Research Article - Clinical Investigation (2017) Volume 7, Issue 3
Constraining Effect of Mild Portal Hypertension against the Negative Influence of Muscle Mass Loss in Cirrhosis after Esophageal Variceal Eradication
- Corresponding Author:
- Hitoshi Maruyama
Department of Gastroenterology
Chiba University Graduate School of Medicine
1-8-1, Inohana, Chuou-ku, Chiba, 260- 8670, Japan
E-mail: maru-cib@umin.ac.jp
Submitted: 04 September 2017; Accepted: 20 September 2017; Published online: 25 September 2017
Abstract
Background: To elucidate the role of muscle mass loss (MML) in the long-term outcome of cirrhosis after the endoscopic eradication of esophageal varices (EV).
Methods and findings: This is a subgroup analysis of 82 prospectively enrolled cirrhosis patients with eradicated EV. A severe portal hypertension (PH) was defined by post-treatment hepatic venous pressure gradient ≥12 mmHg. MML was assessed by skeletal muscle index at the L3 lesion (cm2/m2) with the cut-off values of 38 for women and 42 for men (median observation period, 37.4 months). Twenty-four patients (29.3%) had MML. Multivariate analysis showed that a presence of hepatocellular carcinoma (P<0.0001) and a presence of MML (P=0.002) were significant prognostic factors. In the cohort with severe PH, the survival rate was significantly lower in patients with MML than in those without. However, in the cohort without severe PH, the survival rate showed no difference between patients with MML (100% at 1year, 50% at 3 years and 5 years) and those without (92.3% at 1 year and 3 years, 71.8% at 5 years; P=0.278).
Conclusions: MML is an independent prognostic factor after the eradication of EV in cirrhosis, and the mild PH exerts a constraining effect against the negative influence of MML.
Keywords
Cirrhosis, Portal hypertension, Esophageal varices, Muscle mass loss
Abbreviations
AIH: Autoimmune Hepatitis; APC: Argon Plasma Coagulation; BCAA: Branched-chain Amino Acid; BCLC: Barcelona Clinic Liver Cancer; BMI: Body Mass Index; CI: Confidence Interval; CT: Computed Tomography; EV: Esophageal Varices; HBV: Hepatitis B Virus; HCC: Hepatocellular Carcinoma; HCV: Hepatitis C Virus; HVPG: Hepatic Venous Pressure Gradient; MELD: Model for End-stage Liver Disease; MML: Muscle Mass Loss; NASH: Nonalcoholic Steatohepatitis; OR: Odds Ratio; PBC: Primary Biliary Cholangitis; PH: Portal Hypertension; SD: Standard Deviation; TIPS: Transjugular Intrahepatic Portosystemic Shun
Introduction
Esophageal varices (EV) are a major complication of cirrhosis [1] that are detected in approximately 50% of cirrhosis patients, and newly-formed or worsening varices can be demonstrated in 5% to 15% of them each year, with a third of the patients with EV undergoing a bleeding episode [2-4]. International consensus clearly shows the timing of primary/secondary prophylaxis with the established therapies using medications and/or endoscopy [3,5]. These data meet the needs of medical care after the eradication of EV and can effectively predict the prognosis.
Muscle mass loss (MML) is an impaired muscular status caused by abnormal health conditions and aging [6,7]. It has become widely recognized in the hepatology field because of the significant influence of MML on patients with liver disease. MML is a poor prognostic factor in cirrhosis or hepatocellular carcinoma (HCC) patients [8,9], and a predictor of the development of complications [10,11] and outcomes after surgical treatment [12].
Based on this background, it is possible to develop a hypothesis that there might be a certain effect by MML on the prognosis of patients with EV. However, there is a paucity of data regarding the relationship between the presence of MML and long-term outcomes of patients with EV treated by endoscopy. Therefore, the present study aimed to elucidate the role of MML with respect to the severity of portal hypertension (PH) in the longterm outcome of cirrhosis patients after the endoscopic eradication of EV.
