Review Article - Interventional Cardiology (2013) Volume 5, Issue 4
Current status of nonsurgical septal reduction therapy in hypertrophic obstructive cardiomyopathy
- Corresponding Author:
- Robert M Cooper
Institute of Cardiovascular Medicine & Science, Liverpool Heart & Chest Hospital
Thomas Drive, Liverpool, L14 3PE, UK
Tel: +44 151 228 1616
E-mail: rob.cooper@lhch.nhs.uk
Abstract
Left ventricular outflow tract obstruction pathophysiology ▪ The critical process in left ventricular outflow tract obstruction is systolic anterior motion of the mitral valve (MV). The pathophysiology is a complex interplay of basal hypertrophy, anterior displacement of the anterior papillary muscle and long anterior MV leaflet. This results in systolic anterior motion and MV contact with the basal septum. The longer the MV is in contact with the septum, the greater the obstruction to flow, and therefore the higher the gradient. ▪ Removal of the hypertrophied basal segment opens the left ventricular outflow tract. This alters the hemodynamics and reduces obstruction and, therefore, gradient. Indications & patient selection ▪ Negatively inotropic medications improve symptoms and gradients in the majority of hypertrophic obstructive cardiomyopathy patients. ▪ Patients must have a subaortic gradient of >50 mmHg (at rest or with exercise), suffer from New York Heart Association class III dyspnea and have a septal width sufficient to perform septal reduction therapy without risk of causing a ventricular septal defect (usually >15 mm).
Keywords
alcohol septal ablation, hypertrophic obstructive cardiomyopathy, myectomy, nonsurgical septal reduction therapy, systolic anterior motion of mitral valve
Historical perspective
Hypertrophic cardiomyopathy (HCM) is an inherited disease characterized by otherwise unexplained hypertrophy of the myocardium. It is transmitted in an autosomal dominant pattern with variable penetrance, with an estimated prevalence of 1 in 500 [1]. While the distribution of hypertrophy can be varied, involvement of the basal interventricular septum is common. The classical description of asymmetrical basal hypertrophy narrowing the left ventricular outflow tract (LVOT) contributes to the pathology underlying LVOT obstruction (LVOTO). The prevalence of LVOTO in HCM is 20–30% at rest [2], and up to 70% with provocation [3]. LVOTO is associated with greater levels of dyspnea, a greater incidence of stroke and higher mortality [2].
Early cases of symptomatic hypertrophic obstructive cardiomyopathy (HOCM) were treated surgically, with good results [4–6]. While surgical techniques have advanced and mortality in expert centers has improved, the principle of septal myectomy through direct visualization and incision remains intact. The desire for a lessinvasive option with lower morbidity and faster recovery times were drivers for the development of a nonsurgical solution. This could also provide an option for those with high surgical risk precluding them from myectomy. Case reports of myocardial infarction have been reported to cause resolution of the clinical signs associated with HOCM [7]. Creating a percutaneous localized infarction could therefore alter LVOT hemodynamics. Early work in this field began in 1983, when inflation of an angioplasty balloon in a septal artery was noted to cause reduction in LVOT gradients [8]. The gradients returned when blood supply was restored. A more definitive solution for inducing infarct was therefore required. Some success has been reported with the creation of an infarct using transcoronary alcohol delivery to remove a ventricular arrhythmogenic substrate [9]; this was therefore adapted to target the basal septum. The pioneering nonsurgical septal reduction therapy (NSRT) was performed with transcoronary alcohol septal injection by Sigwart in 1994. A 68-year-old female with HOCM who failed to respond to medical therapy and pacing underwent alcohol septal ablation (ASA) with a good result. Her case and two others were reported in 1995 [10]. She remained healthy 10 years later [11]. An alternative to surgery is now available.
LVOTO pathophysiology
Basal interventricular septum hypertrophy and anterior displacement of the anterior mitral valve (MV) leaflet in systole (systolic anterior motion [SAM]) are the key components to LVOTO in HOCM. Recent MRI-based studies of MV morphology in HCM have highlighted a significantly longer anterior leaflet when compared with controls. This results in coaptation with the posterior leaflet towards its midpoint, leaving a redundant tip [12]. This is now thought to be part of the HCM phenotype.
The asymmetric hypertrophy of the basal septum leads to a narrowed LVOT. This causes rapid acceleration of blood flow within the left ventricle (LV) cavity immediately distal and apical to the MV leaflets. Through Venturi drag effects and forward flow catching the redundant tip, the anterior MV leaflet moves towards the hypertrophied septum. Once mitral–septal contact occurs, the orifice is narrowed further and obstruction to flow develops, resulting in a pressure difference. This pressure difference forces the leaflet further against the septum, further narrowing the orifice, exacerbating the hemodynamic abnormalities. This establishes an amplifying feedback loop until systole is complete. The greater the length of time the anterior MV leaflet is in contact with the septum, the higher the pressure difference [13].
Obstruction results in a pressure drop from the LV to the aorta and the magnitude of the problem will vary with myocardial contractility and loading conditions. In the vast majority of affected patients, the degree of obstruction increases with exercise and, hence, cardiac output is compromised at times of increasing demand. This obstruction has multiple deleterious effects including reduction of forward cardiac output, mitral regurgitation of varying degrees and load-dependent diastolic dysfunction leading to an increase in LV end-diastolic pressure and subsequent coronary flow abnormalities. These factors contribute to symptoms of dyspnea, chest pain, presyncope and syncope [14–16].
ASA reduces the size of the basal interventricular septum by inducing a localized infarction with transcoronary alcohol injection. The myocardium is stunned in the acute phase. Coagulation necrosis then leads to scar formation and subsequent thinning of the myocardium. Therefore, the LVOT widens and can account for a significant improvement in hemodynamics. The effects can continue to improve for several months afterwards. This can partly be explained by the change in diastolic function. The drop in LVOT gradient has been shown to reduce left ventricular hypertrophy distant from the LVOT and therefore cardiac mass [17,18]; this shows that part of the hypertrophy in HOCM is afterload-dependent and not entirely genetically predetermined.
Indications & patient selection
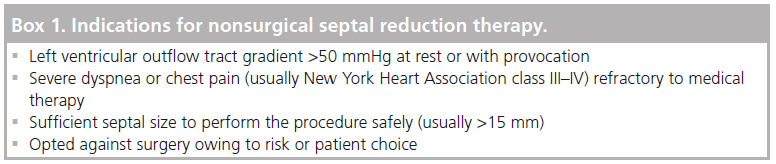
The safety and efficacy of NSRT can be improved with fastidious patient selection and procedural planning. The 2011 American College of Cardiology Foundation (ACCF)/American Heart Association guidelines for the Diagnosis and Treatment of HCM stipulate that there must be a subaortic gradient of at least 50 mmHg, at rest or with provocation [19]. The 2003 American College of Cardiology (ACC)/European Society of Cardiology consensus document on HCM first set this gradient requirement, whilst others have suggested that a resting gradient of >30 mmHg and a rise with provocation to >50 mmHg or >100 mmHg will suffice [18–22]. It is important that provocation should be physiological rather than pharmacological, as dobutamine can produce subaortic gradients in the normal heart [23]. Treating a symptomatic patient with no resting pressure difference, but with an exercise-induced gradient can have a good outcome [24]. Symptom burden is part of selection criteria. Patients must have limiting symptoms that are refractory to medical therapy [20]. Dyspnea, classed as New York Heart Association (NYHA) grade III–V, and, to a lesser extent chest pain and syncope, are indicators for treatment. Medical therapy uses negative inotropes and can include b-blockers, calcium channel blockers and disopyramide. Patients should have hypertrophy of the basal interventricular septum thought to be responsible for causing a gradient. Some quote interventricular width of >18 mm with clear protrusion into the LV cavity, while others state a size of >15 mm will suffice [25–26]. The 2011 ACCF/American Heart Association guidelines simply state that septal thickness is “sufficient to perform the procedure safely and effectively in the judgement of the individual operator” [19].
