Perspective - Interventional Cardiology (2013) Volume 5, Issue 1
Does prior coronary stenting compromise future coronary surgery?
- Corresponding Author:
- Lazar Velicki
University of Novi Sad, Medical Faculty
Novi Sad Serbia & Institute of Cardiovascular Diseases Vojvodina,
Put Doktora Goldmana 4, 21204 Sremska Kamenica, Serbia
Tel: +381 21 4805701
Fax: +381 21 6622059
E-mail: velar@sbb.rs
Abstract
Keywords
CABG;coronary stent;major adverse cardiac events;myocardial revascularization;outcome;PCI;risk assessment.
Introduction
Coronary artery disease (CAD) is currently the leading cause of death globally, and is expected to rise in the near future, despite prevalence decline reported in the developed part of the world. Globally, the proportion of deaths attributed to CAD is expected to grow by 2% between 2004 and 2030 [1]. Coronary artery bypass graft (CABG) and percutaneous coronary interventions (PCIs) are alternative revascularization procedures for patients with multivessel CAD [2]. The potential for adverse factors when the CABG procedure follows PCI is an important consideration when choosing therapy for patients with CAD.
CABG continues to be one of the most commonly performed surgical procedures worldwide and certainly the most scrutinized. Significant improvements have been made over the 40-year long history of surgical treatment of CAD, while the root cause and profile of patients undergoing cardiac surgery has profoundy changed [3,4]. Patients submitted to surgery today are older and present with complex comorbidities, usually requiring more than an isolated CABG procedure. Interventional cardiology – a ‘younger brother’ of cardiac surgery – with a history of 30 years that also underwent significant changes and improvements, both from a technological and philosophical standpoint (when to perform the procedure, how to perform it and what can be expected). PCI has evolved from balloon angioplasty of a single coronary stenosis to multivessel stenting with drug-eluting stents (DES) and treatment of chronic total occlusions with advanced techniques and devices. Coronary stents have evolved from the early concept of providing mechanical support and preventing vessel recoil to becoming ubiquitous devices, and have culminated in the highly sophisticated technology of DES [5].
It is evident from the recent clinical trials, (Box 1) comparing PCI and CABG in patients with multivessel CAD, that CABG offers significant advantages over PCI in terms of rate of repeated revascularization, major adverse cardiac events (MACE) and long-term survival [6–11]. In the recent randomized prospective SYNTAX trial, comparisons of PCI with CABG in left main disease and/or three vessel disease patients have been performed. At 3 years, major adverse cardiac and cerebrovascular events (death, stroke, myocardial infarction [MI], and repeat revascularization; CABG 20.2% vs PCI 28.0%; p < 0.001), repeat revascularization (10.7 vs 19.7%; p < 0.001) and MI (3.6 vs 7.1%; p = 0.002) were elevated in the PCI arm [12]. Major adverse cardiac and cerebral event rates were not significantly different between arms in the left main subgroup (22.3 vs 26.8%; p = 0.20), but were higher with PCI in the three vessel disease subgroup (18.8 vs 28.8%; p < 0.001).
Box1: Studies comparing percutaneous coronary intervention with coronary artery bypass grafting for multivessel coronary artery disease.
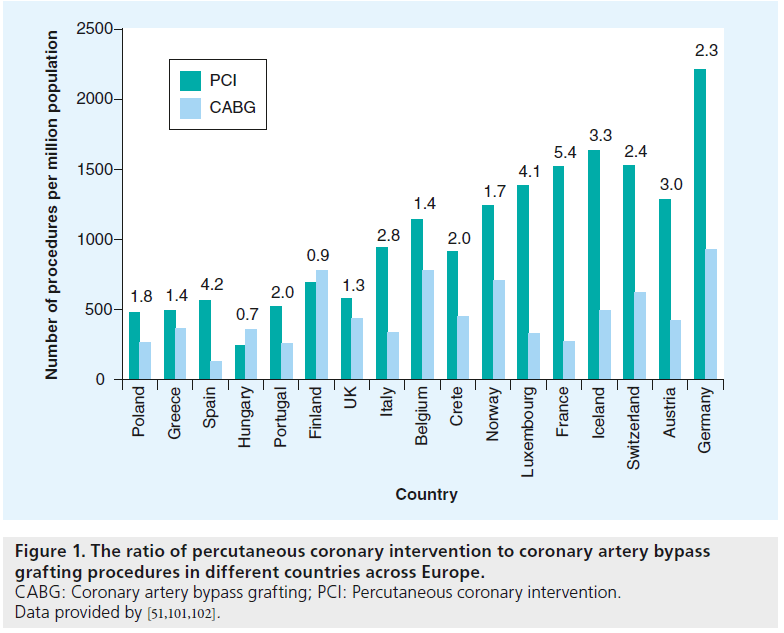
However, the number of performed procedures shows a dramatic change over time,with CABG declining and reaching steady levels only in the most recent years, while the count of PCIs is increasing and patient scope expanding. Event cases involving complex CAD that, until recently, were considered strictly as CABG patients, are now referred for percutaneous treatment. At the same time, aggressive repeated PCI with multiple stent-graft placement has become more common in the ‘stent era’ [13]. In many industrialized countries, the ratio of PCI to CABG now exceeds four to one (Figures 1 & 2) [14].
Figure 1: The ratio of percutaneous coronary intervention to coronary artery bypass grafting procedures in different countries across Europe. CABG: Coronary artery bypass grafting; PCI: Percutaneous coronary intervention. Data provided by [51,101,102].
