRCT group only). Exclusion criteria: cardiac history or symptoms, prior MI or coronary revascularization, abnormal resting electrocardiogram, congestive heart failure, severe arrhythmia or pacemaker, stroke or Transient Ischemic Attack (TIA); increased bleeding risk; impaired renal function; or active medical condition with life expectancy <1 year. A flow diagram of the study groups is shown in Figure 1.
FFRCT-guided
Of 135 patients enrolled in the CTA- FFRCT study, 78 patients were included in the FFRCT-Guided cohort and 57 were excluded as shown in Figure 1. Prior to surgery, calcium scoring and coronary CT Angiography (CTA) imaging were performed in accordance with guidelines with beta-blockade for heart rate control and sublingual nitroglycerin for coronary vasodilation [26]. CTA image datasets were sent to HeartFlow (Redwood City, CA) for computational analysis of FFRCT with results returned within 24 hours. Significant CTA stenosis was defined as ≥ 50%. Lesionspecific coronary ischemia was defined as FFRCT ≤ 0.80 distal to stenosis in >2 mm vessels. Severe ischemia was defined as FFRCT ≤ 0.75 distal to stenosis. Patients with coronary ischemia were evaluated by the Heart team for guidance on patient management and decisions in regard to coronary intervention (PCI, Coronary Artery Bypass Graft (CABG) or optimal medical care) in accord with 2018 ESC/EACTS guidelines on myocardial revascularization [27]. Post-operative medical care was guided by the cardiovascular team in collaboration with each patient’s local medical provider with follow-up at 1, 3, 6, 12 months and every 6 months thereafter.
Medical therapy
Of 145 patients enrolled in the VOYAGER PAD trial, 79 patients were included in the Medical Therapy cohort and 66 were excluded as shown in Figure 1. Following successful revascularization surgery, patients were randomly assigned to receive either 2.5 mg rivaroxaban twice daily plus aspirin or placebo twice daily plus aspirin in addition to guideline-directed medical care [13]. No patient had elective coronary angiography or revascularization. Post-operative medical care was provided by the patient’s local medical provider with follow-up at 1, 3, 6 and 12 months and every 6 months thereafter. Patient compliance with the investigational drug and overall medical therapy was recorded by clinical trial nurses at each visit along with documentation of efficacy and safety outcome measures of the trial. Following publication of the results of VOYAGER PAD trial [13], unblinding of individual patient data at our site revealed that of the 79 patients in this cohort, 37 had received rivaroxaban and 42 had received placebo.
Study endpoints
The primary study endpoint was all-cause death during followup with secondary endpoints of MI and death or MI. Endpoints were recorded locally and adjudicated by an institutional interdisciplinary endpoints committee in accord with Academic Research Consortium-2 consensus document [28].
Statistical analysis
Power calculation for this study was based on all-cause mortality rates reported in the two published studies [13,24] with an estimate that 58 patients would be required in each group to test the hypothesis that mortality with FFRCT analysis would be lower than Medical Therapy (90% power, alpha 0.05, two-sided level of significance). Continuous variables were tested for normal distribution with Shapiro-Wilk test and were expressed as mean ± standard deviation if normally distributed and as the median and inter-quartile range if non-normally distributed. Continuous variables were compared between the groups using Student`s t test and Mann-Whitney U test, respectively. Categorical variables were expressed as count (percentage) and were compared using Chisquare test or Fisher`s exact test as appropriate. Kaplan-Meier survival curves were compared using the log-rank test. Hazard ratios and corresponding 95% confidence intervals were generated using a Cox proportional hazards model adjusted for age, gender, hyperlipidemia, hypertension, diabetes mellitus, smoking history and rivaroxaban therapy. Statistical analyses were performed using IBM SPSS Statistics version 23.0 with two-sided p values and significance defined as p<0.05.