Methods
Study
This is a subgroup analysis of the cohort of the prospective study that includes consecutive cirrhosis patients (March 2008 to December 2014). The study was performed to examine the long-term outcomes of cirrhosis patients with EV eradicated by endoscopic treatment. This study was approved by the ethics committee of Chiba University Hospital, and informed written consent was obtained from all patients. The inclusion criteria were as follows: (1) cirrhosis patients with EV who were scheduled for sclerotherapy combined with argon plasma coagulation (APC), which was a principal treatment for primary/ secondary prophylaxis of EV in our department; and (2) those who were scheduled for the evaluation of the severity of PH by hepatic venous catheterization after the endoscopic treatment. A diagnosis of cirrhosis was based on a combination of biochemical and imaging findings, the latter using both ultrasound and computed tomography (CT). The criteria for primary prophylaxis in this study were the presence of medium-to-large varices and/or red signs on varices.
However, the study excluded following patients: (1) a Child-Pugh C classification or advanced liver cancer (stage C or D according to the Barcelona Clinic Liver Cancer staging system for HCC [13]), since the use of a sclerosant is not recommended for these advanced liver disease patients in Japan; (2) those who underwent a transjugular intrahepatic portosystemic shunt (TIPS); and (3) those using vasoactive drugs, such as β-blockers, which are not approved as a treatment for PH in Japan. The degree of ascites was defined from grade 1 to 3 according to international guidelines [14]. Spleen volume was measured by the sum of the manual trace of spleen on the CT image using image analysis software (HOPE/DrABLE-EX; Fujitsu, Tokyo, Japan).
The endpoint was a prognosis determined by either the time of death, the receipt of a liver transplantation, or a final hospital visit. The observation period was defined as the time interval between the end of the endoscopic treatment and the time of the endpoint.
Endoscopy
Definition of EV: Gastroesophageal varices were classified according to the general rules of the Japan Research Society for Portal Hypertension [15]: small, medium, and large. Red signs were assessed by the presence of red wale markings, cherry red spots, or hematocystic spots. A recurrence of varices was determined by a recurrence of varices and/or red signs on the endoscopic image.
In cases with bleeding or with bleeding in the history, the source of bleeding was examined by endoscope. A variceal bleeding was defined by both the presence of a bleeding history and endoscopic evidence of active bleeding or a fibrin clot on the varices.
Gastroesophageal varices were considered to be the source of the bleeding when no other cause of gastrointestinal bleeding could be identified in patients without any evidence of active bleeding or a fibrin clot.
Endoscopic sclerotherapy: The sclerotherapy was performed according to the previous report [16], under intravenous anesthesia using pentazocine (15 mg; Daiichi Sankyo Co. Ltd., Tokyo, Japan) and flunitrazepam (0.5-1.0 mg; Eisai Co. Ltd, Tokyo, Japan) with careful monitoring of vital signs, such as blood pressure, pulse rate, and oxygen saturation.
After the confirmation of the intravariceal puncture by the presence of withdrawn blood in the injection needle, we injected the sclerosant, which was prepared as a 5% solution (a mixture of equal volumes of 10% ethanolamine oleate and iopamidol; Oldamin; Mochida Pharmaceutical, Tokyo, Japan; Iopamiron 300; Bayer Schering Pharma, Osaka, Japan). Fluoroscopy was used to administer the sclerosant to fill the variceal vascular bed to the left gastric vein and to avoid an excessive injection. The needle puncture was repeated for multiple variceal vessels, using a maximum dose of 20 mL of 5% sclerosant solution at one session.
The sclerotherapy was performed once or twice a week to achieve variceal embolization, and the effect was evaluated before each section by endoscopic ultrasonography using a 12MHz ultrasonic miniature probe system (UM2R; Olympus, Tokyo, Japan). After the confirmation of the embolization of varices, a consolidation therapy was added by APC with the following settings: an argon gas flow rate of 1.4 to 1.8L/min and an arc output of 40 W; with an ICC200/APC300 or a VIO300D/APC2 unit (ERBE Elektromedizin GmbH, Tübingen, Germany) with a flexible APC probe (ERBE Elektromedizin GmbH, Tübingen, Germany). APC was performed one or two times to obtain the whole mucosal coagulation approximately 5 cm from the esophagocardiac junction. Follow-up endoscopic examinations were performed 6 months after treatment, and every 6 months to 1 year thereafter.