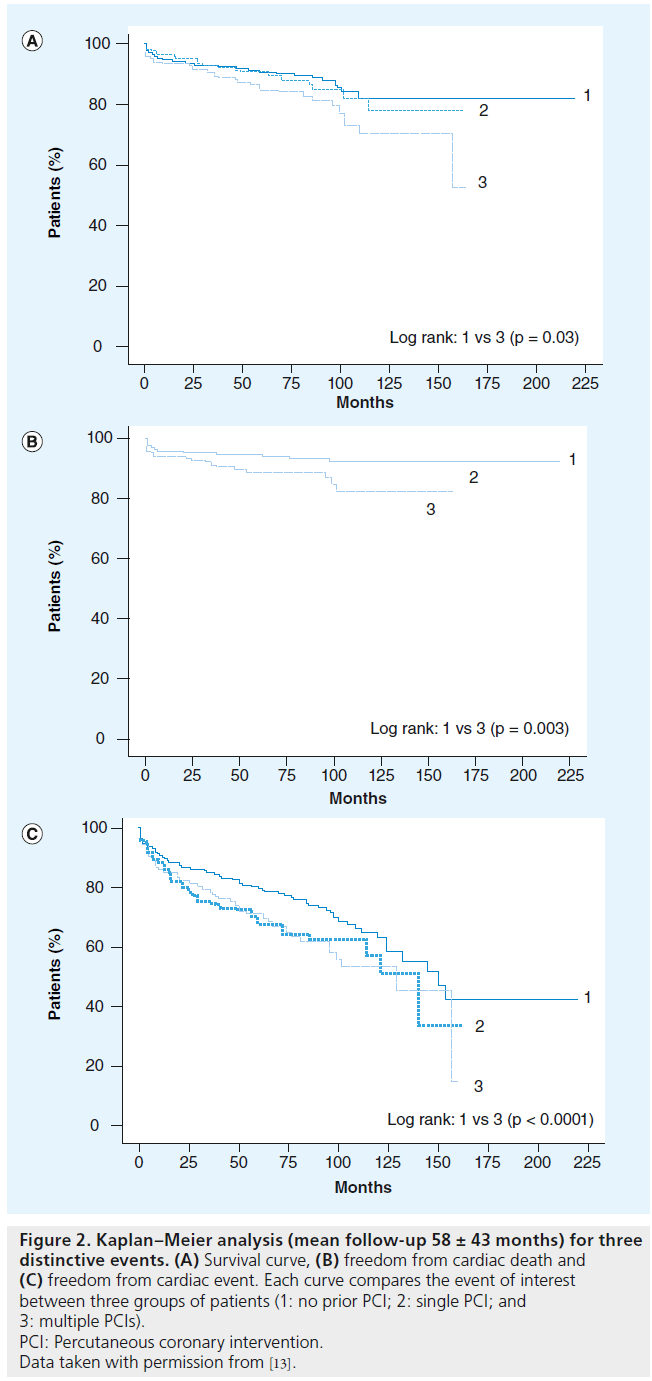
Figure 2: Kaplan–Meier analysis (mean follow-up 58 ± 43 months) for three distinctive events. (A) Survival curve, (B) freedom from cardiac death and (C) freedom from cardiac event. Each curve compares the event of interest between three groups of patients (1: no prior PCI; 2: single PCI; and 3: multiple PCIs). PCI: Percutaneous coronary intervention. Data taken with permission from [13].
Relationship between prior PCI & subsequent CABG
As a result of widespread use of PCI, a greater number of patients are referred to CABG after having prior PCI [15]. Two recent studies found that 6–13% of the patients with implanted baremetal stents (BMS) undergo CABG within 1 year after PCI, and 13–26% within 10 years [16,17]. The risk of any repeat revascularization has been lowered with the introduction of DES. Abbott et al. report the 1-year rate of target-vessel revascularization as 5.0% in DES and 9.2% in BMS patients (p < 0.001), with lower risk of any repeat revascularization (PCI or CABG) in DES patients (adjusted hazard ratio [HR]: 0.38, 95% CI: 0.25–0.60; p < 0.001) [18]. However, with the number of PCIs on the rise, one may expect an increase in the number of patients with prior PCI to be ultimately referred to CABG as a result of long-term PCI failure, incomplete revascularization or disease progression, as has been confirmed by the study of Barakate et al. [19]. As Chocron et al. noticed, the initial choice of PCI as a primary revascularization procedure is reinforced by the perception that patients can safely be referred to surgery after PCI. However, the data regarding CABG outcomes in patients who develop recurrent angina after initial PCI are sparse [15]. Whether there is a relationship between increased perioperative risk during CABG and previous PCI is debatable, and the extent of previous stenting procedures has, so far, not been well studied as a prognostic factor of CABG [20]. Nevertheless, the number of studies exploring the influence of previous PCI on the outcome of subsequent CABG is growing.
One of the first studies that examined the influence of previous PCI on the outcome of subsequent CABG was the study from Thielmann et al. [20]. Although retrospective in nature, the study included 3275 consecutive patients who underwent first-time isolated CABG. The patients were divided into three groups depending on their previous status (group 1: no previous PCI; group 2: single previous PCI; and group 3: multiple previous PCIs). The authors concluded that in patients with a history of multiple PCI sessions, perioperative risk for in-hospital mortality (odds ratio [OR]: 3.01, 95% CI: 1.51–5.98; p < 0.0017) and MACEs (OR: 2.31, 95% CI: 1.45–3.67; p < 0.0004) during subsequent elective CABG is increased. The study found a relationship between the stent load (placement of multiple, usually overlapping stents) and the outcomes of subsequent surgery.
A study from 2008 by Chocron et al., also explored the effect of pre-PCI influence on CABG outcome [15]. For this purpose, the patients were retrieved from the IMAGINE study [21]. A total of 2489 patients were analyzed in this randomized, multicenter international trial. The primary end point was the time to first occurrence of one or more of the following: cardiovascular death or resuscitated cardiac arrest, nonfatal MI, coronary revascularization, unstable angina requiring hospitalization, documented angina not requiring hospitalization, stroke or congestive heart failure requiring hospitalization. The authors found a significant increase in the primary end point in the PCI group (HR: 1.53, 95% CI: 1.17–1.98; p = 0.0016). The authors also concluded that the patients with left ventricular ejection fraction ≥40% and a history of PCI prior to surgery had a worse outcome post-CABG than those with no prior PCI. Although low left ventricular ejection fraction is traditionally regarded as an independent risk factor for heart surgery, limiting the population of patients with prior PCI to those with ejection fraction ≥40% who are at risk of subsequent CABG appears strange.
