Research Article - Interventional Cardiology (2017) Volume 9, Issue 6
Creation of primary arteriovenous fistula:Vascular access for hemodialysis
- Corresponding Author:
- Masao Nunokawa
Cardiovascular Surgery, Kyorin University
6-20-2 Shinkawa, Mitaka-city, Tokyo 181-8611, Japan
Tel: +422475511
E-mail: m_nunokawa@hotmail.com
Submitted: October 09, 2017; Accepted: October 27, 2017; Published online: October 31, 2017
Abstract
Sustainable vascular access (VA) is literally a lifeline for most end-stage renal disease (ESRD) patients in Japan. For the creation of an arterio-venous fistula (AVF) for hemodialysis, I introduce a method performed at our institute that can be referred to by vascular surgeons. The lecture describes the preoperative assessment of targeted veins and arteries, and general condition as well as the importance of communication between the surgeon and the nephrologist. Detailed procedures are depicted and explained.
Keywords
Vascular access, Duplex scan, Anastomosis
Introduction
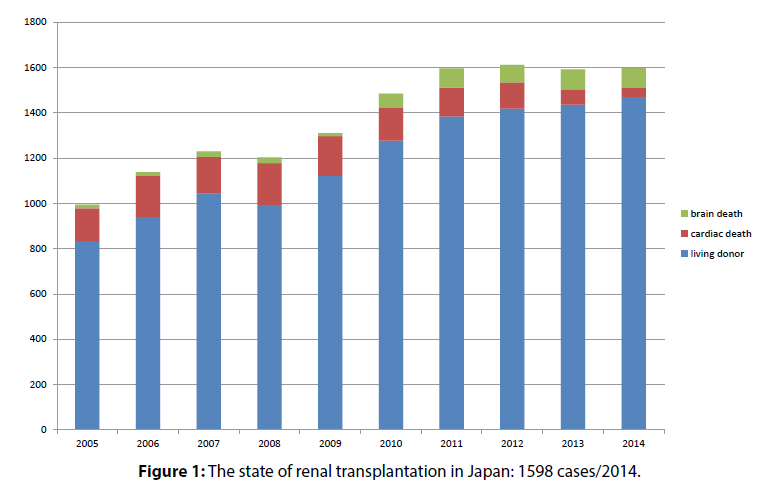
Currently renal transplantation is the best mode of therapy for patients with end-stage renal disease (ESRD); however, only 1,600 operations are performed in Japan annually (Figure 1) [1]. This small number cannot meet the demand: hemodialysis (HD) is widely accepted as a practical and necessary alternative. Safe and sustainable vascular access (VA) is literally a lifeline for most ESRD patients.
Figure 1: The state of renal transplantation in Japan: 1598 cases/2014.
While operating, one should create an arterio-venous fistula (AVF) with enough flow for HD (Table 1). One should pay attention to the anastomosis procedure based on demographics: in young patients, whose vessels may tend to spasm; or in older and diabetic patients, whose vessels are already affected by atheromatous changes. The latter patient may acquire critical limb ischemia that necessitates a distal bypass. In these challenging situations is where the skilled vascular surgeon can play a key role.
| A flow of more than 300 ml/min. is required: Flush the vein with 20 ml heparinized saline in 4 seconds i.e. 300 ml/min. |
| The vein is situated within 5 mm from the skin surface. |
| Sufficient length of the vein allows easy punctures for both “pull” and “return.” |
| No obstruction in the outflow. |
| Assurance of arterial inflow. |
| Good patency: maturation, rare sudden occlusion. |
| Patient’s ease. |
| Few complications. |
Table 1: Useful primary arterio-venous fistula (PAVF).
On the other hand, the latter patients may sometimes complicate with critical limb ischemia, which often indicate distal bypass. In such situations one’s skill in small caliber anastomosis acquired through VA surgery may be very valuable. As for VA, guidelines for HD initiation and VA were published from the Japanese Society for Dialysis Therapy [2,3], KDOQI [4] and the Society for Vascular Surgery [5] are available. Surgeons who use this interventional modality should customize their approach keeping these guidelines in mind.