Results
Study cohort comparison
Baseline characteristics in the two study groups were well balanced with no significant differences in age, gender, risk factors or preoperative ankle-brachial index (Tables 1-4). The primary indication for revascularization surgery was CLTI in 86% of the FFRCT- Guided group and 81% of the Medical Therapy group. Postoperative guideline-directed medical therapy was similar in the two groups with statin therapy in 81% vs. 82%, antihypertensives in 60% vs. 66% and antiplatelets/anticoagulants in 94% vs. 99%. Comparison of the two study groups to the VOYAGER PAD trial population is shown on Table 4.
| Patient characteristics | FFRCT-Guided N=78 | Medical therapy N=79 | p-value |
|---|---|---|---|
| Median age-years, (IQR) | 66 (60-71) | 65 (60-73) | 0.813 |
| Female gender-n, (%) | 16 (21%) | 17 (22%) | 0.877 |
| Risk factors and coexisting conditions-n, (%) | |||
| Hypertension | 57 (73) | 56 (71) | 0.76 |
| Hyperlipidemia | 34 (44) | 29 (38) | 0.446 |
| Current smoker | 27 (35) | 20 (25) | 0.203 |
| Diabetes mellitus | 9 (12) | 17 (22) | 0.093 |
| Cardiac history or symptoms of CAD | 0 | 0 | ns |
| Pre-operative evaluation | |||
| Median ankle-brachial index (IQR) | 0.62 (0.47-0.68) | 0.62 (0.44-0.72) | 0.538 |
| Primary indication for lower-extremity revascularization–n, (%) | |||
| Critical limb threatening ischemia | 67 (86) | 64 (81) | 0.41 |
| Limiting claudication | 11 (14) | 15 (19) | 0.41 |
| Operation performed-n, (%) | |||
| Infra-inguinal surgical revascularization | 78 (100) | 79 (100) | ns |
| Post-operative medications-n, (%) | |||
| Statins | 63 (81) | 65 (82) | 0.808 |
| ACE inhibitor or ARB | 47 (60) | 52 (66) | 0.47 |
| Antiplatelets/anticoagulants | 73 (94) | 78 (99) | 0.117 |
| Insulin | 9 (12) | 17 (22) | 0.093 |
Data are presented as number (%) or mean+Standard Deviation (SD) or median, Abbreviation: IQR: Interquartile Range; CAD: Coronary Artery Disease; ns: Not Specified
Table 1: Baseline characteristics of FFRCT-Guided and Medical Therapy study groups.
Pre-operative cardiac evaluation
Patients in both groups had standard pre-operative assessment and were cleared for elective surgery. The results of additional CTA and FFRCT evaluation in the FFRCT-Guided cohort are shown in Table 2. FFRCT analysis revealed lesion-specific coronary ischemia in 53 patients (68%) and left main ischemia in 8%. Severe coronary ischemia ( FFRCT ≤ 0.75) was present in 47 patients (60%).
| Coronary CTA (n=78) | |
|---|---|
| Calcium score, median (IQR) Agatston score | 857 [349-1879] |
| Calcium score range, (min-max) | 5-4135 |
| Coronary CTA stenosis ≥ 50%-n (%) | 56 (72) |
| Left main | 6 (8) |
| Single vessel stenosis | 24 (31) |
| 2 vessel stenosis | 18 (23) |
| 3 vessel stenosis | 14 (18) |
| Coronary CTA stenosis ≥ 70%-n (%) | 33 (42) |
| FFRCT analysis (n=78) | |
| FFRCT ≤ 0.80 distal to stenosis-n (%) | 53 (68) |
| Mean FFRCT value | 0.69 ± 0.14 |
| Left main | 6 (8) |
| Single vessel ischemia | 21 (27) |
| 2 vessel ischemia | 13 (17) |
| 3 vessel ischemia | 19 (24) |
| FFRCT ≤ 0.75 distal to stenosis-n (%) | 47 (60) |
| Mean FFRCT value | 0.60 ± 0.10 |
| FFRCT>0.80 distal to stenosis-n (%) | 25 (32) |
| Mean FFRCT value | 0.86 ± 0.06 |
Data presented as number (%) or mean+Standard Deviation (SD) or median. Abbreviation: IQR: interquartile range; CTA: CT Angiography; FFRCT: coronary computed tomography angiography-derived fractional flow reserve.