Muscle mass loss
The quantitative assessment of the muscle was made by the CT finding at the L3 region using the SliceOmatic V5.0 software (Tomovision, Montreal, Quebec, Canada) according to the literature [17,18]. Briefly, the sum of the cross-section areas of the skeletal muscle was calculated (cm2), and the formula “cross-sectional muscle area/height2” provided the skeletal muscle index at the L3 lesion with the cut-off values for diagnosing MML: 38 cm2/m2 for women and 42 cm2/m2 for men [19].
Hepatic venous catheterization
Hepatic venous catheterization was performed in the main branch of the right hepatic vein after the eradication of EV. A free and a wedged hepatic venous pressure were measured, and the hepatic venous pressure gradient (HVPG) was calculated. A severe PH was defined by HVPG 12 mmHg or higher according to the literature [20].
Statistical analysis
The study expressed the data as the mean ± standard deviation (SD), median, or percentages. The Student’s t test or the Mann-Whitney U test was used for the analysis of continuous variables, and the chi-square test was used for categorical variables. The Kaplan-Meier method calculated the cumulative survival rate. The study also detected significant factors by using univariate and multivariate analysis with Cox regression analysis. P values less than 0.05 were considered to be significant. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC).
Results
Patient characteristics
The study included a total of 82 patients (age range, 37-83 years; mean age±SD, 64.2±10.4 years; 51 males, 31 females) with EV (small 6, medium 57, and large 19) (Table 1). Fifty-four patients also had gastric varices (small 38, medium 14, and large 2). There were 29 patients with Child-Pugh class A and 53 patients with Child-Pugh class B, with the range of scores 5 to 9 (mean±SD, 6.9±1.2). The model for end-stage liver disease score ranged from 6 to 15 (mean±SD, 9.3±2.2). Forty patients were accompanied with hepatitis virus infection (hepatitis B in 3 and hepatitis C in 37) and 5 of them received antiviral therapy.
| Number | 82 |
| Age | 64.2±10.4 (37-83) |
| Sex (male/female) | 51/31 |
| BMI | 24.7±4.4 (16.0-38.1) |
| Etiology (HBV/HCV/Alcoholic/NASH/PBC/AIH/others) | 3/37/11/12/4/1/14 |
| Antiviral therapy (-/+) | 35/5 |
| Child-Pugh classification (A/B) | 29/53 |
| Child-Pugh score | 6.9±1.2 (5-9) |
| MELD score | 9.3±2.2 (6-15) |
| Na | 137.6±2.7 (131-144) |
| Hepatocellular carcinoma (-/+) | 72/10 |
| Hepatocellular carcinoma , BCLC stage (A/B/C/D) | 6/4/0/0 |
| Esophageal varices (Small/Medium/Large) | 6/57/19 |
| Bleeding from esophageal varices (-/+) | 49/33 |
| Gastric varices (None/Small/medium/large) | 28/38/14/2 |
| Spleen volume (cm3) | 504.9±322.0 (94.3-2321.9) |
| HVPG (mmHg) | 14.9±4.2 (4.2-24.1) |
| BCAA supplementation (-/+) | 30/52 |
Table 1: Patient characteristics
All EV had disappeared as a result of the endoscopic treatment with 1.9±0.8 sclerotherapy treatments (total dose of 5% sclerosant, 14.7±8.5 mL) and 1.6 ± 0.6 APC treatments. The HVPG ranged from 4.2 to 24.1 (14.9±4.2), and a presence of severe portal hypertension was detected in 62 patients (75.6%).
The median observation period was 37.4 months (4.8-105.5 months); the recurrence of EV was found in 30 patients (36.6%), and 10 patients had re-bleeding (12.2%).
MML
Twenty-four patients (29.3%) had MML, 8/29 (27.6%) with Child-Pugh class A and 16/53 (30.2%) with Child-Pugh class B, showing no difference (P=0.81) (Table 2). The frequency of MML was not significantly different between patients with (12/52, 23.1%) and without oral supplementation of branchedchain amino acid (BCAA) (12/30, 40%; P=0.11), with (2/5, 40%) and without antiviral therapy (10/35, 28.6%; P=0.63), and with (7/30, 23.3%) and without variceal recurrence (17/50, 34%; P=0.32). The muscle volume showed no correlation with the HVPG value (r=-0.08, P=0.47), and the frequency of MML showed no difference between patients with severe PH (17/62, 27.4%) and those without (7/20, 35%; P=0.52).