A group from Canada investigated the impact of prior PCI on in-hospital mortality after CABG [22]. A total of 6032 patients formed the final study group, of whom 919 (15.2%) patients had a prior PCI. Rates of in-hospital mortality after CABG were higher in the prior- PCI group (3.6 vs 2.3%; p = 0.02). Using multivariate techniques after propensity score matching, prior PCI emerged as an independent predictor of postoperative in-hospital mortality (3.6 vs 1.7%; p = 0.01). The conclusion drawn by authors is that patients who undergo CABG procedure after previously having had PCI tend to have reduced comorbidity and diminshed CAD; however, they also have more advanced symptoms and carry greater urgency.
The most recent multicenter study on the subject from Mannacio et al. investigated the impact of previous PCI on postoperative outcome and 5-year survival after subsequent CABG [23]. A total of 7855 consecutive patients from four cardiac surgical centers were enrolled, 1021 (13%) of them with a history of previous PCI. The authors identified history of previous PCI to be significantly associated with an increased hospital mortality (OR: 2.8, 95% CI: 1.4–4.8; p = 0.003) and MACE (OR: 2.1, 95% CI: 1.2–3.6; p < 0.0001). Survival at 3 and 5 years was lower in patients with previous PCI compared with the non-PCI patients (97.4 ± 0.01% vs 96.5 ± 0.02% and 94.2 ± 0.03% vs 92.1 ± 0.05%; log-rank test: p = 0.03).
What has been suggested by Thielmann et al. in 2006, is also supported by three more recent publications [20]. Sakaguchi et al. investigated the impact of repeated PCIs on long-term survival after subsequent CABG [13]. Although the number of patients in this study was small (894 patients), the study was able to conclude that the repeated PCI treatment introduces additional risks and affects long-term prognosis for patients who required subsequent CABG. The final confirmation of the significant impact of multiple previous PCIs on the outcome of following CABG came from Massoudy et al. in their multicenter analysis [24]. This comprehensive study summarized data collected from 29,928 consecutive patients who underwent isolated first-time CABG. A total of 10.3% of patients had one prior PCI, and 3.7% of patients had two or more previous interventions. The results showed that a history of two or more previous PCIs was significantly associated with in-hospital mortality (OR: 1.9, 95% CI: 1.3–2.7; p = 0.0016) and MACEs (OR: 1.5, 95% CI: 1.2–1.9; p = 0.0019). Eifert et al. investigated the mid-term influence of previous PCI in patients submitted for subsequent CABG procedures [25]. The outcomes of 200 patients (group A: 100 patients with prior PCI; and group B: 100 patients who underwent primary CABG) were compared in this observational study. Significant differences between the groups were established for the following parameters (group A vs group B): administration of vasoactive inotropes (adrenaline; p = 0.006 and noradrenaline; p = 0.023), level of creatine kinase or troponin I (p = 0.002; p < 0.001), postoperative resuscitation (p = 0.029), intra-aortic balloon pump (p = 0.003), and 30-day mortality (9% in group A vs 1% in group B; p = 0.018). The authors conclude that morbidity, mortality and reoperation rate during mid-term were significantly higher in patients with prior PCI.
Most recently, a research group in Brazil had conducted a study aimed to evaluate risk factors for CABG in patients who have had prior PCI treatments [26]. In this study, a total of 1099 consecutive patients who underwent CABG were included, 14.6% of them with a history of previous PCI. The authors found that the previous PCI group presented with signs of unstable angina more often (16.1 vs 9.9%; p = 0.019). Using multivariate logistic regression analysis, previous PCI emerged as an independent predictor of postoperative in-hospital mortality (OR: 1.94, 95% CI: 1.02–3.68; p = 0.044). After computed propensity score matching based on preoperative risk factors, in-hospital mortality remained higher among patients with previous PCI (OR: 3.46, 95% CI: 1.10–10.93; p = 0.034).
Nevertheless, there are several studies in which no correlation between previous PCI and subsequent CABG has been found. One of them is the study by Yap et al. in which the authors analyzed a pool of patients submitted to firsttime isolated CABG procedures from June 2001 to May 2008 [27]. A total of 13,184 patients were enrolled in the study (1457 patients had history of previous PCI). The results showed that there was no difference in unadjusted in-hospital mortality (1.65 vs 1.55%; p = 0.78) or MACEs (3.0 vs 3.0%; p = 0.99) between patients with and without prior PCI. After adjustment, prior PCI was not a predictor of in-hospital (OR: 1.22, 95% CI: 0.76–2.0; p = 0.41) or midterm mortality at 6-year follow-up (HR: 0.94, 95% CI: 0.75–1.18; p = 0.62). The results from different studies are obviously conflicting and probably reflect discrepancies between study methodologies. Therefore, it is apparent that a prior PCI treatment increases the surgical risk in a subsequent CABG procedure. A multicentric large-scale prospective study is required to determine the real impact of prior PCI on subsequent CABG and to guide therapeutic strategy. When considering prior PCI as a potential risk factor, one should not omit the risks embedded in coronary surgery per se (mortality rate <2%, risk of stroke, renal failure, infection and arrhythmias among others).
The effect of coronary stenting on the native coronary artery
The classical standpoint of the interventional cardiologists: “subsequent CABG may be successfully performed in any patient with a history of previous PCI,” is now being seriously challenged. It is of utmost importance to select patients who may be candidates for PCI, but in whom PCI will not provide successful long-term results and who will, eventually, require surgery as a definitive revascularization option. The interactions between coronary stents (including the procedure of stent implantation) and coronary arteries are numerous, all of which may, to a certain extent, explain the unfavorable outcome of subsequent CABG (Table 1) . Presumably, the presence of a stent might itself induce deleterious consequences inside the coronary artery. Every PCI procedure initiates a cascade of inflammatory reactions [5,28,29], which, together with promoted endothelial hyperplasia, may lead to early or late stent failure (complete or partial stent occlusion resulting from restenosis, thrombosis or any other cause). Coronary stents have been shown to induce an initial acute inflammatory cell response within 0–3 days, as well as a chronic inf lammatory reaction after 2–4 weeks [5]. This proinf lammatory state, coupled with the presence of denuded coronary endothelium, activates cytokines and the complement system, subsequently leading to accumulation of platelets and neutrophils, causing microvascular thrombotic obstruction and/or distal microembolization [20,30]. The late post-stenting structural changes, originally found in the coronary artery segment covered with stent, may spread beyond the stenting site and affect the distal coronary artery section, the target area of a subsequent bypass graft anastomosis [24]. The exact mechanism of how these remote structural changes occur is still a matter of debate, but it is most likely related to an increased circulating level of proinflammatory cytokines resulting in persistent low-grade inflammation associated with endothelial dysfunction and reduced vasomotor function distal to the lesion [5]. Endothelial dysfunction is connected to decreased availability of vasculoprotective agents such as nitric oxide, prostacyclin and antioxidant systems. It has been shown that plasma levels of nitric oxide in post-PCI patients (3–6 months after PCI) are significantly reduced [31]. It has also been suggested that the stent deployment leads to a synergistic interaction between the stent and the atherosclerotic plaque, potentiating the inflammatory reaction already taking place in the coronary arterial wall.