NICE, in the UK, states that NSRT can be performed in HOCM patients with symptoms refractory to medical therapy. They make a requirement that this should only be performed in specialist units with clinicians who have adequate training [101]. This was re-enforced in the ACCF/ACC guidelines, stating that an operator must have experience of at least 20 procedures or work in a center with a cumulative procedural volume of 50 patients [19]. The learning curve in ASA can require the performance of at least 40 procedures [27].
It must be clear before progressing to NSRT that no other indication for cardiac surgery exists. MV disease and significant coronary artery disease requiring bypass grafting must be ruled out. If coronary artery disease exists, an individual approach can be adopted and percutaneous intervention considered. In a small number of patients, abnormal MV anatomy or a subaortic ring will be present and responsible for a large part of the LVOT gradient. Iatrogenic infarct of the basal septum in these patients will not affect the hemodynamics and should not be performed; they are better suited to surgery.
Patient demographics play a significant role in treatment selection. As the long-term sequelae of inducing a myocardial scar is still under debate, careful consideration should be exercised when proceeding with young patients; ASA is generally not performed in children [20,28–30]. Given the choice, most patients will favor ASA over cardiac surgery [31]. This seems to be driven by the fear of incision, general anesthesia and prolonged recovery periods. Patients do respond to guidance from medical personnel and must be aware that NSRT is not always the correct choice.
Many patients referred for the procedure do not need invasive solutions and can be treated with more intensive medical therapy. A total of 37% of 249 patients referred for septal reduction therapy (including myectomy) were satisfactorily treated with increased oral medication in one reported series from a tertiary referral center [32].
The generally agreed indications for proceeding to NSRT are highlighted in Box 1.
ASA: the procedure
Most operators favor right femoral arterial access. The radial artery has also been shown to be a viable option [33]. Simultaneous monitoring of LV and central aortic pressures should be performed using catheters placed at the LV apex and ascending aorta. This will give continuous LVOT gradient readings throughout the procedure. This usually requires two arterial access points. Catheters placed in the LV should be ‘end hole’ and have pressure portals at the distal point only. Pigtail catheters have multiple portals; the proximal holes may lie above the level of obstruction and provide falsely low gradient readings. LVOT gradient should be measured during Valsalva maneuver and following an ectopic beat for the Brockenbrough–Braunwald phenomenon. An alternative method of using a single arterial puncture and measuring continuous gradients using a central aortic catheter and a pressure wire located in the LV apex is also possible. Occassionally, a high ectopic burden or runs of ventricular tachycardia prevent continuous monitoring of the gradient.
A venous sheath should be sited to allow the insertion of a temporary pacing wire at the right ventricle (RV) apex prior to any injection. Septal pacing must be avoided as early activation of the septum can increase LVOTO. A second venous sheath can be used to insert an intracardiac echocardiography (ICE) catheter; a full validation study of the usefulness of ICE is underway.
Heparin is used to prevent thromboembolic complication. A guide catheter should be positioned at the ostium of the left (or very rarely the right) coronary artery and a guidewire advanced to the septal artery of choice. A softer wire has been proven to reduce coronary complication but, occasionally, a stiffer wire will be needed [34]. An over-the-wire balloon is then advanced into the septal artery; the balloon must be compatible with alcohol injection. A slightly oversized balloon is preferred to overexpansion of an undersized balloon. The balloon should be placed proximally in the septal artery and be shown not to impinge on the epicardial artery, so as not to risk dissection or occlusion of the parent vessel. Even partial occlusion of the left anterior descending artery (LAD) can promote collateral flow from septal arcade systems to the mid or distal LAD.
The guidewire is then removed and a small amount of angiographic contrast (1–2 ml) is injected into the septal artery beyond balloon occlusion. The test injection should ensure no reflux of dye into the epicardial artery. Careful inspection for collateral connection to distal territories should be performed. Alcohol injection into such arteries will result in distal, unwanted and ineffective infarction.
Myocardial contrast echocardiography (MCE) is used to demonstrate the area of myocardium supplied by the chosen vessel. Contrast should be injected with continuous echocardiographic screening. An obvious opacification of the area of the septum involved in the contact point for SAM will be seen if the artery is the correct choice. If contrast is seen elsewhere, an alternative artery will need to be explored.
An alternative approach without the use of MCE relies on the change in gradient measurements with occlusion of the chosen septal artery. Septal arteries originating from the LAD are balloon occluded in a stepwise manner. If a ≤30% gradient drop was observed, an alternative vessel was chosen. A drop in the LVOT gradient of >30% with balloon occlusion was used to identify vessels supplying the target area [35]. This method has largely been superceded since the introduction of contrast echocardiography.
Once the target artery has been satisfactorily identified, a small volume (1–3 ml) of absolute alcohol is injected slowly in 1-ml increments. Analgesia can be given as injection of alcohol can cause some discomfort. The volume of alcohol injected will depend on the effect on the LVOT gradient and the area of myocardium opacified with contrast injection. There is evidence to suggest that small doses (1–2 ml) of alcohol can have as equally effective haemodynamic results as large (2–4 ml) doses [36,37]. Balloon occlusion should be maintained for at least 5 min. A final angiographic image is taken to show the septal branch no-reflow and rule out any unwanted coronary artery damage. The patient is kept under coronary care supervision for 24–48 h with the temporary pacing wire in situ. If no complete heart block is present at that time it can be removed. The authors advocate the use of temporary permanent pacing systems up to 2 weeks postprocedure to allow intrinsic conduction to recover. This may reduce the need for permanent system implantation. Hospital stay is 4–5 days if no complications are observed.
Changes in the methodology of ASA have not been as pronounced as those observed in other procedures in cardiology. The introduction of MCE was a significant milestone that reduced procedural complications [38,39]. The recognition that small doses of alcohol can be effective may allow operators to secure the same hemodynamic effect with less risk [37]. Power Doppler imaging shows promise in visualizing the target area following myocardial contrast injection and warrants further exploration [37,40].
Results
▪ Survival
HCM patients with resting LVOTO have a higher mortality than matched HCM patients without a gradient [2]. Recent case series have suggested that removing the gradient may have a beneficial effect on survival. Of 173 ASA patients, with mean age of 64 years, followedup for a median time of 5.7 years, survival was no different to that of the general, non-HCM population [41]. The survival was identical to those treated with surgical myectomy in the same time period. Residual LVOTO was a predictor of mortality in this series.
The largest series of ASA patients studied for survival (n = 465) over a mean of 8.4 years showed a 1-, 5- and 10‑year survival of 99, 94 and 90%, respectively. This compares favorably to that of the age- and sex-matched general non-HCM population figures of 99, 93 and 84% respectively [Jensen MK et al., Pers. Comm.].
A further study comparing survival following invasive treatment with ASA versus conservative management found a benefit with ASA. This was explained by noncardiac death [42].
These recent series will go some way to reassuring operators that removing the LVOT gradient with ASA has a beneficial effect on long-term outcome.