| Muscle mass loss | P value | ||
|---|---|---|---|
| - | + | ||
| Number | 58 | 24 | - |
| Age | 63.4±11.0 | 65.9±8.8 | 0.33 |
| Sex (male/female) | 37/21 | 14/10 | 0.65 |
| BMI | 26.3±4.0 | 20.7±2.3 | <0.0001 |
| Etiology (HBV/HCV/Alcoholic/NASH/PBC/AIH/others) | 1/27/7/11/2/0/10 | 2/10/4/1/2/1/4 | 0.39 |
| Antiviral therapy (-/+) | 25/3 | 10/2 | 0.63 |
| Child-Pugh score | 6.9±1.2 | 7.0±1.2 | 0.64 |
| MELD score | 9.4±2.2 | 8.8±2.1 | 0.25 |
| Child-Pugh classification (A/B) | 21/37 | 8/16 | 0.81 |
| Na | 137.3±2.6 | 138.2±2.9 | 0.19 |
| Hepatocellular carcinoma (-/+) | 52/6 | 20/4 | 0.47 |
| Esophageal varices (Small/Medium/Large) | 5/39/14 | 1/18/5 | 0.78 |
| Bleeding from esophageal varices (-/+) | 35/23 | 14/10 | 0.87 |
| Recurrence of esophageal varices (-/+) | 33/23 | 17/7 | 0.32 |
| BCAA supplementation (-/+) | 18/40 | 12/12 | 0.11 |
| Number of death (-/+) | 41/17 | 10/14 | 0.014 |
| Spleen volume (cm3) | 522.2±342.5 | 463.0±267.9 | 0.45 |
| HVPG (mmHg) | 14.9±4.2 | 14.9±4.3 | 0.95 |
| HVPG (<12/≥12) | 13/45 | 7/17 | 0.52 |
Table 2: Comparison of clinical findings between patients with and without muscle mass loss
Prognosis
Thirty-one patients died during the study period, 17 of hepatic failure 8 of HCC, 1 of gastrointestinal bleeding, 1 of esophageal cancer, and 4 of unknown cause. The cumulative overall survival rate was 92.6% at 1 year, 71.4% at 3 years, and 56.5% at 5 years after the eradication of EV. Univariate analysis showed that a presence of HCC (odds ratio [OR] 5.438, 95% confidence