Table 2: Pre-operative evaluation in FFRCT-Guided group.
Post-operative management
In the FFRCT-Guided group, 43 of 53 patients (81%) with lesionspecific coronary ischemia underwent coronary angiography within 3 months following surgery. Elective coronary revascularization was performed in 29 patients (37% of entire group, 55% of those with silent ischemia) with PCI in 25 and CABG in 4 patients (Figure 1). A representative patient example of coronary revascularization is shown in Figure 2. In the Medical Therapy group all patients were treated medically and no patient had elective coronary revascularization. No patient in either group had a significant bleeding complication during the follow up period.
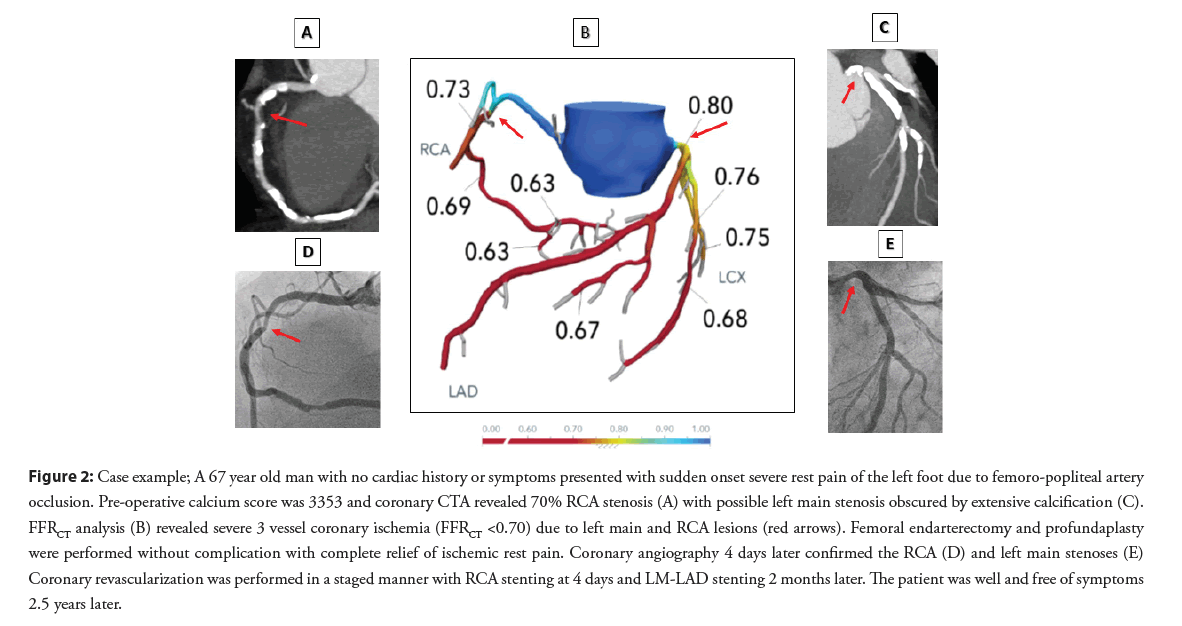
Figure 2: Case example; A 67 year old man with no cardiac history or symptoms presented with sudden onset severe rest pain of the left foot due to femoro-popliteal artery occlusion. Pre-operative calcium score was 3353 and coronary CTA revealed 70% RCA stenosis (A) with possible left main stenosis obscured by extensive calcification (C). FFRCT analysis (B) revealed severe 3 vessel coronary ischemia (FFRCT <0.70) due to left main and RCA lesions (red arrows). Femoral endarterectomy and profundaplasty were performed without complication with complete relief of ischemic rest pain. Coronary angiography 4 days later confirmed the RCA (D) and left main stenoses (E) Coronary revascularization was performed in a staged manner with RCA stenting at 4 days and LM-LAD stenting 2 months later. The patient was well and free of symptoms 2.5 years later.