▪ Risk of ventricular arrhythmia
The medium-term risk of ventricular arrhythmia has been studied with conflicting results. Some observational studies provide the reassurance that the risk of sudden cardiac death (SCD) in those treated with alcohol is not significantly higher than those without an iatrogenic myocardial infarction [43–45]. A registry of all ASA patients with implantable cardioverter defibrillators (ICDs) for primary prevention highlighted the annual incidence of appropriate ICD discharge as 2.8% [28]. A comprehensive registry of 465 ASA patients in Denmark and Germany identified the SCD risk over 8.4 years as 0.5% per annum, with 16 sudden deaths and three appropriate ICD discharges (representing a 2.7% appropriate ICD discharge rate) [Jensen MK et al., Pers. Comm.]. The majority of ICD series identify an appropriate discharge rate of 2.5–3.0% per annum post-ASA.
The multicenter HCM ICD registry in North America highlighted an increased risk of appropriate ICD activation post-ASA. A fourfold increase in appropriate ICD treatment was observed with event rates of 10.3% per year compared with 2.6% per year in those treated with myectomy [46]. The ASA group, however, consisted of only 17 patients and four events; the low numbers may contribute to an eye-catching difference in this report.
Meta-analyses have not indicated any difference between ASA and septal myectomy in the medium-term incidence of SCD or all-cause mortality [47,48]. The overall trend in SCD and ventricular tachycardia risk in reported series appears to favor no clear increase in arrhythmia post-ASA.
▪ Symptom & gradient resolution
The first series of 18 patients to undergo ASA was reported in 1997 [49]. This was promising and showed that the procedure had the potential to reduce LVOT gradients, reduce symptom burden and increase exercise tolerance. A larger series of patients were reported in 1999, a total of 50 patients were followed-up for 7 months [35], showing a clear improvement in LVOT gradients, interventricular septal size, VO2MAX and pulmonary artery pressures. Perhaps more importantly, the change in patient symptoms was reproduced, with NYHA status improving from a mean of 3 to 1.7.
Following these breakthrough studies, multiple early patient series were reported and were summarized in a systematic review collated in 2006 [50]. In total, 2959 patients were summarized from 42 published studies, although the authors accept that some were duplicated by involvement in more than one report. A baseline assessment revealed a mean age of 53.5 years, a NYHA class of 2.9 despite medical therapy, and peak LVOT gradients of 65 mmHg at rest and 125 mmHg on provocation. Mean follow-up was 12.7 months. Early mortality (defined as within 30 days) was reported to be 1.5%, with late allcause mortality at 0.5% (0–9.3%). NYHA status improved significantly to 1.2 (p < 0.001). A reduction in angina burden was also seen; Canadian Cardiovascular Society score reduced from 1.9 to 0.4 (p < 0.001). Echocardiographic gradients reduced to 15 mmHg at rest and 31 mmHg with provocation (both p < 0.001). Repeat procedures were required in 7% of patients.
A 2011 report from the Multicenter North American registry detailed procedures in 874 patients. All had a resting gradient of ≥30 mmHg or provocable gradient of ≥60 mmHg with advanced symptoms of exertional dyspnea and/or angina despite medical therapy [51]. Mean follow-up duration was 26 months. A total of 78% of patients suffered NYHA class III or IV dyspnea prior to the procedure, with a gradient at rest measuring 70 mmHg, and 99 mmHg on provocation. Following ASA, 72.5% of patients were classified as NYHA class I, 23% as NYHA class II, 3.9% as NYHA class III and 0.65% as NYHA class IV. Repeat procedure was required in 12.8% of patients. Mean peak resting gradients postprocedure reduced to 35 mmHg. The Scandinavian multicenter study included 279 patients with similar results, with NYHA class III/IV breathlessness falling from 94 to 21% and outflow tract gradients falling from 58 to 12 mmHg. Those with persisting NYHA class III/IV breathlessness had a high prevalence of comorbidities including chronic obstructive pulmonary disease and valve disease [52].
number of series of medium-term results are now available, with time periods ranging from 25–141 months (Table 1) [34,35,37,43,51–58]. Improvement in dyspnea is observed in most cases, with a clear trend towards lower LVOT gradients. The procedure does not provide uniform improvement for all, however, with large recent series displaying a mean postprocedure resting gradient of 35 mmHg (a gradient that some would claim justifies repeat treatment), and persistent NYHA class III dyspnea in 21% of patients [52,59]. There is therefore scope for improvement in the treatment and subsequent outcomes for HCM patients with LVOTO refractory to medical therapy.
| Study | Follow-up | n | Age at | Pre-ASA | Post-ASA | Complications | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (year) | (months) | ASA | NYHA | Rest | NYHA | Resting | Death at | Death at | Death: | ||
| (years) | |||||||||||
| class† | gradient | class† | gradient | ASA (%) | follow-up | cardiac | |||||
| (mmHg) | (mmHg) | (%) | cause (%) | ||||||||
| Seggewiss | 58 | 100 | 53 | 2.8 | 76 | 1.6 | 0 | 1 | 4 | 2 | [54] |
| et al. (2007) | |||||||||||
| Kwon et al. | 96 | 55 | 63 | 96% ≥3 | 70 | 17% ≥3 | 31 | – | 24 | 2 | [53] |
| (2008) | |||||||||||
| Fernandes | 55 | 579 | 54 | 2.8 | 77 | 1.4 | <10 | – | 9 | 4 | [34] |
| et al. (2008) | |||||||||||
| Kuhn et al. | 25 | 329 | 58 | – | 54 | – | 21 | 1.8 | 8.9 | 3 | [57] |
| (2008) | |||||||||||
| Lyne et al. | 141 | 12 | 49 | 2.7 | 70 | 1 | 3 | 0 | 24 | 16 | [55] |
| (2010) | |||||||||||
| Klopotowski | 111 | 61 | 48 | – | – | – | – | 0 | 5 | 1.6 | [45] |
| et al. (2010) | |||||||||||
| Jensen et al. | 42 | 77 | 61 | 97% ≥3 | 60 | 9% ≥3 | 12 | 0 | 16 | 6.5 | [43] |
| (2011) | |||||||||||
| Nagueh | 26 | 874 | 55 | 78% ≥3 | 70 | 4.5% ≥3 | 35 | – | 9.3 | 2.8 | [51] |
| et al. (2011) | |||||||||||
| Jensen et al. | 44 | 279 | 59 | 94% ≥3 | 58 | 21% ≥3 | 12 | 0.3 | 12.2 | – | [52] |
| (2011) | |||||||||||
| Veselka et al. | 84 | 76 | 55 | 2.8 | 74 | 1.5 | 18–24 | 0 | 9 | – | [37] |
| (2011) | |||||||||||
| Sorajja et al. | 68 | 177 | 64 | 100% ≥3 | 73 | 67% =1 | 11 | 1.1 | 14.7 | 2.8 | [41] |
| (2012) | |||||||||||
ASA: Alcohol septal ablation; NYHA: New York Heart Association
Table 1. Long-term results from observational studies postalcohol septal ablation.
Comparison to myectomy
According to the 2011 ACC/ACCF guidelines, surgical myectomy remains the treatment of choice for HOCM with symptoms of heart failure. Surgery has a class IIa recommendation, while ASA is class IIb [19]. This is based on excellent survival, symptom resolution, abolition of gradient and low operative mortality at centers of excellence [60]. The enthusiasm for ASA over the past decade has resulted in fewer centers performing septal myectomy, especially in Europe [61]. The performance of myectomy in lower volume centers is associated with higher risk; the US Society for Cardiovascular Angiography and Intervention Registry data up to 2011 highlighted an operative mortality of 5.3%. A total of 242 myectomy procedures were performed in 97 centers at a rate of 2.5 operations per center per decade [62]. This compares with a mortality of 0.5% in high-volume centers [60]. It is possible to set up a dedicated service de novo and produce good results, but occasional myectomy in otherwise experienced surgeons should be discouraged [63].