interval [CI] 2.493-11.861, P<0.0001), serum NA concentration (OR 0.857, 95% CI 0.741- 0.990; P=0.036), and a presence of MML (OR 2.446, 95% CI 1.220-4.907, P=0.012) were significant factors for prognosis. Multivariate analysis showed that a presence of HCC (OR 5.277, 95% CI 2.307-12.068; P<0.0001) and a presence of MML (OR 3.081, 95% CI 1.491-6.370; P=0.002) were significant prognostic factors.
Prognosis with respect to MML and PH
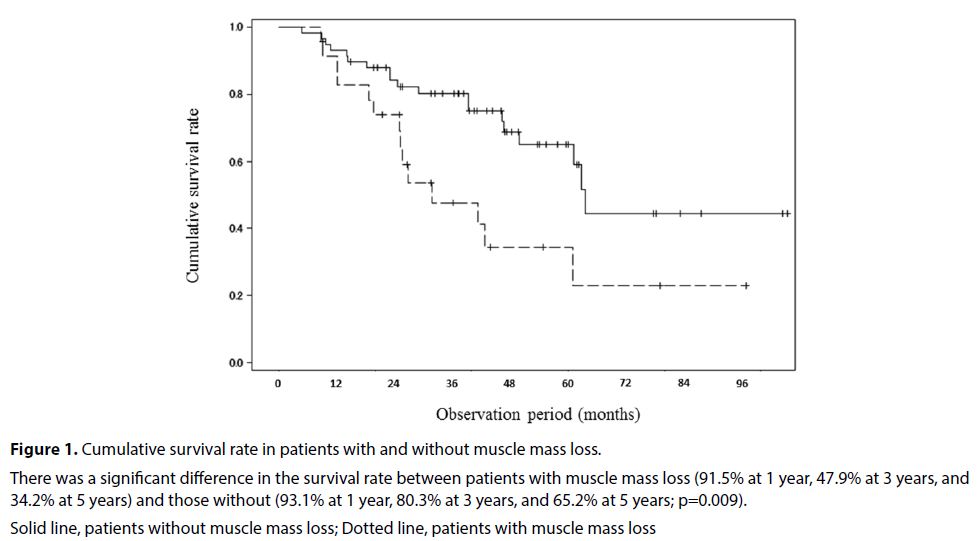
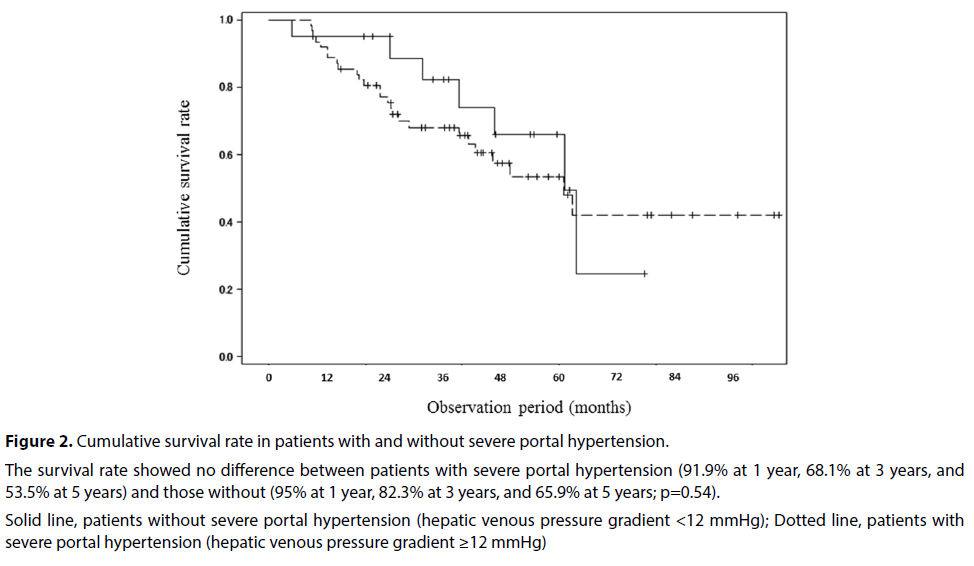
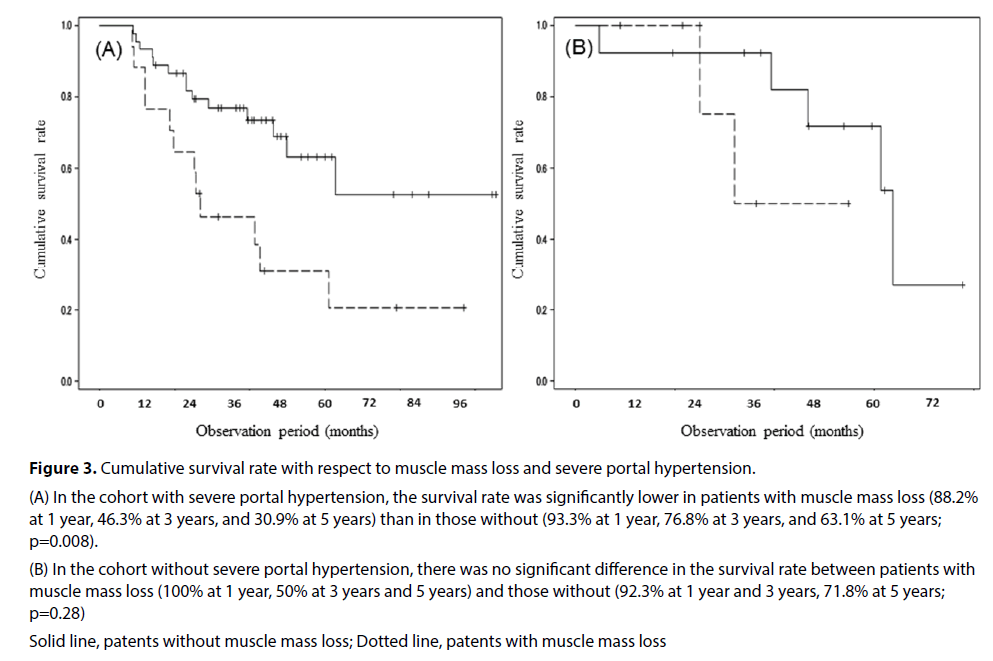
There was a significant difference in the survival rate between patients with MML (91.5% at 1 year, 47.9% at 3 years, and 34.2% at 5 years) and those without (93.1% at 1 year, 80.3% at 3 years, and 65.2% at 5 years; P=0.009) (Figure 1). The survival rate showed no difference between patients with severe PH (91.9% at 1 year, 68.1% at 3 years, and 53.5% at 5 years) and those without (95% at 1 year, 82.3% at 3 years, and 65.9% at 5 years; P=0.54) (Figure 2). In the cohort with severe PH, the survival rate was significantly lower in patients with MML (88.2% at 1 year, 46.3% at 3 years, and 30.9% at 5 years) than in those without (93.3% at 1 year, 76.8% at 3 years, and 63.1% at 5 years; P=0.008) (Figure 3a). However, in the cohort without severe PH, there was no significant difference in the survival rate between patients with MML (100% at 1 year, 50% at 3 years and 5 years) and those without (92.3% at 1 year and 3 years, 71.8% at 5 years; P=0.28) (Figure 3b).