Primary and secondary endpoints
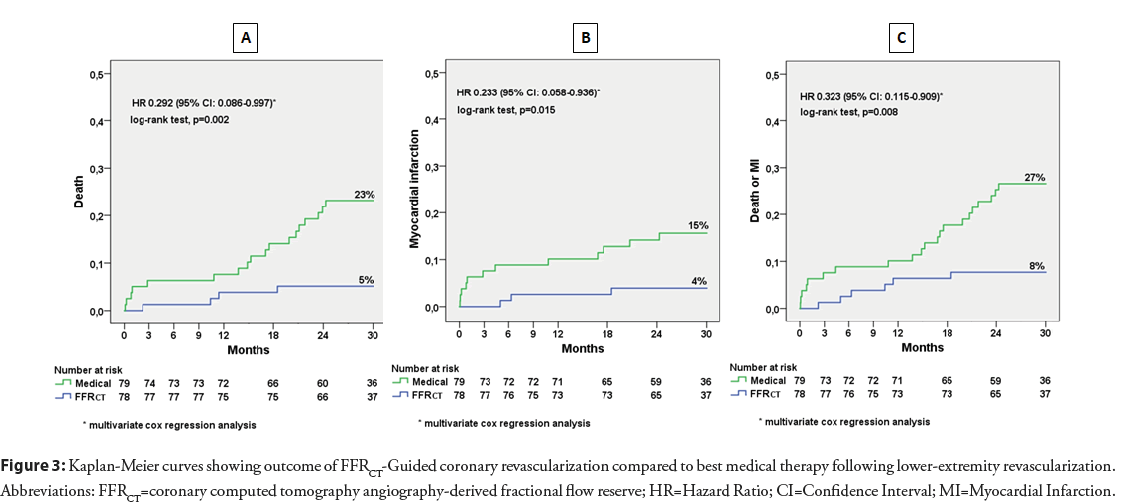
Median follow-up was 29.8 months (IQR 26.6-31.2 months) in FFRCT-Guided and 29.9 months (IQR 25.9-32.0 months) in Medical Therapy. The primary and secondary endpoints are shown in Table 3. There were 4 deaths (5.1%) in the FFRCT-Guided cohort (one cardiac, one stroke and 2 cancer deaths) and 18 deaths (22.8%) in the Medical Therapy cohort (9 cardiac and 9 cancer deaths). In the FFRCT-Guided cohort there were 3 MIs (3.8%)-one was procedure-related during PCI and 2 occurred spontaneously in patients who had declined elective PCI and were successfully treated with urgent PCI. In the Medical Therapy cohort there were 12 MIs (15.2%)-9 were fatal, two were successfully treated with urgent PCI and one was treated medically. Cumulative incidence of death, MI and death or MI (Kaplan- Meier estimates) are shown in Figure 3. Compared to Medical Therapy, the FFRCT-Guided cohort had a significantly lower risk of death (5.1% vs. 22.8%, log-rank p=0.002), MI (3.8% vs. 15.2%, log-rank p=0.015) and death or MI (3.8% vs. 15.2%, log-rank p=0.008). Multivariate Cox regression modeling, with adjustment for age, gender, hyperlipidemia, hypertension, diabetes mellitus, current smoking and rivaroxaban therapy showed significantly lower risk of death (HR: 0.292; 95% CI: 0.086-0.997; p=0.049), MI (HR: 0.233; 95% CI: 0.058-0.936; p=0.040) and death or MI (HR: 0.323; 95% CI: 0.115-0.909; p=0.032) (Table 3).
| Cumulative incidence | P value KM Log-rank | Univariate Cox analysis HR (95% CI) | p Value | Multivariable adjusted* Cox HR (95% CI) | p value | ||
|---|---|---|---|---|---|---|---|
| FFRCT-guided (n=78) | Medical therapy (n=79) | ||||||
| Death, n (%) | 4 (5.1) | 18 (22.8) | 0.002 | 0.210 (0.01-0.620) | 0.005 | 0.292 (0.086-0.997) | 0.049 |
| Myocardial infarction, n (%) | 3 (3.8) | 12 (15.2) | 0.015 | 0.237 (0.067-0.839) | 0.026 | 0.233 (0.058-0.936) | 0.04 |
| Death or MI, n (%) | 6 (7.7) | 21 (26.6) | 0.008 | 0.267 (0.108-0.663) | 0.004 | 0.323 (0.115-0.909) | 0.032 |
Abbreviations: *Adjusted for age, gender, hyperlipidemia, hypertension, diabetes mellitus, smoking and rivaroxaban therapy; FFRCT: coronary computed tomography angiography-derived fractional flow reserve; CI: Confidence Interval; MI: Myocardial Infarction; HR: Hazard Ratio.