The 2012 report of the Mayo Clinic experience from Sorajja et al. described outcome up to 5.7 years in 173 patients undergoing ASA and in an age- and sex-matched group undergoing surgical myectomy [41]. The proportion of patients alive and free from severe symptoms in the ablation group was 78 versus 73% in the myectomy group (p = 0.26). Procedural mortality occurred in two ASA patients and in one full myectomy patient. Both patients who died in the ASA group presented with cardiac failure. Pacemaker requirement was higher in the ablation group than in the myectomy group (20.3 vs 2.3%). The median 30-day LVOT gradient was better in the myectomy group (11 vs 5 mmHg, a small but statistically significant difference). This nonrandomized study shows that, in appropriately selected patients, outcome from ASA can be comparable to myectomy with regard to survival and symptom resolution.
Meta-analyses have compared symptom burden, complication rate and mortality between ASA and myectomy. A meta-analysis evaluating nonrandomized studies comparing ASA and myectomy at individual institutions identified five such studies, with 183 patients undergoing ASA and 168 undergoing myectomy [64]. The age of the ASA cohort was slightly higher at 54.4 ± 6.3 years versus 45.0 ± 4.4 years in the myectomy group. Baseline NYHA status and echocardiographic characteristics were comparable between groups. Symptom improvement was similar, with NYHA reducing to 1.5 ± 0.3 in the ASA group and 1.3 ± 0.2 in the myectomy cohort. Reduction in LVOT gradient was slightly superior in the myectomy group (resting LVOT gradient was 10.8 vs 18.2 mmHg). Requirement of permanent pacing was higher in the ASA group at 18.4 versus 3.3% in the myectomy cohort [64]. An updated meta-analysis of comparative observational studies in 2010 had higher numbers, with 380 patients undergoing ASA and 326 patients undergoing myectomy [47]. The data support the previous conclusion that the risk of mortality was similar at shortand long-term follow-up, and that reduction of symptoms was comparable.
The only significant differences to come out of a detailed statistical analysis were a higher incidence of pacemaker implantation and right bundle branch block (BBB) in the ASA group and a small, yet significantly higher, residual LVOT gradient in the ASA group [47].
Leonardi et al. selected 19 ASA case series and eight independent myectomy case series for analysis [48]. The follow-up period for ASA was significantly shorter and, again, the ASA population was slightly older (55 vs 44 years). Baseline echocardiographic data were similar but with a statistically larger mean interventricular width in myectomy patients of 23 mm versus 21 mm. After adjustment for baseline characteristics, the odds ratio for treatment effect on mortality (all-cause and SCD) in ASA was 0.28 (95% CI: 0.16–0.46). This compares to a ratio of 0.32 (95% CI: 0.11–0.97) in myectomy. Although not statistically significant, the outcome favored ASA [48].
The numbers in these comparative metaanalyses are much lower than the total number of ASA procedures performed worldwide and reported on [50], and perhaps reflect the small numbers of centers actively performing myectomy. This undoubtedly has an effect on choice of treatment. If one has no center to offer myectomy due to dwindling numbers of operators, the expertise is then in ASA, and this can become the safer and more effective option.
Meta-analyses of nonrandomized studies and case series are the highest level of evidence we currently have to work with when comparing these two strategies of septal reduction. The prospect of a randomized comparison has often been raised, but now seems unlikely [65]. The patient population requiring septal reduction therapy is small. There is a low incidence of HCM in the general population, and only a small proportion of these patients have LVOTO refractory to medical therapy. This, coupled with the very low event rate observed over medium-term follow-up, currently would mean that a study to detect differences in mortality would be extremely difficult to power.
Predictors of outcome in ASA
Ability to predict symptomatic outcome has been investigated with limited success [27,66,67]. This may be due to relatively low numbers. Reduced ejection fraction before ASA predicts mortality [51]. Systolic dysfunction often reflects an advanced stage in disease progression with poor outcome [68].
A multivariate analysis highlighted that patients undergoing ASA with ≥three of the following features had a superior 4-year survival free of death and severe symptoms; gradient of <100 mmHg, septal hypertrophy of <18 mm, age of >65 years and LAD diameter of <4.0 mm [67].
The time to gradient reduction following septal artery occlusion during ASA correlates well with the magnitude of final response. A rapid correction of LV gradients predicts a good result with minimal residual pressure difference [69]. Operator experience of >50 procedures is an independent predictor of survival free of severe symptoms [67].
Postprocedural use of b-blockers, higher septal thickness and need for repeat procedure also predicted mortality [51]. Those with a higher gradient at baseline and postprocedure have a higher risk of unsatisfactory outcome and need for further procedure [66].
Periprocedural complications of ASA
Early series described a high rate of pacemaker implantation, with reports of 40% of patients requiring permanent systems [35]. The introduction of MCE has reduced this rate to approximately 10–15% [50–52]. MCE has a class I recommendation in the ACC/European Society of Cardiology guidelines [20]. Predictors of heart block are first degree atrioventricular block, left BBB, lack of use of MCE, injection of alcohol by bolus rather than infusion, injection of more than one septal artery and female sex [70]. However, patients with long-term pacing seem to have a similar outcome clinically and hemodynamically to those without pacing following ASA [71].
The incidence of right BBB following ASA has been reported as being up to 54% [70]. The high incidence of right BBB at ASA probably explains why left BBB is a predictor of complete heart block [71]. There have been reports of numerous cases of delayed heart block post-ASA. Therefore, normal conduction at discharge does not necessarily mean that implantation of a pacemaker will never be indicated [71].
Other periprocedural complications have a significantly lower frequency. Early series reported ventricular fibrillation in 2.2%, LAD dissection in 1.8% and pericardial effusion in 1.8% of patients [50]. The improvement in complication rates in recent years may be attributable to refinements in procedure and operator technique. More recently, periprocedural ventricular arrhythmia was seen in 0.02% and LAD dissection in 0.01% of patients [51].
Alternative methods of NSRT
Alternative approaches to NSRT have been sought to overcome the complications and limitations of ASA. An alternative substance for arterial injection is one approach. This is an attempt to reduce the risk of alcohol escape, causing unwanted distal infarction. The immediate polymerization of glue lends itself to more accurate and proximal distribution [72,73]. In a series of 18 patients, no complications (including heart block) were observed [73]. Glue has a different effect on the myocardium; it simply restricts blood flow causing ischemia and subsequent infarction. Alcohol, however, causes tissue injury by a direct necrotizing effect, with acute dehydration and fixation of surrounding tissues. The early results from the Cyanoacrylate studies are encouraging, with a reduction in LVOT gradient from 75.8 to 18.0 mmHg and a reduction in symptom burden with NYHA status falling from a mean of 3.1 to 2.2 at 6 months. Three of the 18 patients showed a recurrence of LVOT gradients at 6 months with associated symptoms, having shown a good response initially. Cyanoacrylate injection for NSRT may be appropriate for those with septal collateralization to distal arteries. This is a cohort previously thought to be inappropriate for NSRT in the form of ASA due to the risk of alcohol escape into undesired tissues.