In general, Japanese patients should be treated according to the Japanese guidelines, but useful material from the guidelines of the United States should be referred to depending on the situation (Table 2).
| Advanced age of hemodialysis patients. |
| Increasing proportion of diabetic cases. |
| Arterial change with renal sclerosis. |
| →Concomitant arterial sclerosis: change of calcium metabolism due to hemodialysis; induced acceleration of arterial calcification. |
| → Critical limb ischemia: need for skill in performing distal bypass, mastering of small caliber anastomosis through the procedures of PAVFs. |
Table 2: Vascular surgeons facing difficult cases.
Recently large sections on VA have been included in vascular surgery textbooks in the US. They describe complex and variable VA with artificial grafts (AVG) [6] as is common in the US, which may be referred to by Japanese surgeons, but the basic procedure is still autologous AVF and the textbooks recommend that easy adoption of AVG should be avoided.
Methods
Timing of HD initiation and VA surgery
Before the first use of an HD access site, AVFs require relatively longer amounts of time to mature sufficiently. Meanwhile, AVG operations should occur just before the initiation of HD because the patency of AVG will fall as time passes.
As per the US guidelines [4], AVFs should be made more than 6 months before and AVGs be made 3 to 6 weeks before the anticipated start of HD. As per the Japanese guidelines [3], the former should be made at least 2 to 4 weeks before and the latter 3 to 4 weeks before the anticipated start of HD.
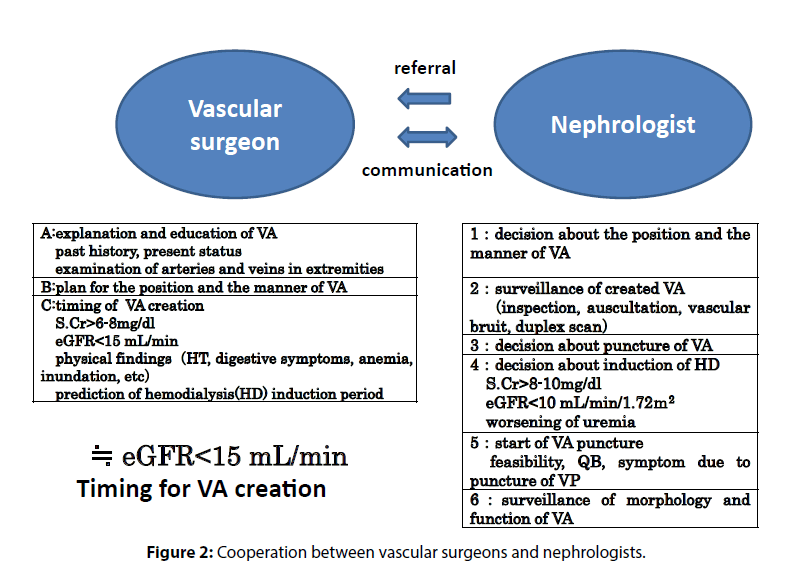
The nephrologist should estimate the initiation time of individual ESRD cases and should refer the patients to a vascular surgeon accordingly. A patient should be referred when the eGFR falls below 15 ml/min/1.72 m2, and when the severity of symptom increase, considering with or without diabetes. The vascular surgeon should assess the site and the type of VA and should decide the timing of the surgery depending on approach chosen (Figure 2).
Results and Discussion
Estimation of general condition and vascular status
The patient’s heart must tolerate the increased volume load after AV fistula creation. After treatment of circulatory overspill, a patient with LVEF lesser than 30% is not a candidate for AVF or AVG; instead, the patient will be a candidate for transposition of the brachial artery. As for AVG, patients with severe mitral regurgitation or ischemic heart disease requiring treatment are not candidates [7].
Systemic infection must be treated before surgery. Marked edema may impede venous evaluation. Dehydration, malnutrition and hypotension may reduce the patency of the PAVF. These conditions should be treated, even following initiation of HD through double-lumen catheter and improvement of general condition.