Table 3: Primary and secondary endpoints.
Figure 3: Kaplan-Meier curves showing outcome of FFRCT-Guided coronary revascularization compared to best medical therapy following lower-extremity revascularization. Abbreviations: FFRCT=coronary computed tomography angiography-derived fractional flow reserve; HR=Hazard Ratio; CI=Confidence Interval; MI=Myocardial Infarction.
Discussion
In this matched cohort analysis of symptomatic PAD patients with no cardiac history or coronary symptoms who had undergone infra-inguinal revascularization surgery, more than one in 5 patients treated with best medical therapy alone died during the 2.5 year follow up period. By contrast, only one in 20 patients who had evaluation for the presence of silent coronary ischemia with selective post-operative coronary revascularization died during the follow up period. This represents a more than 70% reduction in the risk of death in PAD patients following lower-extremity surgical revascularization. Similarly, the risk of myocardial infarction was reduced by 77%.
High mortality with best medical therapy following lowerextremity revascularization
The high mortality (22.8%) in the Medical Therapy cohort is consistent with two-year mortality rates of 20%-40% reported from large, current experiences of medically treated patients following open and endovascular revascularization [2-5]. The highest mortality rates occur in patients with chronic limb threatening ischemia [1,2,3]. A recent meta-analysis of 12 observational studies with more than 17,000 CLTI patients reported a one-year mortality rate of 21% following lower-extremity revascularization [5]. In our study, more than 80% of patients had CLTI and all patients had open surgical infra-inguinal revascularization. While endovascular procedures are gaining prominence for infra-inguinal procedures, there is no evidence that this has reduced mortality [1,2,5]. A recent analysis of all-cause mortality in a randomized trial of endovascular infra-inguinal revascularization in 2289 patients with CLTI or claudication found that one-year mortality was 10% and 2.5 year mortality was 25%, similar to the Medical Therapy cohort in this study [3].
Low mortality with FFR-guided coronary revascularization
The low rates of death (5.1%) and MI (3.8%) in the FFRCT-guided cohort with coronary revascularization are consistent with evidence that FFR-guided coronary revascularization reduces death and MI in patients with stable CAD [16]. A meta-analysis of individual patient data from 2400 subjects with stable coronary lesions in 3 randomized trials of FFR-guided PCI vs. medical therapy showed a 30% reduction in the hard endpoints of death or MI with coronary revascularization over a 5 year period [17]. The Swedish Coronary Angiography and Angioplasty Registry of 23,860 patients with stable angina pectoris, found that FFR-guided PCI was associated with a 19% reduction in 5 year mortality compared to angiography-guided revascularization [28,29]. The benefit of FFR-guided revascularization has also been shown in patients with stable coronary disease and no coronary symptoms [18]. In the FAME 2 trial, which randomized patients with FFR evidence of coronary ischemia to PCI or medical therapy, 98 patients had asymptomatic (silent) ischemia, 11% of the total. Patients with silent ischemia randomized to PCI had a three-fold lower rate of death or MI compared to patients randomized to medical therapy (9.4% vs. 31.1%, p=0.006) [18].