The injection of microspheres to restrict blood flow has also been investigated. Initial experience with polyvinyl alcohol foam particles was reported in 2004 with encouraging initial results. Gradients reduced from 83 ± 32 to 31 ± 35 mmHg (mean ± standard deviation) with improvement in mean NYHA from 3.3 to 1.3. Also of note was the lack of periprocedural complication in 18 patients [74].
The success of coil embolization of noncoronary arteries led to the concept being adapted for the treatment of HOCM [75]. A pilot study investigating the feasibility of coronary artery coil embolization was performed in 20 patients with drug-refractory symptoms attributable to HOCM [76]. A total of 18 patients suffered class III NYHA breathlessness at onset. Ten patients improved to class I and a further six to class II, while two found no improvement. The reduction in LVOT gradients was relatively modest compared with most ASA studies at 80–36 mmHg.
One patient died during admission from a confirmed ventricular septal defect postprocedure and subsequent operative complications. This patient was proposed to have had a large myocardial infarction, diagnosed by high enzyme release and which was thought to be the cause of the septal defect. No patients required the implantation of a pacemaker [76]. This is encouraging with respect to the lack of conduction system damage, but the relatively small drop in gradients and periprocedural death may counteract this in terms of overall attraction to coil embolization as a treatment modality.
The rich collateralization of the septum may explain the difficulties in effectively reducing long-term gradients by ischemia alone. Intracoronary glue and coil embolization have a similar methodology to covered stents and simple balloon occlusion in this respect. These therapies have not been adopted owing to the limited impact on gradients in the long term.
A significant limitation of ASA is the reliance on the coronary anatomy to provide access to a target for ablation. In total 5–8% of patients have no septal vessel suitable for injection, due to the inability to identify or instrument an artery supplying the basal hypertrophy at the SAM–septal contact point [57,77]. It is possible to bypass this problem by accessing the septum from the ventricles and providing a direct treatment. A feasibility study in canines assessed the role of a combined ICE and alcohol delivery catheter [78]. The catheter was placed in the right ventricle (RV) in nine canines, and the LV in three. ICE was used to guide the operator to insert a needle directly into the basal septum of the beating heart. Once in situ, a microbubble was injected to ensure safe positioning of the needle tip in the myocardium. A varying dose of alcohol was then injected (0.5, 1.0 and 1.5 ml) and the canines were observed for 3 h prior to sacrifice. No arrhythmia developed during this time. The operators were able to observe the depth of injection in real-time during the procedure with ICE. Histological analysis confirmed the scar to be in the desired area, and the size of the scar correlated to the volume of alcohol injected [78]. While showing promise, the procedure has not yet been trialed in humans.
An alternative, more advanced approach for direct treatment is septal radiofrequency ablation [79,80]. Lawrenz et al. performed endocardial radiofrequency ablation of septal hypertrophy (ERASH) in a cohort of 19 adults, eight of whom had ineffective ASA due to inappropriate coronary anatomy or spillover of contrast into collateral branches [79]. All patients were offered, but declined, surgery. Ablation was performed from the RV septum in ten patients and LV septum in nine patients. Although follow-up imaging showed a minor reduction in the magnitude of septal hypertrophy of 22.6–21.4 mm, deep scars were visible up to 28 mm within the septum on cardiac MRI in some patients. This is in contrast to ASA where a more marked change in septal thickness in diastole is seen. Despite a modest effect on septal width, a reduction in resting gradients from 77.7 to 26.5 mmHg and provoked gradients from 157.5 to 64.2 mmHg was observed. NYHA status reduced from a mean of 3.0 to 1.7. Four patients required permanent pacing and remained reliant at 6 months. One pericardial tamponade due to perforation from RV pacing wire required surgical correction [79].
ERASH has also been successfully used in the management of LVOTO in children with success [80]. A total of 32 children with a mean age of 11.1 years (2.9–17.5 years) underwent ERASH from the LV. Symptomatic improvement was remarkable. Prior to treatment, 88% of patients suffered excess lethargy, 50% suffered breathlessness, 25% had nonarrhythmic syncope and 9% reported anginal chest pain. Over a median follow-up of 48 months (3–144 months) all symptoms were resolved, bar one child (3%) with excess lethargy. Echocardiographic gradients reduced from 97 (30–144 mmHg) to 32 mmHg (0–140 mmHg) at most recent follow-up. One procedural death occurred. At cessation of ablation LVOT gradients were noted to have increased due to tissue edema at the site of ablation, LV dysfunction ensued and the patient died 3 days later. Two patients required pacemaker implantation, one of whom was not reliant 1 week later.
Reduction of septal thickening in systole is proposed to be responsible for the reduced gradient. This akinesia of the basal septum prevents narrowing of the LVOT in systole, reducing SAM and, therefore, obstruction. The flexibility of ERASH appeals to physicians dealing with HOCM, and early results are comparable with results of early ASA series [35]. With a clear learning curve for operators performing ASA [81], this indicates significant promise for ERASH.
Conclusion
NSRT in the form of ASA has evolved to provide relief from symptoms attributable to LVOTO in the majority of patients in an appropriately selected group. While initially viewed as an alternative to myectomy only in patients unfit for surgery, the balance of preference has changed for some operators [21,82,83]. The improved outcomes of ASA are comparable with myectomy [48,64]. The complication rate has much improved and its availability is much more widespread. Mediumterm survival is better than matched HCM controls, and is even better than general populationmatched controls in some series [Jensen MK et al., Pers. Comm.] [41]. The risk of ventricular arrhythmia has not been proven to be significantly elevated post-ASA versus nontreated HCM and myectomy patients [Jensen MK et al., Pers. Comm.] [43–45,47,48].
We will never be able to conduct a randomized controlled trial to compare survival between myectomy and ASA to provide a higher level of evidence [65], but a wealth of observational data are now available [11,29,34,35,43,50–58,76,77]. While ASA can provide relief to the majority of appropriately selected HOCM patients, up to 25–30% of patients in the largest series are still significantly symptomatic or have persisting LVOT gradients >30 mmHg [51,52]. A further 5–8% of patients selected for ASA cannot undergo the procedure due to limitations of septal arterial supply [57,77].
Newer methods of NSRT therapy may provide greater options for specific subsets of patients within the HOCM cohort [73,76,79,80], and as these procedures develop, they may well provide a valuable adjunct to the management of HOCM patients. Currently, ASA remains our major contributor to NSRT.
Future perspective
Progress in the performance of ASA has stalled in recent years. Techniques have not advanced at the same rate as procedures in other fields of cardiology. The significant limitations in current practice can be improved by adapting ASA and incorporating alternative forms of NSRT into the care of HOCM patients.
Improved imaging and preprocedural planning offers promise in the refinement of ASA. Computerized tomography angiography has the potential to identify and describe septal arteries in the target myocardium before entering the catheterization laboratory. This may improve the localization of the infarct and, hence, the effect on gradients. This may also allow us to identify septals originating from unexpected parent epicardial arteries allowing alcohol delivery in some of the 5–8% currently unable to receive treatment.
Transthoracic echocardiogram images in the laboratory can be poor as the patient is lying prone. The operator and radiographer must also be careful not to irradiate the sonographer during image acquisition. Guiding ASA with intracardiac echocardiography has been investigated and warrants further exploration [84]. Better visualization of the myocardial contrast and intraprocedural effects of balloon occlusion on LVOT gradients and SAM may help to refine the localization of the infarct, improving long-term outcome.
There will always be a group of patients who cannot undergo ASA due to limitations in equipment design and anatomical variants. These patients may now be offered treatment through alternative methods, with radiofrequency ablation being the most likely methodology.