Figure 1: Cumulative survival rate in patients with and without muscle mass loss.
There was a significant difference in the survival rate between patients with muscle mass loss (91.5% at 1 year, 47.9% at 3 years, and 34.2% at 5 years) and those without (93.1% at 1 year, 80.3% at 3 years, and 65.2% at 5 years; p=0.009).
Solid line, patients without muscle mass loss; Dotted line, patients with muscle mass loss
Figure 2: Cumulative survival rate in patients with and without severe portal hypertension.
The survival rate showed no difference between patients with severe portal hypertension (91.9% at 1 year, 68.1% at 3 years, and 53.5% at 5 years) and those without (95% at 1 year, 82.3% at 3 years, and 65.9% at 5 years; p=0.54).
Solid line, patients without severe portal hypertension (hepatic venous pressure gradient <12 mmHg); Dotted line, patients with severe portal hypertension (hepatic venous pressure gradient ≥12 mmHg)
Figure 3: Cumulative survival rate with respect to muscle mass loss and severe portal hypertension.
(A) In the cohort with severe portal hypertension, the survival rate was significantly lower in patients with muscle mass loss (88.2% at 1 year, 46.3% at 3 years, and 30.9% at 5 years) than in those without (93.3% at 1 year, 76.8% at 3 years, and 63.1% at 5 years; p=0.008).
(B) In the cohort without severe portal hypertension, there was no significant difference in the survival rate between patients with muscle mass loss (100% at 1 year, 50% at 3 years and 5 years) and those without (92.3% at 1 year and 3 years, 71.8% at 5 years; p=0.28)
Solid line, patents without muscle mass loss; Dotted line, patents with muscle mass loss
Discussion
Prediction of the prognosis is an important issue in the management of cirrhosis patients. To the best of our knowledge, this may be the first study to report the significant influence of MML as an independent prognostic factor for cirrhosis after the eradication of EV. Furthermore, although the precise mechanism remains undetermined, there is an interaction between portal hemodynamics and muscle atrophy, which is that the mild PH exerts a constraining effect against the negative influence of MML. It should be determined whether the application of vasoactive medication or TIPS could reduce the portal pressure and in turn reduce the influence of MML.
The present study detected HCC as a significant prognostic factor other than MML, which may be plausible according to the literature [21]. However, the HVPG was not a statistically significant factor for prognosis. One of the reasons may be a potential bias of the patient population; this study was performed in patients with cirrhosis accompanied by EV, mostly of moderate or high grade. In other words, the patient’s condition suggests the presence of a certain degree of PH, evidenced by a mean HVPG of 14.9 mmHg and 75.6% patients with HVPG 12 mmHg or higher. In fact, investigators suggested that the link between the severity of PH and the prognosis of cirrhosis patients is contentious, and the influence of portal pressure may depend on the additional patient condition factors such as liver function and/or compensation/decompensation [22,23].