Medical therapy vs. selective coronary revascularization of PAD patients
Mortality rates in randomized trials of medical therapy in PAD patients (approximately 3% per year) [10-12] are similar to the rates reported in randomized trials of patients with stable CAD [16-18] and are considerably lower than mortality rates in broad clinical experiences of lower-extremity revascularization for PAD (10%- 13% per year) [1-5]. In the VOYAGER PAD trial of rivaroxaban vs. placebo, in both groups at 3 years, cumulative death and MI rates were only 9.4% and 4.3%, respectively [13] compared to 22.8% and 15.2% in the Medical Therapy cohort of this study, which was a subset of the VOYAGER trial. This disparity is likely due to important differences in the study populations as shown on Table 4. In VOYAGER 31% of patients had symptomatic CAD and 11% had prior MI, while the Medical Therapy cohort in this study had no known CAD. Furthermore, most patients in VOYAGER PAD had infra-inguinal revascularization for claudication (77%) whereas in the Medical Therapy cohort most revascularizations were for CLTI (81%). In a randomized trial of lower-extremity revascularization that included 1480 CLTI and 809 claudication patients, all-cause mortality was threefold higher in CLTI compared to claudication patients (33% vs. 10% at 2.5 years) [3]. Randomized trials of new medical therapies in PAD patients, such as rivaroxaban and PCSK-9 inhibitors, have primarily focused on patients with claudication and demonstrated clinical benefit using composite cardiovascular, cerebrovascular and peripheral endpoints [10- 13]. In the VOYAGER PAD trial, the benefit of rivaroxaban was driven by fewer acute limb events with no reduction in all-cause mortality [13]. Thus, the consistent failure of new medical therapy trials to demonstrate a survival benefit in PAD patients may be due to a primary focus on low risk claudication patients with many having known CAD and prior coronary revascularization with relatively low mortality rates.
| VOYAGER PAD | Medical therapy |
FFRCT-guided |
|
|---|---|---|---|
| Patient characteristics | |||
| Number of patients | 6564 | 79 | 78 |
| Months of follow-up, median | 28 | 30 | 30 |
| Age (median, years) | 67 | 65 | 66 |
| Female gender (%) | 26 | 22 | 21 |
| Hypertension | 81% | 71% | 73% |
| Hyperlipidemia | 60% | 38% | 44% |
| Current smoker | 35% | 25% | 35% |
| Diabetes mellitus | 40% | 22% | 12% |
| Symptomatic CAD | 31% | 0% | 0% |
| History of MI | 11% | 0% | 0% |
| Pre-op ABI, median | 0.56 | 0.62 | 0.62 |
| LE revascularization for claudication | 77% | 19% | 14% |
| LE revascularization for CLTI | 23% | 81% | 86% |
| Endovascular procedure | 65% | 0% | 0% |
| Open surgical procedure | 35% | 100% | 100% |
| Post-op medical therapy | |||
| Statin therapy | 80% | 82% | 81% |
| Antihypertensives | 63% | 66% | 60% |
| Aspirin | 99% | 99% | 94% |
| Rivaroxaban | 50% | 47% | 0% |
| Endpoints | |||
| Death | 9.40% | 22.80% | 5.10% |
| Myocardial infarction | 4.30% | 15.20% | 3.80% |
| Death or MI | Not reported | 26.60% | 7.70% |
Table 4: Patient characteristics of VOYAGER PAD trial compared to Medical Therapy and FFRCT-Guided cohorts.
Most patients in both arms of this study had high risk CLTI. In the FFRCT-guided cohort silent coronary ischemia was identified by study protocol-directed coronary CTA and FFRCT evaluation prior to lower-extremity revascularization. The finding of ischemiaproducing coronary stenosis in 2 of 3 patients was unexpected in view of the absence of cardiac symptoms. Of surprise was the severity of ischemia with 60% having FFRCT values ≤ 0.75 with a mean FFRCT value of 0.60 ± 0.10. In addition, 8% of patients had left main ischemia. The high mortality risk of left main disease is well known as is the increasing risk of adverse cardiac events in patients with lower FFR values [30]. CAD patients with FFR values below 0.70 are at highest risk and patients with low FFR values derive the greatest benefit from coronary revascularization [31]. However, the significance of FFRCT-defined coronary ischemia in stable PAD patients needing surgery was unknown in this study and lower-extremity revascularization was guided by the pressing clinical need for limb salvage. While FFRCT analysis did not alter the decision to proceed with revascularization surgery, the results identified high risk patients and facilitated early cardiology engagement in patient management including selective coronary revascularization in the post-operative period with the objective of improving long-term survival. While previous studies found no long-term survival benefit with coronary revascularization prior to elective peripheral vascular surgery [15], this study demonstrated a survival benefit with selective coronary revascularization of patients with silent coronary ischemia after clinically indicated lower-extremity revascularization.