Success from ASA is clearly linked to higher volume operators and centers [19,27]. Given the relatively low volume of patients appropriate for ASA, the establishment of specified ‘centers of excellence’ will increase operator volume and, hence, improve patient outcomes. Centralization of outcomes and complications through national databasing was identified as an important missing facility by the ACCF/American Heart Association guidelines [19]. Combining outcomes for all centers performing ASA will allow for conclusions about the procedure with more confidence and allow collaboration with prospective developments, allowing advancement in technique and, ultimately, better patient outcomes.
Financial & competing interests disclosure
RH Stables has a research grant for the investigation of the use of intracardiac echocardiography in the performance of alcohol septal ablation (investigator initiated studies from Biosense Webster: IIS 191). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Left ventricular outflow tract obstruction pathophysiology
▪ The critical process in left ventricular outflow tract obstruction is systolic anterior motion of the mitral valve (MV). The pathophysiology is a complex interplay of basal hypertrophy, anterior displacement of the anterior papillary muscle and long anterior MV leaflet. This results in systolic anterior motion and MV contact with the basal septum. The longer the MV is in contact with the septum, the greater the obstruction to flow, and therefore the higher the gradient.
▪ Removal of the hypertrophied basal segment opens the left ventricular outflow tract. This alters the hemodynamics and reduces obstruction and, therefore, gradient.
Indications & patient selection
▪ Negatively inotropic medications improve symptoms and gradients in the majority of hypertrophic obstructive cardiomyopathy patients.
▪ Patients must have a subaortic gradient of >50 mmHg (at rest or with exercise), suffer from New York Heart Association class III dyspnea and have a septal width sufficient to perform septal reduction therapy without risk of causing a ventricular septal defect (usually >15 mm).
Results
▪ Alcohol ablation symptomatic resolution is good for the majority of patients.
▪ Recent data have suggested that medium-term survival following alcohol ablation is sufficient. The fear of infarct-induced ventricular tachycardia has not been proven above predicted ventricular tachycardia/sudden cardiac death rates for a matched hypertrophic cardiomyopathy population.
▪ Myectomy remains an excellent option in selected patients in high-volume centers. Many more alcohol ablation procedures are performed internationally.
Periprocedural complications of alcohol septal ablation
▪ The procedure of alcohol septal ablation appears to be very safe in experienced centers.
Future perspective
▪ Alcohol septal ablation in its current form still has restricting factors and therefore room for improvement.
▪ We are at the mercy of coronary artery anatomy and must identify the correct artery for injection from invasive angiography alone in most settings.
▪ We then must rely on the equipment available to access and balloon occlude the chosen artery, which is often of very small caliber.
▪ We must also bear in mind that target area of myocardium may have dual supply from more than one coronary vessel.
▪ Injection of fluid into coronary arteries is not under the direct control we desire.
▪ We must bear in mind confounding factors such as variable run off, septal arcades and vessels draining directly into the left and right ventricular cavities when injecting contrast and alcohol.
▪ Better imaging of the myocardium in the catheterization laboratory is desirable as accurate identification of the area supplied by myocardial contrast could improve localization of the iatrogenic infarct after alcohol injection.
▪ Owing to these limitations the prospect of a direct treatment to the LV basal septum without reliance on septal artery anatomy and fluid dynamics is an attractive prospect.
▪ Centers providing treatment to hypertrophic obstructive cardiomyopathy patients should have the benefit of offering all septal reduction therapies.
▪ Patients should be discussed at a multidisciplinary meeting to decide the best methodology of treatment for each individual.
▪ There is no blanket prescription of reduction therapy that is appropriate to all and each patient should have personalized treatment.
References
Papers of special note have been highlighted as:
▪ of interest
- Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet 363(9424), 1881–1891 (2004).
- Maron MS, Olivotto I, Betocchi S et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N. Engl. J. Med. 348(4), 295–303 (2003).
- Maron MS, Olivotto I, Zenovich AG et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation114(21), 2232–2239 (2006).
- Woo A, Williams WG, Choi R et al.Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation 111(16), 2033–2041 (2005).
- Nagueh SF, Ommen SR, Lakkis NM et al. Comparison of ethanol septal reduction therapy with surgical myectomy for the treatment of hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 38(6), 1701–1706 (2001).
- Qin JX, Shiota T, Lever HM et al. Outcome of patients with hypertrophic obstructive cardiomyopathy after percutaneous transluminal septal myocardial ablation and septal myectomy surgery. J. Am. Coll. Cardiol. 38(7), 1994–2000 (2001).
- Come PC, Riley MF. Hypertrophic cardiomyopathy. Disappearance of auscultatory, carotid pulse, and echocardiographic manifestations of obstruction following myocardial infarction. Chest 82(4), 451–454 (1982).
- Sigwart U, Grbic M, Payot M, Essinger A, Sadeghi H. Ventricular wall motion during balloon occlusion. In: Ventricular Wall Motion. Sigwart U, Heintzen PH (Eds).George Theime, NY, USA, 206–210 (1983).
- Brugada P, de Swart H, Smeets JL, Wellens HJ. Transcoronary chemical ablation of ventricular tachycardia. Circulation 79(3), 475–482 (1989).
- Sigwart U. Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. Lancet 346(8969), 211–214 (1995).
- Vecht JA, Dave R, Vecht RJ. Alcohol septal ablation: the first patient in 1994. Br. J. Cardiol. 13(1), 62–64 (2006).
- Maron MS, Olivotto I, Harrigan C et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 124(1), 40–47 (2011).
- Sherrid MV, Chu CK, Delia E, Mogtader A, Dwyer EM Jr. An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 22(3), 816–825 (1993).
- Maron BJ, Maron MS, Wigle ED, Braunwald E. The 50-year history, controversy, and clinical implications of left ventricular outflow tract obstruction in hypertrophic cardiomyopathy from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy: from idiopathic hypertrophic subaortic stenosis to hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54(3), 191–200 (2009).
- Maron BJ, Nishimura RA, Danielson GK. Pitfalls in clinical recognition and a novel operative approach for hypertrophic cardiomyopathy with severe outflow obstruction due to anomalous papillary muscle. Circulation 98(23), 2505–2508 (1998).
- Ommen SR, Nishimura RA. What causes outflow tract obstruction in hypertrophic cardiomyopathy? Heart 95(21), 1725–1726 (2009).
- Mazur W, Nagueh SF, Lakkis NM et al. Regression of left ventricular hypertrophy after nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. Circulation 103(11), 1492–1496 (2001).
- Lakkis N, Plana JC, Nagueh S, Killip D, Roberts R, Spencer WH 3rd. Efficacy of nonsurgical septal reduction therapy in symptomatic patients with obstructive hypertrophic cardiomyopathy and provocable gradients. Am. J. Cardiol. 88(5), 583–586 (2001).
- Gersh BJ, Maron BJ, Bonow RO et al. 2011 ACCF/AHA Guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 124(24), 2761–2796 (2011).
- Maron BJ, McKenna WJ, Danielson GK et al. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J. Am. Coll. Cardiol. 42(9), 1687–1713 (2003).
- Parakh N, Bhargava B. Golden jubilee of hypertrophic cardiomyopathy: is alcohol septal ablation the gold standard? Cardiovasc. Revasc. Med. 10(3), 172–178 (2009).
- Seggewiss H. Medical therapy versus interventional therapy in hypertropic obstructive cardiomyopathy. Curr. Control Trials Cardiovasc. Med. 1(2), 115–119 (2000).