Surgeons choose VA site and manner depending on the patency of arteries and veins of both upper extremities. Meticulous examination through inspection and palpation reveal the condition of the skin and joints. Doctors should take a thorough history of venipuncture, upper extremity injury, and chest injury/deformity (Table 3).
| Observation of the upper extremities |
|---|
| Swelling (suspicious of the occlusion of the central veins). |
| Hemiplegia (create VA on the paralytic side). |
| Contracture of the elbow joint. |
| Skin conditions: dryness, redness and infection. |
Table 3: Creation of a useful primary arteriovenous fistula (PAVF)-1
The most important findings of the arteries are pulsations of the brachial, radial, and ulnar arteries, and verification of the patency of the palmer arch with Allen’s maneuver. The entire length of the veins should be verified to be patent and without stenosis under dilation with a tourniquet.
If one is anxious about the creation of PAVFs in patients with the above-mentioned physical findings, vascular duplex scan is a good method to confirm the nature of the vessels (Table 4) [8].
| Aim of ultrasound examination |
|---|
| Mapping of the veins that are obscure under visual inspection. |
| Obtaining information on the basilic vein and the deep veins in the upper arm. |
| Measuring diameters of the vessels at the anastomotic site. |
| Identification of thickness of the vessel (especialy artery) wall and extent of calcification. |
| Measuring flow volume and velocity of the artery. |
Table 4: Creation of a useful primary arterio-venous fistula (PAVF)-2.
Suturing calcified arteries is somewhat difficult. The artery must be patent with original forward flow, such that the peak systolic velocity of normal radial or ulnar artery is around 50 cm/sec. If the diameter of the artery is less than the acceptable diameter of 1.5 mm, the anastomosis site should be modified to the proximal site.
As to the veins, a diameter of 2.0 mm or larger under tourniquet and the patency of proximal side are needed. If the vein is located deeper than 5 mm from the skin surface, it should be trans positioned to a shallower side or an artificial prosthesis may be considered.
Central venous obstruction may cause early occlusion of the VA or venous hypertension. Thus, if central venous obstruction is revealed, creation of the VA on the contralateral side should be considered (Table 5).
| Suspected of the central vein obstruction |
|---|
| Marked edema with laterality in case of VA in the extremity. |
| Developed collateral venous pathway in the upper arm and the chest. |
| Past histories of catheterization in the central veins or pacemaker implantation. |
| Post-operative state of breast cancer. |
| Other past histories of surgery in the upper extremity or the neck |
Table 5: Creation of a useful primary arterio-venous fistula (PAVF)-3.
Practice of VA
Basic policy for the selection of VA site
1. Start as distal as possible to preserve more proximal portions for future use.
2. Because the patency of the artificial graft is low and the complication rate of autologous fistulas is very small, autologous VA should be tried first. Sometimes AVF on the upper arm is more valuable than AVG on the forearm.
3. Usually the non-dominant upper extremity should be selected as the first site because of the feasibility for HD and the tolerance for infection.
PAVF on the forearm
1. Anastomosis between the radial artery and the cephalic vein: The first site of the candidate should be at the wrist or at the so-called Tabatière. Anastomosis of the cephalic vein onto the ulnar artery or the brachial artery is also a candidate.
2. The basilic vein could be anastomosed onto the radial artery, the ulnar artery, or the brachial artery. The vein may sometimes be placed in tunneled transposition manner or loop-shaped.
PAVF on the upper arm
1. The cephalic vein is anastomosed onto the brachial artery or the proximal radial artery at the antecubital fossa.
2. As the basilic vein is situated in the deep layers, it is usually placed a in tunneled transposition manner for the feasibility of puncture.
3. Each vein should be anastomosed as distal as possible to avoid blood steal phenomenon.
4. Occasionally transposition of the brachial deep vein is considered as candidate.
Basic procedure of the AVF
1. Anastomosis should be made between arterial-side and venous-end. Side-to-side anastomosis has a higher probability of causing venous hypertension.
2. By flushing with heparinized saline from the venous end, venous diameter, patency, and the status of tributaries should be verified.
3. Just after the anastomosis, the flow may decrease because of vascular spasms. Though this may resolve spontaneously, a careful procedure is needed to preserve the sensitive intima and avoid thrombosis.