| Effect | Possible mechanisms |
|---|---|
| In-stent thrombosis | More often seen when ≥1 stent used, long stented segments of coronary artery, drug-eluting stents, |
| bifurcation lesion stenting | |
| Incomplete revascularization | Due to poor run-of, full metal jacket, unsuitable distal part of native coronary artery (small diameter or |
| diffuse distal coronary disease) | |
| Multiple percutaneous coronary | Impaired native blood flow through collaterals, multiple mini-myocardial infarctions, multiple |
| interventions | mini-embolic events in coronary circulation causing lowering of the left ventricular ejection fraction |
| Loss of time | Delay of definitive revascularization procedure (coronary artery bypass grafting which causes |
| progression of coronary artery disease) | |
| Local and remote stent effects | Inflammatory process, endothelial dysfunction, increased proliferative activity, biohumoral response |
Table 1. Possible mechanisms of negative effects of previous coronary stenting on subsequent coronary artery bypass grafting.
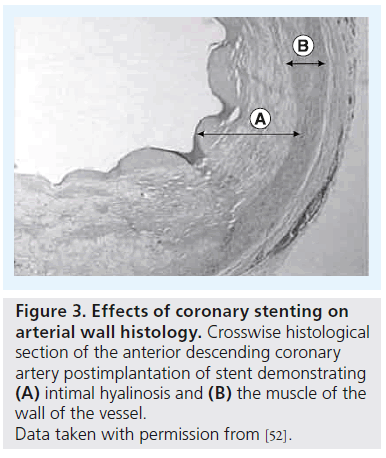
Another important concern is the effect of active drugs released from DES and their interaction with the arterial wall (Figure 3). Although primarily aimed at preventing vascular smooth muscle cell proliferation and migration, these drugs also impair re-endothelialization and induce tissue factor expression, resulting in a prothrombogenic environment [32]. DES, but not BMS, are associated with acetylcholine- or exercise-induced paradoxical coronary vasoconstriction of the adjacent vessel segments [33,34]. Despite the manifesting antiproliferative effect of sirolimus (rapamycin), resulting in reduction of the endothelial growth and intrastent restenosis, the inf lammatory response seems to be aggravated at the stent extremities (edge effect), producing an exacerbation of the inflammatory response [35].
Figure 3: Effects of coronary stenting on arterial wall histology. Crosswise histological section of the anterior descending coronary artery postimplantation of stent demonstrating (A) intimal hyalinosis and (B) the muscle of the wall of the vessel. Data taken with permission from [52].
In such circumstances, surgeons are faced with a necessity to create anastomosis at the very distal part of the vessel which may be significantly deteriorated by remote stent effects (poor run-off). In fact, the reason why CABG offers a survival advantage for multivessel and left main-stem CAD is because bypass grafts are placed in the mid-coronary vessel – where CABG not only protects the entire zones of vulnerable proximal myocardium against ‘culprit’ lesion but also offers prophylaxis against new lesions in diffusely diseased endothelium [14]. Although, rarely seen these days, ‘full metal jacket’ (overstenting of almost a complete coronary artery that is diffusely diseased) [36] allows creation of the bypass anastomosis at the very distal part of the coronary artery which may not offer adequate run-off, resulting in low patency rate. More importantly, an increasing number of patients with a full-metal jacket diseased left anterior descending artery (LAD) are being referred for CABG, creating a challenging problem to the cardiac surgeon, precluding complete revascularization in some patients, while rendering others inoperable [37]. With the recent refinements in percutaneous techniques, aggressive repeated PCI with overlapping stent placement has become more common, affecting up to 30% of patients and has resulted in an increase in the number of high-risk ‘stent-loaded’ patients who are referred to cardiac surgeons [38]. Coronary side-branch obstruction or occlusion resulting from multiple and overlapping stents may lead to compromised collateral flow causing focal infarctions [39]. It has been estimated that,on average, 5% of the total left ventricular mass may undergo irreversible myocardial injury due to focal infarctions related to stent implantation [40]. As a result of this, the patient’s risk profile may change, converting him/her to a higher risk patient subgroup that could lead to a higher mortality rate in CABG patients with a history of previous PCIs [24].
Surgical dilemma: what to do with patients with prior PCI?
A significant number of patients presenting with ST-elevation myocardial infarction (STEMI) will undergo primary PCI as a standard treatment strategy [41,42]. According to the guidelines, only the infarction-causing artery should be revascularized in these settings and the patient should be evaluated for suitability for further percutaneous treatment or referral to cardiac surgery as a definitive revascularization option. Consequently, a considerable number of patients are referred to cardiac surgery having had a previously implanted coronary stent in a STEMI setting, which is now patent and functional, requiring antiplatelet therapy. Preoperative use of antiplatelet drugs such as aspirin and clopidogrel may cause excess bleeding and perioperative discontinuation of these medications may cause in-stent thrombosis and adversely influence the outcome.