- Pellikka PA, Oh JK, Bailey KR, Nichols BA, Monahan KH, Tajik AJ. Dynamic intraventricular obstruction during dobutamine stress echocardiography. A new observation. Circulation 86(5), 1429–1432 (1992).
- Gietzen FH, Leuner CJ, Obergassel L, Strunk-Mueller C, Kuhn H. Role of transcoronary ablation of septal hypertrophy in patients with hypertrophic cardiomyopathy, New York Heart Association functional class III or IV, and outflow obstruction only under provocable conditions. Circulation 106(4), 454–459 (2002).
- Holmes DR Jr, Valeti US, Nishimura RA. Alcohol septal ablation for hypertrophic cardiomyopathy: indications and technique. Catheter. Cardiovasc. Interv. 66(3), 375–389(2005).
- Rigopoulos AG, Seggewiss H. A decade of percutaneous septal ablation in hypertrophic cardiomyopathy. Circ. J. 75(1), 28–37 (2010).
- van der Lee C, Scholzel B, ten Berg JM, Geleijnse ML, Idzerda HH, van Domburg RT et al. Usefulness of clinical, echocardiographic,and procedural characteristics to predict outcome after percutaneous transluminal septal myocardial ablation. Am. J. Cardiol. 101(9), 1315–1320 (2008).
- Cuoco FA, Spencer WH 3rd, Fernandes VL et al. Implantable cardioverter-defibrillatortherapy for primary prevention of sudden death after alcohol septal ablation of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 52(21), 1718–1723 (2008).
- Lawrenz T, Obergassel L, Lieder F et al. Transcoronary ablation of septal hypertrophy does not alter ICD intervention rates in high risk patients with hypertrophic obstructive cardiomyopathy. Pacing Clin. Electrophysiol. 28(4), 295–300 (2005).
- Noseworthy PA, Rosenberg MA, Fifer MA et al. Ventricular arrhythmia followingalcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 104(1), 128–132 (2009).
- Fifer MA. Controversies in cardiovascular medicine. Most fully informed patients choose septal ablation over septal myectomy. Circulation 116(2), 207–216 (2007).
- Rothman RD, Baggish AL, O’Callaghan C et al. Management strategy in 249 consecutivepatients with obstructive hypertrophic cardiomyopathy referred to a dedicated program. Am. J. Cardiol. 110(8), 1169–1174 (2012).
- Cuisset T, Franceschi F, Prevot S et al. Transradial approach and subclavian wired temporary pacemaker to increase safety of alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: the TRASA trial. Arch. Cardiovasc. Dis. 104(8–9), 444–449 (2011).
- Fernandes VL, Nielsen C, Nagueh SF et al. Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007. JACC Cardiovasc. Interv. 1(5), 561–570 (2008).
- Gietzen FH, Leuner CJ, Raute-Kreinsen U et al. Acute and long-term results aftertranscoronary ablation of septal hypertrophy (TASH). Catheter interventional treatment for hypertrophic obstructive cardiomyopathy. Eur. Heart J. 20(18), 1342–1354 (1999).
- Veselka J, Duchonova R, Palenickova J et al. Impact of ethanol dosing on the long-term outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy: a single-center prospective, and randomized study. Circ. J. 70(12), 1550–1552 (2006).
- Veselka J, Tomasov P, Zemanek D. Long-term effects of varying alcohol dosing in percutaneous septal ablation for obstructive hypertrophic cardiomyopathy: a randomized study with a follow-up up to 11 years. Can. J. Cardiol. 27(6), 763–767 (2011).
- Nagueh SF, Lakkis NM, He ZX et al. Role of myocardial contrast echocardiography during nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 32(1), 225–229 (1998).
- Faber L, Seggewiss H, Fassbender D et al. Percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: acute results in 66 patients with reference to myocardial contrast echocardiography. Z. Kardiol. 87(3), 191–201 (1998).
- Zemanek D, Svab P, Veselka J. Power Doppler myocardial contrast echocardiography in alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Clin. Cardiol. 33(12), E82 (2010).
- Sorajja P, Ommen SR, Holmes DR Jr et al. Survival after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 126(20), 2374–2380 (2012).
- Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph-Edwards A et al. Long-term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J. Am. Coll. Cardiol. 58(22), 2313–2321 (2011).
- Jensen MK, Havndrup O, Hassager C et al. Survival and sudden cardiac death after septal ablation for hypertrophic obstructive cardiomyopathy. Scand. Cardiovasc. J. 45(3), 153–160 (2011).
- Lawrenz T, Obergassel L, Lieder F et al. Transcoronary ablation of septal hypertrophy does not alter ICD intervention rates in high risk patients with hypertrophic obstructive cardiomyopathy. Pacing Clin. Electrophysiol. 28(4), 295–300 (2005).
- Klopotowski M, Chojnowska L, Malek LA et al. The risk of non-sustained ventriculartachycardia after percutaneous alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Clin. Res. Cardiol. 99(5), 285–292 (2010).
- Maron BJ, Spirito P, Shen WK et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 298(4), 405–412 (2007).
- Agarwal S, Tuzcu EM, Desai MY et al. Updated meta-analysis of septal alcohol ablation versus myectomy for hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 55(8), 823–834 (2010).
- Leonardi RA, Kransdorf EP, Simel DL, Wang A. Meta-analyses of septal reduction therapies for obstructive hypertrophic cardiomyopathy: comparative rates of overall mortality and sudden cardiac death after treatment. Circ. Cardiovasc. Interv. 3(2), 97–104 (2010).
- Knight C, Kurbaan AS, Seggewiss H et al. Nonsurgical septal reduction for hypertrophic obstructive cardiomyopathy: outcome in the first series of patients. Circulation 95(8), 2075–2081 (1997).
- Alam M, Dokainish H, Lakkis N. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy: a systematic review of published studies. J. Interv. Cardiol. 19(4), 319–327 (2006).
- Nagueh SF, Groves BM, Schwartz L et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy a multicenter North American registry. J. Am. Coll. Cardiol. 58(22), 2322–2328 (2011).
- Jensen MK, Almaas VM, Jacobsson L et al. Long-term outcome of percutaneous transluminal septal myocardial ablation in hypertrophic obstructive cardiomyopathy: a Scandinavian multicenter study. Circ. Cardiovasc. Interv. 4(3), 256–265(2011).
- Kwon DH, Kapadia SR, Tuzcu EM et al. Long-term outcomes in high-risk symptomatic patients with hypertrophic cardiomyopathy undergoing alcohol septal ablation. JACC Cardiovasc. Interv. 1(4), 432–438 (2008).
- Seggewiss H, Rigopoulos A, Welge D, Ziemssen P, Faber L. Long-term follow-up after percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Clin. Res. Cardiol. 96(12), 856–863 (2007).
- Lyne JC, Kilpatrick T, Duncan A, Knight CJ, Sigwart U, Fox KM. Long-term follow-up of the first patients to undergo transcatheter alcohol septal ablation. Cardiology 116(3), 168–173 (2010).
- Sorajja P, Valeti U, Nishimura RA et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation 118(2), 131–139 (2008).
- Kuhn H, Lawrenz T, Lieder F et al. Survival after transcoronary ablation of septal hypertrophy in hypertrophic obstructive cardiomyopathy (TASH): a 10 year experience. Clin. Res. Cardiol. 97(4), 234–243 (2008).