4. The arteriotomy should be 7 mm in length. This shorter length will reduce the risk of steal phenomenon.
5. To prevent anastomotic thrombosis, intravenous or direct arterial heparinization may be feasible. Special attention should be paid to avoid intimal injury of the affected arteries when direct arterial injection is selected.
6. Anastomosis is performed using a continuous suture with non-absorbable 7-0 double-armed monofilament. Continuous sutures may prevent dilation of the anastomosis.
7. After finishing the anastomosis, the near major branch should be ligated to allow more flow into the vein trunk and to prompt maturation of the VA vein.
8. A few days after the operation, the patient should be urged to perform the ball-grasp exercise to increase the VA venous flow.
Basic procedure of the AVG
1. The size of the arteriotomy is determined by the shape of the anastomosis. The flow is regulated by the graft diameter.
2. A prosthetic graft of 6 mm diameter is used
3. Injecting heparinized saline directly into the graft should be avoided to prevent seroma formation.
4. Anastomosis is performed using continuous suture with non-absorbable double-armed 6-0 or 7-0 monofilament.
5. Judicious sterile technique is needed to prevent prosthesis infection.
Practice of primary A-V fistula in the distal forearm at our institution
Medical management should be performed according to best institutional practice. However, if specific techniques are of interest, readers may adopt these.
Preoperative evaluation of the vessels
Easy and simple examination of the outpatient: After the usual physical examination, we percuss the veins to verify venous patency. The veins are pre-dilated with a rubber tourniquet at the upper arm. Then the examiner places a finger on the proximal side of the vein and gently percusses the distal side, causing the pulse to conduct up to the proximal side. If the pulse felt is very distinct, there is no obstruction between the proximal and distal sides of the vein. When this sensation is questionable, ultrasound evaluation of the veins is essential.
Skin incision: site and shape of the anastomosis
Other than cases in which the obvious superiority of the anastomosis in the Tabtière is recognized, the usual Radio-Cephalic fistula is chosen. A transverse incision perpendicular to the axis is made just proximal to the radial styloid process. If the vein is located very far from the artery, a long bow-shaped oblique incision or transposition of the vein may be needed.
Dissection of the subcutaneous tissue
Usually the trunk of the cephalic vein is situated beneath a thin fascia in deeper layers of the subcutaneous tissue. After the fascia is approached, the divided subcutaneous tissue is retracted gently and a vessel loop is placed around the vein at the distal side of the fascia incision. The vein is straightened by retraction of the vessel loop and mobilized by dividing the tissue on top of the vein up to the distal and proximal side. In this maneuver, the veins tend to spasm and tributaries may appear longer than usual, the tributaries are ligated 1.5 mm away from the vein to avoid waist formation when the vein dilates.
Before arterial dissection, local anesthetic is injected into the surrounding fibrous sheath. The cut surface of the needle tip is turned downward, avoiding careless arterial puncture.
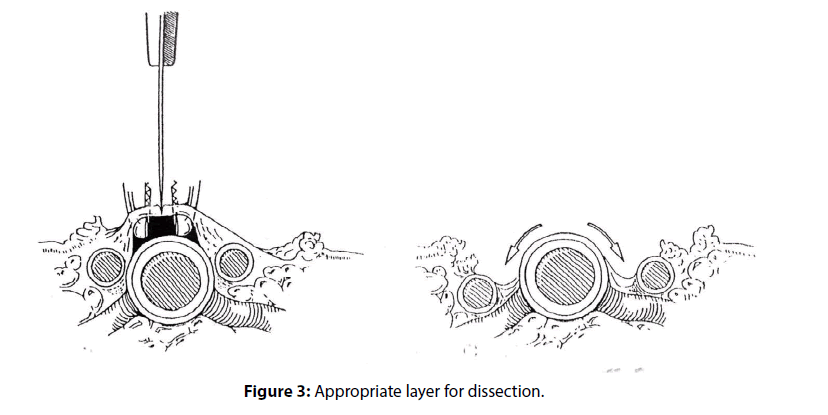
A thin layer of periarterial tissue contains concomitant veins which run parallel to the artery on either side. By dividing this layer on the anterior aspect, the artery is exposed without damaging concomitant veins (Figure 3).