Limitations
Limitations of this study include the fact that patients were drawn from two consecutive studies with different study designs. The VOYAGER PAD study enrolled patients up to 10 days after successful lower-extremity revascularization. Thus, high risk patients with post-operative MI, or other complications were excluded from the VOYAGER PAD trial, leaving a lower risk study population. By contrast, patients in the FFRCT-Guided cohort were enrolled before revascularization surgery in order to undergo pre-operative coronary CTA and FFRCT evaluation. All enrolled patients who had infra-inguinal revascularization were included in the FFRCT cohort irrespective of the FFRCT result and regardless of whether an adverse post-operative event occurred. This may have resulted in an imbalance in the study populations in favor of Medical Therapy. On the other hand, the Medical Therapy group had a higher number of diabetic patients (17 vs. 9) which was not statistically significant (p=0.09) but which may have contributed to a higher mortality in this group. The numerical difference in diabetes was taken into account in the multivariate Cox proportional modeling, which was adjusted for diabetes mellitus as well as other baseline variables of age, gender, hyperlipidemia, hypertension and rivaroxaban therapy. It should be noted that patients in both studies were cared for by the same clinical team with no changes to patient care protocols during the course of the two studies. However, since the studies were not conducted in the same time frame, alterations in practice patterns may have occurred which may have influenced results. The risk of selection bias in this study was limited by the fact that FFRCT analysis was not available during the time frame of the VOYAGER PAD trial. Nonetheless, despite the possibility of imbalance in the study populations, there was a clear difference in outcome with reduced death and MI in the FFRCT cohort. While the results of this single center study are promising, they are hypothesis generating and cannot be generalized. Prospective controlled trials are needed to define the role of selective coronary revascularization of PAD patients with silent coronary ischemia following lowerextremity revascularization.
Conclusion
Pre-operative evaluation of PAD patients with no cardiac symptoms undergoing lower-extremity revascularization using CT-derived FFR reveals a high prevalence (68%) of unsuspected, silent coronary ischemia. Selective post-operative coronary revascularization of patients with silent ischemia resulted in fewer deaths and myocardial infarctions and improved two-year survival (95%) compared to patients treated with best medical therapy alone (77%). Prospective controlled studies are needed to further define the role of FFRCT guided management of patients undergoing lower-extremity revascularization.
Acknowledgments
The authors wish to acknowledge Dace Jakovicka, Erika Sprindzuka, Ligita Zvaigzne for help with data collection.
Funding
The Latvian Council of Science, project nr. 2018/2-0295 provided research grant for this project.
References
- Fridh EB, Andersson M, Thuresson M, et al. Amputation rates, mortality and pre-operative comorbidities in patients revascularised for intermittent claudication or critical limb ischemia: A population based study. Eur J Vasc Endovasc Surg. 54(4): 480-486 (2017).
- Sigvant B, Kragsterman B, Flakenberg M et al. Contemporary cardiovascular risk and secondary drug treatment patterns in peripheral artery disease patients undergoing revascularization. J Vasc Surg. 64(4): 1009-17 (2016).
- Nordanstig J, James S, Manne Andersson, et al. Mortality with paclitaxel-coated devices in peripheral artery disease. N Engl J Med. 383: 2538-46 (2020).
- Simons JP, Baril DT, Goodney PP, et al. The effect of postoperative myocardial ischemia on long-term survival after vascular surgery. J Vasc Surg. 58(6): 1600-1608 (2013).
- Wubbeke LF, Naves CC, Daemen JH, et al. Mortality and major amputation after revascularization in octagenarians versus non-octagenarians with chronic limb threatening ischemia: A systemic review and meta-analysis. Eur J Vasc Endovasc Surg. 60: 231-241 (2020).