One of the more common scenarios that present a challenge for cardiac surgeons when evaluating therapy options includes: the diffuse triple vessel disease patient with ongoing STEMI caused by occlusion of the LAD, subsequently treated with stent deployment in the LAD (infarction artery), but where lesions are still present in the remainder of the vessels. The main issue is what to do with the LAD, keeping in mind two things: first, there is a functional stent in the proximal segment of the LAD with diffuse coronary disease; and second, knowing that the left internal mammary artery (LIMA)-to-LAD is superior to any other kind of revascularization approach. There is no easy answer. One would have to compromise between the long-term success and possible early graft failure due to competitive flow (although the mammary artery poses an intrinsic mechanism of flow auto-regulation which would probably end up in a ‘string-sign’ pattern), and leaving the LAD, sacrificing the long-term success with probable repeated PCIs which, as previously described, raises the risk of subsequent CABG procedures. In other words, the coronary artery that was previously treated by PCI will be left untouched in the subsequent CABG, exposing the patient to risks of subsequent restenosis [13].
Careful decision-making in the setting of multivessel STEMI is mandatory, implying an individualized treatment approach. Treatment strategies vary widely from an aggressive approach, which treats all significant lesions in the acute phase of primary PCI, to a conservative approach, with primary PCI of only the infarct-related artery and subsequent medical therapy (unless recurrent ischemia occurs) (Box 2) [43,44]. In some patients with severely affected coronary arteries (more than one critical stenosis >90% that would require multiple stent deployment covering long coronary artery segments), emergency surgical revascularization may prove to be the best option, avoiding the initial coronary stenting. Another, potentially appealing option is to perform hybrid revascularization in a STEMI setting, attaching LIMA to LAD and deploying DES in other arteries.
Perhaps the worst case scenario is when confronted with a diffusely diseased LAD with multiple overlapping stents covering all of its length. The challenge is then to achieve complete revascularization without compromising long-term results. Several authors have successfully demonstrated an alternative surgical approach for resolving this challenging scenario [45]. Forced surgical extraction of one or more coronary stents is mandatory in order to create a portion of artery amenable to revascularization. This is usually accomplished using a technique similar to open coronary endarterectomy after which an on-lay vein or LIMA patch is sewn. The denuded endothelium after stent extraction could, however, enhance myofibrocyte proliferation, and act as a scaffold for new thrombus formation, thus the use of clopidogrel and aspirin in this case may help to decrease the risk of graft failure [37].
The influence of implanted stents (especially DES) on the arterial and vein conduits, if any, remains unknown. A study by Gaudino et al. had found that 5 years after CABG, patients with previous PCI had a lower saphenous vein graft patency rate (45 vs 7.5%; p < 0.001) and a higher incidence of recurrent ischemia and recatheterization [46]. It has been reported that the graft occlusion rate of CABG is superior to the restenosis rate of PCI [13]. The conclusion can be drawn that the prognosis of the coronary artery, once treated with PCI and left untouched at subsequent CABG, is worse off than a coronary artery that was not treated with PCI and was bypassed in subsequent CABG.
Having established that prior PCI is a de facto risk factor for subsequent CABG procedures, one may want to look at patient profiles that might be linked with this unfavorable relationship. The results from several studies are summarized in Table 2. Obviously, many risk factors (patient related, procedure related, drug related, coronary artery anatomy and pathology) may influence the success or failure of specific procedures, thus emphasizing the need for adequate patient selection according to corresponding procedure type.
| Study (year) | Main results | Ref. |
|---|---|---|
| Barakate et al. | There was a higher incidence of unstable angina among previous PTCA patients (70 vs 52%; p < 0.05). | [19] |
| (2003) | Furthermore, the authors reported that a greater number of patients in this group had NYHA class IV | |
| symptoms, despite previous intervention | ||
| Hassan et al. (2005) | Patients with prior PCI were at lower risk in comparison with patients undergoing de novo CABG in terms of | [22] |
| age, history of recent MI, ventricular dysfunction and coronary disease burden. By contrast, they were more | ||
| likely to have Canadian Cardiovascular Society class IV symptoms and to arrive at the operating table with | ||
| urgent status. In other words, patients with a previous history of PCI are likely to present with the symptoms | ||
| related to unstable angina and some form of acute coronary syndrome | ||
| Thielmann et al. | Multiple stenting per se and the presence of previous MI influence the outcome of the following CABG with | [20] |
| (2006) | no specific difference in patient profile pattern. The proportion of patients with Canadian Cardiovascular | |
| Society class III–IV symptoms is virtually the same among the groups (no previous PCI – 11%, single previous | ||
| PCI – 13%, multiple previous PCIs – 13%; p = 0.4), while the incidence of previous MI shows a significant | ||
| difference (no previous PCI – 34%, single previous PCI – 36%, multiple previous PCIs – 47%; p < 0.0001) | ||
| Massoudy et al. | There are significant differences among different patient populations (in terms of prior PCI) regarding: | [24] |
| (2009) | peripheral vascular disease (highest in ≥2 PCIs group; p < 0.0001), chronic obstructive pulmonary disease | |
| (highest in ≥2 PCIs group; p =0.0019), hypertension (highest in ≥2 PCIs group; p < 0.0001), chronic | ||
| smoking (highest in ≥2 PCIs group; p < 0.0001), hyperlipidemia (highest in ≥2 PCIs group; p < 0.0001), | ||
| previous MI (highest in 1 PCI group; p < 0.0001) |
Table 2. The published studies that report on patient profiles that might be linked to an increased risk of coronary artery bypass grafting after prior percutaneous coronary intervention.
Prior PCI as a risk factor in risk assessment models
With the growing evidence that previous PCI adversely influences the outcome of subsequent CABG, we may expect that prior PCI emerges as a risk factor in new outcome prediction and risk stratification models in cardiac surgery. So far, only the Society of Thoracic Surgeons risk model incorporates prior PCI performed within the 6 h before the surgery as an independent predictor [47]. The paper from Bonaros et al., evaluated the success of common risk stratification models in cardiac surgery in predicting the perioperative outcome of CABG in patients with previous PCI [48]. Statistical discrimination for a 30-day mortaility rate was better in the non-PCI group (area under the curve: 0.875 vs 0.552 in the PCI group). Logistic EuroSCORE predicted 30-day mortality in the non-PCI group (95% CI: 0.806–0.934; p = 0.0004), but not in the PCI group (95% CI: 0.301–0.765; p = 0.8). The authors concluded that the EuroSCORE and the Society of Thoracic Surgeons model were inaccurate in predicting perioperative mortality after CABG in patients with a history of elective PCI; there is a need for modification of the risk model in order to improve risk assessment for surgical candidates with prior PCI. By implementing ‘prior PCI’ as an additional risk factor to the risk stratification model, the patients and their families would be given more objective information about the severity of the disease and the risk the specific surgical intervention carries, especially in light of current knowledge of stent-to-surgery interaction.