Without identifying a suitable dissecting layer or through crude motions, the artery may easily spasm. Paired branches of the artery are also ligated as venous tributaries’ logation.
End-of -vein to side-of-artery anastomosis
Because the vein is completely dissected and approximated to the artery in an oblique course, the anastomotic incision site of the vein is distal to that of the artery. One should estimate the final anastomotic shape and should estimate the fitting length of the vessels, then make longitudinal anastomotic incisions on both sides of the vessels.
The venous incision is made from the distal opening along the line confronting the artery leaving the posterior wall still connected to avoid twisting the vein at this point.
Occasionally, one can utilize a large dorsal tributary as material for the heal patch-plasty. Cutting between the open ends of the veins gives additional length to the heel using a tributary, thus, providing the branch end with an additional suture bite and preventing anastomotic stenosis.
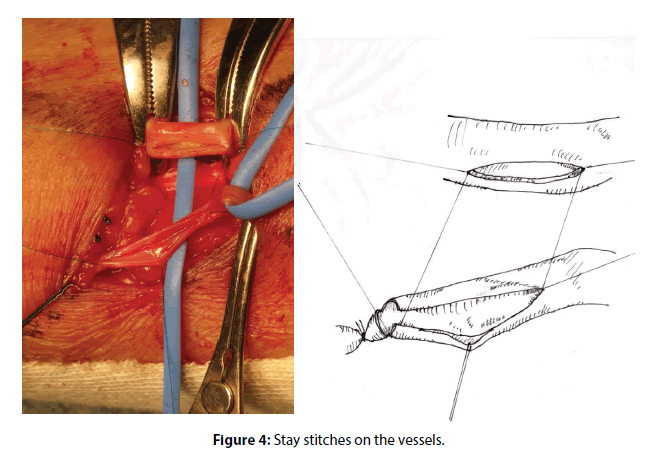
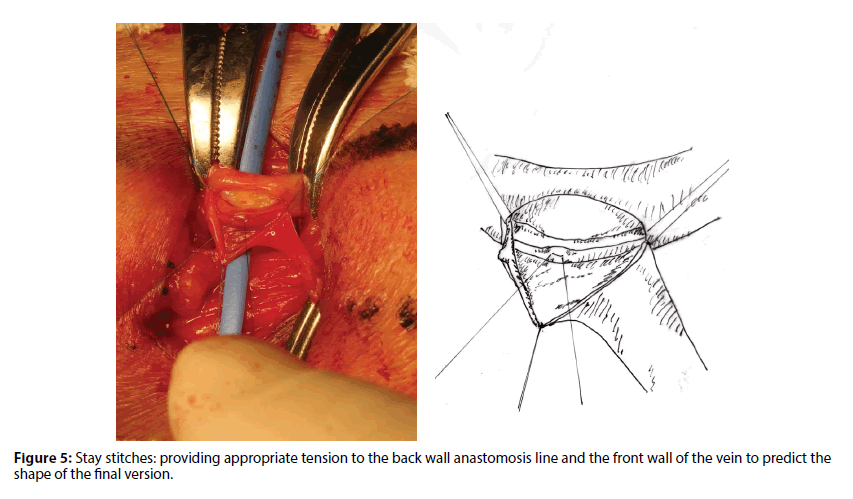
A 7-mm longitudinal incision is made on the artery. The stay sutures are placed at three points using 7-0 polypropylene monofilament: at the heel point, the needle enters the artery from the inside outward and another end enters the vein from inside outward. At the toe point, the needle enters the vein from outside inward and it enters the artery from inside outward. The third stay suture is placed at the midway point of the front wall of the vein. Imagining the final shape of the anastomosed vein, the end of the vein is trimmed, and the back wall is divided under suitable tension to the vessels being retracted gentry by the stays (Figure 4).
The main purpose of the stay suture is to minimize the chance of grasping the vessels proper and to apply appropriate tension especially to the vein maintaining the original shape and length of the vein. The stay technique is especially useful in the creation of AVF: under gentle tension onto the heel and the toe, both vessels approximate naturally and the anastomotic line becomes visible along a straight line (Figure 5).