- Hur DJ, Kizilgul M, Aung WW, et al. Frequency of coronary artery disease in patients undergoing peripheral artery disease surgery. Am J Cardiol. 110 (5): 736-740 (2012).
- Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 55:305-368 (2018)
- Thiney M, Schiava ND, Ecochard R, et al. Effect on mortality and cardiovascular events of adherence to guideline-recommended therapy 4 years after lower extremity arterial revascularization. Ann Vasc Surg. 52: 138-146 (2018).
- Berger JS, Hiatt WR. Medical therapy in peripheral artery disease. Circulation 126(4): 491-500 (2012).
- Hiatt WR, Fowkes FGR, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 376: 32-40 (2017).
- Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease, the COMPASS trial. J Am Coll Cardiol. 71(20): 2306-15 (2018).
- Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipopotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease. Circulation. 137(4): 338-350 (2018).
- Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 382(21): 1994-2004 (2020).
- Wijeysundera HC, Bennell MC, Feng Qiu, et al. Comparative-effectiveness of revascularization versus routine medical therapy for stable ischemic heart disease: A population-based study. J Gen Intern Med. 29(7): 1031-9 (2014).
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 351(27): 2795-80417 (2004).
- Xaplanteris P, Fournier S, Pijls NHJ, et al. Five-Year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 379(3): 250-259 (2018).
- Zimmermann FM, Omerovic E, Fournier S, et al. Fractional flow reserve-guided percutaneous coronary intervention vs. medical therapy for patients with stable coronary lesions: Meta-analysis of individual patient data. Eur Heart J. 40: 180-186 (2019).
- Fournier S, Kobayashi Y, Fearon WF, et al. Asymptomatic patients with abnormal fractional flow reserve treated with medication alone or with PCI. J Am Coll Cardiol. 74(12): 1642-1644 (2019).
- Norgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomographic angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 63(12): 1145-1155 (2014).
- Bom MJ, Schumacher SP, Driessen RS, et al. Non-invasive procedural planning using computed tomography-derived fractional flow reserve. Catheter Cardiovasc Interv. 97(4): 614-622 (2021).
- Norgaaard BL, Terkelsen CJ, Mathiassen ON, et al. Coronary CT angiographic and flow reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol 72(18): 2123-2134 (2018).
- Patel MR, Norgaard BL, Fairbairn TA, et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT. The ADVANCE Registry. JACC Cardiovasc Imaging. 13: 97-105 (2020).
- Krievins D, Zellans E, Erglis A, et al. High prevalence of asymptomatic ischemia-producing coronary stenosis in patients with critical limb ischemia. Vasc Dis Manag. 15: E96-E101 (2018).
- Krievins D, Zellans E, Latkovskis G, et al. Pre-operative diagnosis of silent coronray ischemia may reduce post-operative death and myocardial infarction and improve survival of patients undergoing lower-extremity surgical revascularization. Eur J Vasc Endovasc Surg 60(3): 411-420 (2020).
- Krievins D, Zellans E, Latkovskis G, et al. Diagnosis of silent coronary ischemia with selective coronary revascularization might improve 2-year survival of patients with critical limb-threatening ischemia. J Vasc Surg. 2021.
- Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr 10(6): 435-449 (2016).
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 40(2): 87-165 (2019).
- Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials. Circulation. 137(24): 2635-2650 (2018).
- Volz S, Dworeck C, Redfors B, et al. Survival of patients with angina pectoris undergoing percutaneous coronary intervention with intracoronary pressure wire guidance. J Am Coll Cardiol. 75(22):2785-99 (2020).
- Johnson NP, Toth GG, Lai D, et al. Prognostic value of fractional flow reserve: Linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 64:1641-54 (2014).
- Ciccarelli G, Barbato E, Toth GG, et al. Angiography versus hemodynamics to predict the natural history of coronary stenoses: Fractional flow reserve versus angiography in multivessel evaluation 2 substudy. Circulation. 137(14): 1475-85 (2018).