Hybrid care
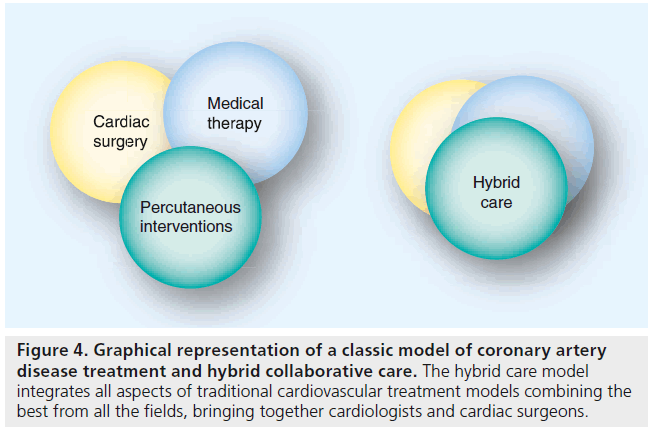
The role of cardiac surgery as a support in cases of failed PCI or rescue procedures has become more evident than ever. Although, apparently running in parallel tracks, the two disciplines will intersect on cases of patients who in the past have received coronary stents, and, due to progression of the CAD, require surgical revascularization; and vice versa (the patients who were submitted to the CABG procedure and later on require deployment of the coronary stent to one of the grafts). However, the field that will, in the future, bring cooperation and integration between cardiologists and cardiac surgeons, will be hybrid revascularization (Figure 4). Hybrid revascularization combines the best of both approaches: surgical (internal mammary artery on the LAD artery outperforms any other revascularization approach by far) and interventional (minimally invasive approach, DES deployment). Essentially, stents are substitutes for saphenous vein grafts for non- LAD lesions, thus making it possible to execute LIMA-to-LAD bypass using a less invasive, less traumatic approach [49]. The long-term effects of DES use on vascular healing and remodeling remain unknown and this is what may be considered as a ‘weak-link’ in the final outcome of this hybrid procedure. With the amount of evidence we currently have, no one can predict what kind of interaction may arise between the native coronary artery, stents and arterial/vein conduits. So far, one thing is certain – stenting for multivessel disease may result in increased morbidity and mortality after later CABG – and for this reason our own surgical colleagues may wish to curb their enthusiasm for hybrid CABG procedures that subject patients to multivessel stenting so that they can perform a minimally invasive LIMA-to-LAD anastomosis [50].
Figure 4: Graphical representation of a classic model of coronary artery disease treatment and hybrid collaborative care. The hybrid care model integrates all aspects of traditional cardiovascular treatment models combining the best from all the fields, bringing together cardiologists and cardiac surgeons.
Despite having been established as standard treatments for CAD, the question of which technology is superior in the long term remains largely unanswered. Developments are so rapid that before long-term outcomes can be assessed, a new technology would likely replace the most recent one. Thus, meaningful head to head comparisons can only be made for short- to mid-term outcomes. For this reason, current guidelines recommend the establishment of a Heart team – an instance comprised of, at least, an interventional cardiologist and a heart surgeon, organized in order to assess and recommend the best revascularization strategy.
Conclusion
There has been an exponential growth in PCIs with stent implantation even in cases where CABG demonstrates superior long-term results (the cases of multivessel coronary disease). The influence of previous PCI on CABG has been observed by cardiac surgeons in daily practice. It has been suggested that previous PCI might be considered as a risk factor with a negative impact on subsequent CABG procedure. Although data from different observational studies found a significant correlation between the outcome of the surgery and the presence of coronary stent, no definitive conclusion can be made and this is still considered as controversial. Both cardiologists and cardiac surgeons should make every effort to know more about CABG and PCI mutually as this may improve patient selection in terms of adequate revascularization procedure. Collaborative decision-making in all but clearcut cases is imperative. Another alternative is to create unified training programs for the care of patients with heart disease that will incorporate all aspects of cardiac disease management including diagnosis, interventional radiology, interventional cardiology, electrophysiology and cardiac surgery.
Future perspective
The premise that CABG surgery can be safely performed in patients with prior coronary stenting may not hold true. Further largescale studies are urgently needed to address the problem of prior PCI influence on subsequent CABG as the number of these patients exponentially grows. It is certain, however, that the number of patients submitted to PCI will continue to rise, resulting in reduced caseload for cardiac surgeons. Eventually, surgeons will become less experienced and unprepared to deal with patients with severe forms of CAD, not amenable to percutaneous treatment. In order to optimize the resources and to maximize the results, a new training (hybrid cardiovascular care) should be established.
Acknowledgement
The author would like to thank V Miladinov for the translation of this manuscript.
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Relationship between prior percutaneous coronary intervention & subsequent coronary artery bypass grafting
▪ In patients with multivessel coronary artery disease, coronary artery bypass grafting (CABG) offers significant advantages over percutaneous coronary intervention (PCI) in terms of rate of repeat revascularization, major adverse cardiac events and long-term survival.
▪ A growing number of patients are referred to CABG after having prior PCI, approximately 6–13% of patients with implanted bare-metal stents undergo CABG within 1 year after PCI, and 13–26% within 10 years.
▪ Subsequent CABG may be needed as a result of PCI failure after an extended time, an incomplete revascularization or disease progression.
The effect of coronary stenting on native coronary artery
▪ The presence of a stent might itself induce deleterious consequences inside the coronary artery.
▪ Every PCI procedure initiates a cascade of inflammatory reactions which, together with promoted endothelial hyperplasia, may lead to early or late stent failure.
▪ Altered vasomotor reactivity after stent implantation occurs due to decreased levels of nitric oxide.
▪ Coronary stenting may lead to microvascular thrombotic obstruction or distal microembolization caused by accumulation of platelets and neutrophils.
▪ Compromised collateral flow that causes focal infractions may result from coronary side-branch obstruction, or an occlusion from multiple, overlapping stents.