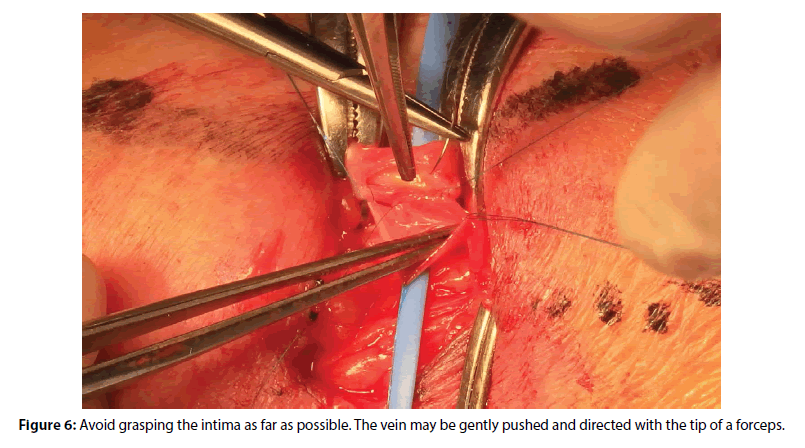
The anastomosis is started using a double-armed suture starting at the midway point of the back wall. The first two stitches of the continuous suture are placed in the artery inside-out and are placed in the vein outside-in, and the suture is left untied. With another end held by rubber-tipped forceps, the running suture begins and subsequent stiches go to the proximal corner handling the thread from inside. Under suitable tension by the suture and the stay, the running suture is placed easily while catching precisely the adventitia of the artery and the intima of the vein. If one does not prefer the backhand grip of the needle driver, one should change position with the assistant. The principle of handling veins is to avoid picking up the intima by forceps. Forceps may be used to dilate the arterial corner inserting the tip into the lumen (Figure 6) or to push and direct from the outside during suturing. The only acceptable grabbing is of the perivascular loose connective tissue using fine forceps.
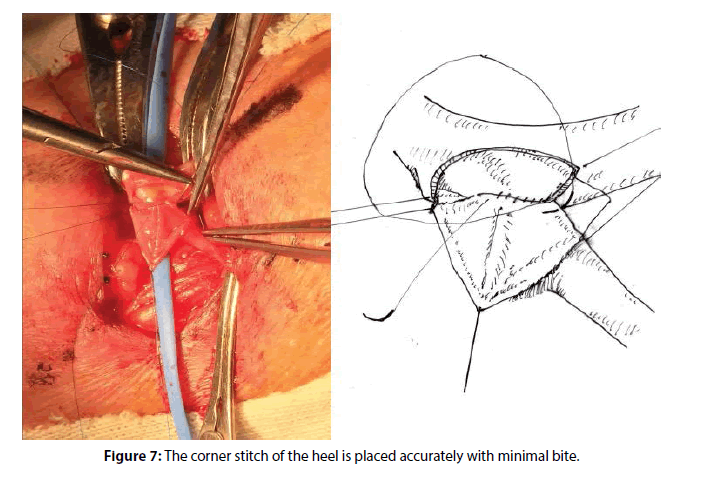
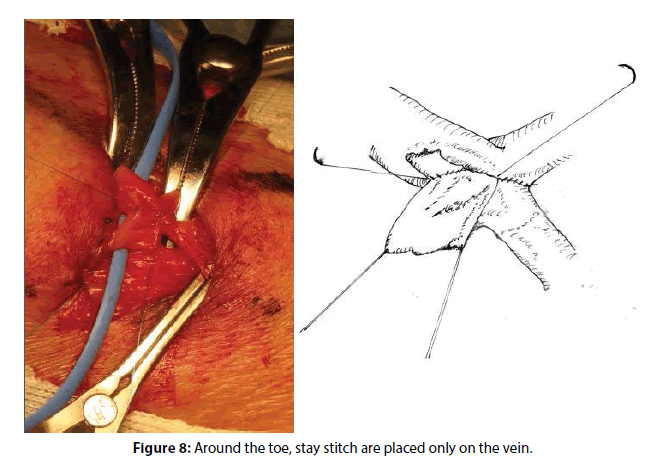
The proximal corner is the most important and delicate site of the sutures. The stay thread at the arterial corner is taken off and the arterial wall around the corner is exposed thoroughly by pulling the venous side corner suitably (Figure 7). Because large bites in the corner may cause narrowing of the lumen, the sutures are placed by taking small and exact bites of the vessels with minimal travel. The handling of the thread is changed to from outside and the suturing is continued until the stay at the midway point of the front wall (Figure 8).