- Klopotowski M, Chojnowska L, Malek LA et al. The risk of non-sustained ventriculartachycardia after percutaneous alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Clin. Res. Cardiol. 99(5), 285–292 (2010).
- Nagueh SF, Groves BM, Schwartz L et al. Alcohol septal ablation for the treatment of hypertrophic obstructive cardiomyopathy a multicenter North American registry. J. Am. Coll. Cardiol. 58(22), 2322–2328 (2011).
- Dearani JA, Ommen SR, Gersh BJ, Schaff HV, Danielson GK. Surgery insight: septal myectomy for obstructive hypertrophic cardiomyopathy – the Mayo Clinic experience. Nat. Clin. Pract. Cardiovasc. Med. 4(9), 503–512 (2007).
- Maron BJ, Yacoub M, Dearani JA. Controversies in cardiovascular medicine. Benefits of surgery in obstructive hypertrophic cardiomyopathy: bring septal myectomy back for European patients. Eur. Heart J. 32(9), 1055–1058 (2011).
- Naidu SS, Wang Y. Comparative in hospital safety and cost of alcohol septal ablation versus isolated surgical myectomy for symptomatic hypertrophic obstructive cardiomyopathy: insights from the multi center PREMIER database. Presented at: 2011 TCT: Transcatheter Cardiovascular Therapuetics Conference. SanFrancisco, CA, USA, 7–11 November 2011.
- Iacovoni A, Spirito P, Simon C et al. A contemporary European experience with surgical septal myectomy in hypertrophic cardiomyopathy. Eur. Heart J. 33(16), 2080–2087 (2012).
- Alam M, Dokainish H, Lakkis NM. Hypertrophic obstructive cardiomyopathy-alcohol septal ablation vs. myectomy: a meta-analysis. Eur. Heart J. 30(9), 1080–1087 (2009).
- Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Will there ever be a randomized trial? J. Am. Coll. Cardiol. 50(9), 831–834 (2007).
- Chang SM, Lakkis NM, Franklin J, Spencer WH 3rd, Nagueh SF. Predictors of outcome after alcohol septal ablation therapy in patients with hypertrophic obstructive cardiomyopathy. Circulation 109(7), 824–827 (2004).
- Sorajja P, Binder J, Nishimura RA et al. Predictors of an optimal clinical outcome with alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Catheter. Cardiovasc. Interv. 81(1), e58–e67 (2012).
- Harris KM, Spirito P, Maron MS et al. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation 114(3), 216–225 (2006).
- Almasood A, Garceau P, Woo A, Rakowski H, Schwartz L, Overgaard CB. Time to significant gradient reduction following septal balloon occlusion predicts the magnitude of final gradient response during alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. JACC Cardiovasc. Interv. 4(9), 1030–1034 (2011).
- Chen AA, Palacios IF, Mela T et al. Acute predictors of subacute complete heart block after alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Am. J. Cardiol. 97(2), 264–269 (2006).
- Chang SM, Nagueh SF, Spencer WH 3rd, Lakkis NM. Complete heart block: determinants and clinical impact in patients with hypertrophic obstructive cardiomyopathy undergoing nonsurgical septal reduction therapy. J. Am. Coll. Cardiol. 42(2), 296–300 (2003).
- Matos GF, Hammadeh R, Francois C, McCarthy R, Leya F. Controlled myocardial infarction induced by intracoronary injection of n-butyl cyanoacrylatein dogs: a feasibility study. Catheter. Cardiovasc. Interv. 66(2), 244–253 (2005).
- Oto A, Aytemir K, Okutucu S et al. Cyanoacrylate for septal ablation in hypertrophic cardiomyopathy. J. Interv. Cardiol. 24(1), 77–84 (2011).
- Gross CM, Schulz-Menger J, Kramer J et al. Percutaneous transluminal septal artery ablation using polyvinyl alcohol foam particles for septal hypertrophy in patients with hypertrophic obstructive cardiomyopathy: acute and 3-year outcomes. J. Endovasc. Ther. 11(6), 705–711 (2004).
- Lanzino G, Kanaan Y, Perrini P, Dayoub H, Fraser K. Emerging concepts in the treatment of intracranial aneurysms: stents, coated coils, and liquid embolic agents. Neurosurgery 57(3), 449–459 (2005).
- Durand E, Mousseaux E, Coste P et al. Non-surgical septal myocardial reduction by coil embolization for hypertrophic obstructive cardiomyopathy: early and 6 months follow-up. Eur. Heart J. 29(3), 348–355 (2008).
- Faber L, Welge D, Fassbender D, Schmidt HK, Horstkotte D, Seggewiss H. One-year follow-up of percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy in 312 patients: predictors of hemodynamic and clinical response. Clin. Res. Cardiol. 96(12), 864–873 (2007).
- Guo R, Qian J, Yang Y et al. A new strategy for septal ablation with transendocardial ethanol injection using a multifunctional intracardiac echocardiography catheter: a feasibility study in canines. Catheter. Cardiovasc. Interv. 78(2), 316–323 (2011).
- Lawrenz T, Borchert B, Leuner C et al. Endocardial radiofrequency ablation for hypertrophic obstructive cardiomyopathy: acute results and 6 months’ follow-up in 19 patients. J. Am. Coll. Cardiol. 57(5), 572–576 (2011).
- Sreeram N, Emmel M, de Giovanni JV. Percutaneous radiofrequency septal reduction for hypertrophic obstructive cardiomyopathy in children. J. Am. Coll. Cardiol. 58(24), 2501–2510 (2011).
- Kimmelstiel CD, Maron BJ. Role of percutaneous septal ablation in hypertrophic obstructive cardiomyopathy. Circulation 109(4), 452–456 (2004).
- Yacoub MH. Surgical versus alcohol septal ablation for hypertrophic obstructive cardiomyopathy: the pendulum swings. Circulation 112(4), 450–452 (2005).
- Hess OM, Sigwart U. New treatment strategies for hypertrophic obstructive cardiomyopathy: alcohol ablation of the septum: the new gold standard? J. Am. Coll. Cardiol. 44(10), 2054–2055 (2004).
- Pedone C, Vijayakumar M, Ligthart JM et al. Intracardiac echocardiography guidance during percutaneous transluminal septal myocardial ablation in patients with obstructive hypertrophic cardiomyopathy. Int. J. Cardiovasc. Intervent. 7(3), 134–137(2005).
- Nonsurgical reduction of the myocardial septum: NICE interventional procedure guidance (2004). http://publications.nice.org.uk/non-surgical-reduction-of-the-myocardial-septum-ipg40
▪ Original report of the first patients to undergo alcohol septal ablation, a controversial procedure at the time.
▪ Set of guideline statements on the care of hypertrophic cardiomyopathy patients. Good summary of the data to date. Details the place of alcohol septal ablation in the treatment of those with hypertrophic obstructive cardiomyopathy in the USA.
▪ One of the few randomized controlled trials in hypertrophic obstructive cardiomyopathy treatment. Provides reassurance that low doses of alcohol can generate favorable hemodynamic results.
▪ Excellent report detailing survival trends in patients who have undergone alcohol septal ablation.
▪ Radiofrequency ablation has the potential to significantly contribute to patient treatment in the long term. This paper, along with Sreeram et al. [80], provides the first description of a new step in the evolution of the treatment of hypertrophic obstructive cardiomyopathy.
▪ Radiofrequency ablation has the potential to significantly contribute to patient treatment in the long term. This paper, along with Lawrenz et al. [79], provides the first description of a new step in the evolution of the treatment of hypertrophic obstructive cardiomyopathy.
▪ Website