Surgical dilemma: what to do with patients with prior PCI
▪ There is a relationship between the stent load and the outcome of subsequent surgery.
▪ A history of two or more previous PCIs is significantly associated with in-hospital mortality and major adverse cardiac events.
▪ Different surgical options are available: to create anastomosis in the distal segment (results may be compromised by poor run-off), to create sequential bypass or to perform forced extraction of the coronary stent.
Prior PCI as a risk factor in risk assessment models
▪ Prior PCI should be incorporated in outcome prediction and risk stratification models in cardiac surgery.
Hybrid care
▪ Close collaboration between cardiologists and cardiac surgeons is mandated when performing hybrid revascularization procedures.
▪ The Heart team is a group of healthcare professionals assembled to assess the medical condition of the specific patient and make recommendations on revascularization strategy
Conclusion
▪ Both cardiologists and cardiac surgeons should make every effort to know more about CABG and PCI mutually as this may improve patient selection in terms of adequate revascularization procedure.
▪ Collaborative decision-making in all but clear-cut cases is imperative.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Molina JA, Heng BH. Global trends in cardiology and cardiothoracic surgery – an opportunity or a threat? Ann. Acad. Med. Singapore 38(6), 541–545 (2009).
- Hlatky MA, Boothroyd DB, Bravata DM et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet 373(9670), 1190–1197 (2009).
- Mihajlovic B, Nicin S, Cemerlic-Adjic N et al. Trends of risk factors in coronary surgery. Srp. Arh. Celok. Lek. 138(9–10), 570–576(2010).
- Mihajlovic B, Nicin S, Kovacevic P et al. Evaluation of results in coronary surgery using EuroSCORE. Srp. Arh. Celok. Lek.139(1–2), 25–29 (2011).
- Gomes WJ, Buffolo E. Coronary stenting and inflammation: implications for further surgical and medical treatment. Ann. Thorac. Surg. 81(5), 1918–1925 (2006).
- SoS Investigators. Coronary artery bypass surgery versus percutaneous coronary intervention with stent implantation in patients with multivessel coronary artery disease (the Stent or Surgery trial): a randomised controlled trial. Lancet 360(9338), 965–970 (2002).
- Booth J, Clayton T, Pepper J et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery trial (SoS). Circulation 118(4), 381–388 (2008).
- Hannan EL, Racz MJ, Walford G et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N. Engl. J. Med. 352(21), 2174–2183(2005).
- Hannan EL, Wu C, Walford G et al.Drug-eluting stents vs. coronary-artery
- Hannan EL, Wu C, Walford G et al. Drug-eluting stents vs. coronary-artery
- Serruys PW, Morice MC, Kappetein AP et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N. Engl. J. Med. 360(10), 961–972 (2009).
- Kappetein AP, Feldman TE, Mack MJ et al. Comparison of coronary bypass surgery with drug-eluting stenting for the treatment of left main and/or three-vessel disease: 3-year follow-up of the SYNTAX trial. Eur. Heart 32(17), 2125–2134 (2011).
- Sakaguchi G, Shimamoto T, Komiya T. Impact of repeated percutaneous coronary intervention on long-term survival after subsequent coronary artery bypass surgery.
- Taggart DP. Coronary artery bypass graft vs. percutaneous coronary angioplasty: CABG on the rebound? Curr. Opin. Cardiol. 22(6), 517–523 (2007).
- Chocron S, Baillot R, Rouleau JL et al. Impact of previous percutaneous transluminal coronary angioplasty and/or stenting revascularization on outcomes after surgical revascularization: insights from the imagine study. Eur. Heart J. 29(5), 673–679 (2008).
- Hoffman SN, TenBrook JA, Wolf MP, Pauker SG, Salem DN, Wong JB. A meta-analysis of randomized controlled trials comparing coronary artery bypass graft with percutaneous transluminal coronary angioplasty: one- to eight-year outcomes. J. Am. Coll. Cardiol. 41(8), 1293–1304(2003).
- Mercado N, Wijns W, Serruys PW et al. One-year outcomes of coronary artery bypass graft surgery versus percutaneous coronary intervention with multiple stenting for multisystem disease: a meta-analysis of individual patient data from randomized clinical trials. J. Thorac. Cardiovasc. Surg.130(2), 512–519 (2005).
- Abbott JD, Voss MR, Nakamura M et al. Unrestricted use of drug-eluting stents compared with bare-metal stents in routine clinical practice: findings from the National Heart, Lung, and Blood Institute Dynamic Registry. J. Am. Coll. Cardiol. 50(21), 2029–2036 (2007).
- Barakate MS, Hemli JM, Hughes CF, Bannon PG, Horton MD. Coronary artery bypass grafting (CABG) after initially successful percutaneous transluminal coronary angioplasty (PTCA): a review of 17 years experience. Eur. J. Cardiothorac. Surg. 23(2), 179–186 (2003).
- Thielmann M, Leyh R, Massoudy P et al. Prognostic significance of multiple previous percutaneous coronary interventions in patients undergoing elective coronary artery bypass surgery. Circulation 114(Suppl. 1), I441–I447 (2006). +
- Warnica JW, Gilst WV, Baillot R et al. Ischemia Management with Accupril post bypass Graft via Inhibition of angiotensin coNverting enzyme (IMAGINE):a multicentre randomized trial – design and rationale. Can. J. Cardiol. 18(11), 1191–1200 (2002).
- Hassan A, Buth KJ, Baskett RJ et al.The association between prior percutaneous coronary intervention and short-term outcomes after coronary artery bypass grafting. Am. Heart J. 150(5), 1026–1031 (2005).
- Mannacio V, Di Tommaso L, De Amicis V et al. Previous percutaneous coronaryinterventions increase mortality and morbidity after coronary surgery. Ann. Thorac. Surg. 93(6), 1956–1962 (2012).
- Massoudy P, Thielmann M, Lehmann N et al. Impact of prior percutaneous coronary intervention on the outcome of coronary artery bypass surgery: a multicenter analysis. J. Thorac. Cardiovasc. Surg. 137(4), 840–845(2009).
- Eifert S, Mair H, Boulesteix AL et al. Mid-term outcomes of patients with PCI prior to CABG in comparison to patients with primary CABG. Vasc. Health Risk Manag. 6, 495–501 (2010).