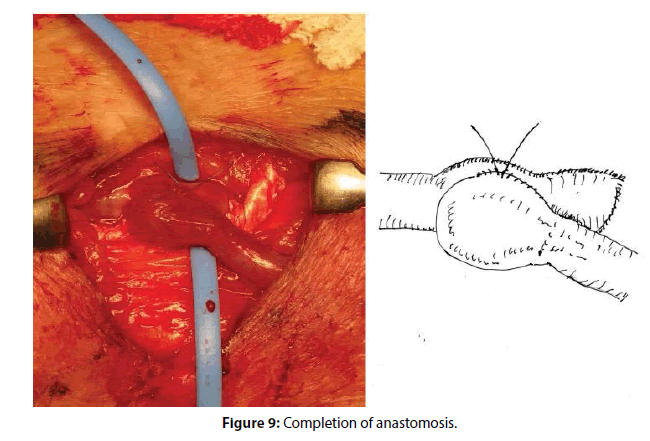
Next, the suture of the distal side of the back wall begins. While the principle is the same as that for the proximal side, if the venous travel of the stitch is somewhat larger than the arterial one around the distal corner, the final shape may resemble a “lion’s hand” (Figure 9).
After unclamping the artery, spontaneous hemostasis of the anastomosis will usually be obtained by applying a finger-tip softly on to the bleeding point for about 5 minutes instead of an additional suture. If the bleeding remains, adequate hemostasis procedure is performed after re-clamping the artery.
Conclusion
Fistula flow can be low due to arterial spasm; however, venous spasm around the edge of dissection is thought to be the main cause of low flow. In this situation, as the vein near the anastomosis is filled with blood and the flow is very slow, intima injury may easily evoke thrombosis of the anastomosis. The application of a local anesthetic may induce gradual venous dilation; papaverine hydrochloride is used for rapid effect. Under prophylactic systemic heparinization of 2000 units, recovery of the spastic vessels occurs, often with well audible murmurs and palpable thrills several hours afterword.
Acknowledgements
The author would like to acknowledge Drs. Yutaka Hosoi and Yoshifumi Nishino for their skilled and photogenic maneuver in the AVF operation.
References
- The Japan Society for Transplantation. Annual progress report from the Japanese Renal Transplant Registry: number of renal transplantation in 2014 and follow-up survey. Jap. Soc. Clin. Re. Transplant: Ishoku. 50:138-155 (2015).
- Japanese Society for Dialysis Therapy. Clinical Guideline for Hemodialysis Initiation for Maintenance Hemodialysis. Ther. Apher. Dial. 19: 93–107 (2015).
- Japanese Society for Dialysis Therapy. 2011 update/Guidelines of Vascular Access Construction and Repair for Chronic Hemodialysis. Ther. Apher. Dial. 19: 1–39 (2015).
- National Kidney Foundation. Kidney Dialysis Outcomes Quality Initiative. The NKF-KDOQI Clin Pract. Guidelines (2006).
- Sidawy AN, Spergel LM, Besarab A, et al. The Society for Vascular Surgery: Clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J. Vasc. Surg. 48 (5 Suppl): 2S-25S (2008).
- Huber TS, Woo K, Rowe VT, et al. Hemodialysis Access. In Rutherford’s Vascular Surgery. ELSEVIER. Saunders: Philadelphia. pp: 1082-1152 (2015).
- Ohhira S, Kukita K, Amano I, Naitoh H. Vascular Access: Practice of creation, maintenance and repair. Chugaiigakusha: Tokyo. pp: 456 (2007).
- Haruguchi H. Vascular Access; The textbook of ultrasound examination. Ishiyaku. Publishers: Tokyo. pp: 2565 (2011).