- Lisboa LA, Mejia OA, Dallan LA et al. Previous percutaneous coronary intervention as risk factor for coronary artery bypass grafting. Arq. Bras. Cardiol. 99(1), 586–595 (2012).
- Yap CH, Yan BP, Akowuah E et al. Does prior percutaneous coronary intervention adversely affect early and mid-term survival after coronary artery surgery? JACC Cardiovasc. Interv. 2(8), 758–764 (2009).
- Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation 105(25), 2974–2980 (2002).
- Toutouzas K, Colombo A, Stefanadis C. Inflammation and restenosis after percutaneous coronary interventions. Eur. Heart J. 25(19), 1679–1687 (2004).
- Thielmann M, Neuhauser M, Knipp S et al. Prognostic impact of previous percutaneous coronary intervention in patients with diabetes mellitus and triple-vessel disease undergoing coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 134(2), 470–476 (2007).
- Wykretowicz A, Dziarmaga M, Szczepanik A, Guzik P, Wysocki H. Prospective evaluation of hydroperoxide plasma levels and stable nitric oxide end products in patients subjected to angioplasty for coronary artery disease. Int. J. Cardiol. 89(2–3), 173–178 (2003).
- Nickenig G, Sinning JM. Response to drug-eluting stents do we need drugs to recompense drug elution? J. Am. Coll. Cardiol. 54(24), 2330–2332 (2009).
- Hofma SH, van der Giessen WJ, van Dalen BM et al. Indication of long-term endothelial dysfunction after sirolimus-eluting stent implantation. Eur. Heart J. 27(2), 166–170 (2006).
- Togni M, Windecker S, Cocchia R et al. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J. Am. Coll. Cardiol. 46(2), 231–236 (2005).
- Gomes WJ, Giannotti-Filho O, Hossne NA Jr, Catani R, Buffolo E. Inflammatory reaction after sirolimus-eluting stent implant. Ann. Thorac. Surg. 80(5), 1903–1904 (2005).
- Aoki J, Ong AT, Rodriguez Granillo GA et al. ‘Full metal jacket’ (stented length > or = 64 mm) using drug-eluting stents for de novo coronary artery lesions. Am. Heart J. 150(5), 994–999 (2005).
- Atoui R, Mohammadi S, Shum-Tim D. Surgical extraction of occluded stents: when stenting becomes a problem. Interact. Cardiovasc. Thorac. Surg. 9(4), 736–738(2009).
- Lee CW, Park KH, Kim YH et al. Clinical and angiographic outcomes after placement of multiple overlapping drug-eluting stents in diffuse coronary lesions. Am. J. Cardiol. 98(7), 918–922 (2006).
- Alfonso F, Hernandez C, Perez-Vizcayno MJ et al. Fate of stent-related side branches aftercoronary intervention in patients with in-stent restenosis. J. Am. Coll. Cardiol. 36(5), 1549–1556 (2000).
- Selvanayagam JB, Porto I, Channon K et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation 111(8), 1027–1032 (2005).
- Hillis LD, Smith PK, Anderson JL et al. Special Articles: 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Anesth. Analg. 114(1), 11–45 (2012).
- Kolh P, Wijns W. Essential messages from the ESC/EACTS guidelines on myocardial revascularization. Eur. J. Cardiothorac.Surg. 41(5), 983–985 (2012).
- Kornowski R. Completeness of revascularization in patients with ST-Elevation acute myocardial infarction. Catheter. Cardiovasc. Interv. 72(7), 934–936 (2008).
- Widimsky P, Holmes DR Jr. How to treat patients with ST-elevation acute myocardial infarction and multi-vessel disease? Eur. Heart J. 32(4), 396–403 (2011).
- Giordano V, Grandjean JG. There is always hope after PCI and stenting. J. Cardiovasc. Med. (Hagerstown) 13(11), 766–768 (2012).
- Gaudino M, Luciani N, Glieca F et al. Patients with in-stent restenosis have an increased risk of mid-term venous graft failure. Ann. Thorac. Surg. 82(3), 802–804 (2006).
- Shahian DM, O’Brien SM, Filardo G et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1 – coronary artery bypass grafting surgery. Ann. Thorac. Surg. 88(Suppl. 1), S2–S22 (2009).
- Bonaros N, Vill D, Wiedemann D et al. Major risk stratification models do not predict perioperative outcome after coronary artery bypass grafting in patients with previous percutaneous intervention. Eur. J. Cardiothorac. Surg. 39(6), e164–e169 (2011).
- Vassiliades TA, Kilgo PD, Douglas JS et al. Clinical outcomes after hybrid coronary revascularization versus off-pump coronary artery bypass: a prospective evaluation. Innovations (Phila) 4(6), 299–306 (2009).
- Lazar HL. Detrimental effects of coronary stenting on subsequent coronary artery bypass surgery: is there another flag on the field?J. Thorac. Cardiovasc. Surg. 138(2), 276–277(2009)
- BJCardio Staff. Intervention: who to treat and how? Br. J. Cardiol. 17(Suppl. 3), S5–S8 (2010).
- Fragomeni LS, Falleiro RP, Hoppen G, Krahl G. Coronary artery bypass grafts in patients with coronary stents. Braz.J. Cardiovasc. Surg. 20(4), 371–376 (2005).
- BCIS Audit Returns of Interventional Procedures 2001. www.bcis.org.uk/resources/documents/ Bcis01.ppt#332,1,BCIS Audit Returns of Interventional Procedures 2001
- Access to Cardiac Care in the UK. A Report on Recent Trends, Variations in Access and Future Need. www.bcs.com/documents/C1D_Access_to_ Cardiac_Care_in_the_UK_-_UK_by_ Country_&_English_SHA_-_FINAL2.pdf
▪ Study that compares the outcome of different revascularization modalities.
▪▪ One of the first studies to establish the impact of multiple previous percutaneous coronary interventions on coronary artery bypass grafting.
▪▪ Large-scale, multicenter study that evaluates the effect of a prior previous percutaneous coronary intervention on the outcome of a subsequent coronary artery bypass grafting procedure.
▪ Websites