The prognosis in patients with MML in our study was comparable to or slightly worse than that in patients with sarcopenia in the literature: 85% at 1 year and 63% at 3 years [8], and 63% at 1 year and 51% at 3 years [24], probably due to the different patient characteristics or the different cut-off values for MML. In fact, the present study used novel diagnostic cutoff values to determine MML, which were recently proposed for Japan [19]. The prevalence of MML, which may be termed presarcopenia because we did not use muscle power criterion, was 29.3% in our study cohort. A previous study reported a similar frequency of presarcopenia: 24.4% (103/422) of cirrhosis patients with Child-Pugh class A, 37.7% (89/236) of those with Child-Pugh class B, and 37.1% (23/62) of those with Child-Pugh class C [25]. Meanwhile, the prevalence of sarcopenia in the Western study is higher than ours: 41% of the patients listed for liver transplantation [24], 40% [18] and 66.2% [26] both in pre-orthotopic liver transplantation patients. The frequency of muscle volume depletion may depend on the race, patient population, and diagnostic criteria for muscle volume quantification. However, a possible reason for the lower frequency of MML in our study may be a potential bias in the patient selection for endoscopic treatment, which is not basically applied for patients with Child- Pugh class C. At the same time, the adequacy of the cut-off values for MML need to be validated in the future.
Recurrence of EV may be a problem that needs to be properly managed. Investigators have reported the anatomical and/or hemodynamic factors related to EV as the predictive parameters for recurrence: severe-type peri-esophageal collateral veins and large perforating veins of the esophagus [27], poor development of para-esophageal veins [28], the high velocity and branch type in the left gastric vein [29], and a posttreatment area of submucosal vessels in the cardia [16]. The present study reported that no significant relation between MML and EV recurrence exists, suggesting the importance of a local factor and not a systemic factor to predict post-treatment EV recurrence. A recent research has demonstrated the benefit of oral supplementation with BCAA on the prognosis of sarcopenia patients [8]. Although the frequency of MML was not significantlydifferent between patients with and without oral supplementation of BCAA in our study, the effect of such nutritional support should be prospectively investigated in the future to discover if it may contribute to suppressing the negative effect of MML in cirrhosis after variceal treatment.
There are some limitations to our study. The first is that it is not a prospectively performed study, and the sample size is relatively small. A further study with a large patient population is needed to validate our data. The second is that the substantial influence of MML may be elucidated by a comparison with control patients who have EV without any treatment. However, since the international consensus strongly recommends the application of primary/secondary prophylaxis for moderate/severe EV or bleeders, a prospective comparison may be difficult based on the ethical aspect.
Conclusion
In conclusion, MML is an independent prognostic factor after the eradication of EV in cirrhosis patients, and the mild PH exerts a constraining effect against the negative influence of MML. The data encourage us to maintain or enhance the muscle volume/power before the advancement of PH.
Competing and Conflicting Interests
Authors have no conflict of interest.
References
- Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol 38: 266-272 (2003).
- Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med 353: 2254-2261 (2005).
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 46: 922-938 (2007).
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 362: 823-832 (2010).
- Sarin SK, Kumar A, Angus PW, Baijal SS, Chawla YK, Dhiman RK, et al. Primary prophylaxis of gastroesophageal variceal bleeding: consensus recommendations of the Asian Pacific Association for the Study of the Liver. Hepatol Int 2: 429-439 (2008).
- Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB. Sarcopenia: etiology, clinical consequences, intervention, and assessment. Osteoporos Int 21: 543-559 (2010).
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39: 412–423 (2010).
- Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 31: 193-199 (2015).
- Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol 63: 131-140 (2015).
- Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Fujimoto Y, Ogawa K, et al. Muscle steatosis is an independent predictor of postoperative complications in patients with hepatocellular carcinoma. World J Surg 40: 1959-1968 (2016).
- Iritani S, Imai K, Takai K, Hanai T, Ideta T, Miyazaki T, et al. Skeletal muscle depletion is an independent prognostic factor for hepatocellular carcinoma. J Gastroenterol 50: 323-332 (2015).
- Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl 20: 640-648 (2014).
- Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 35: 519-524 (2002).
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol 53: 397-417 (2010).
- Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc 22: 1-9 (2010).
- Kondo T, Maruyama H, Kiyono S, Sekimoto T, Shimada T, Takahashi M, et al. Eradication of esophageal varices by sclerotherapy combined with argon plasma coagulation: effect of portal hemodynamics and longitudinal clinical course. Dig Endosc 28: 152-161 (2016).
- Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 9: 629-635 (2008).
- Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10: 166-173 (2012).
- Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46: 951–963 (2016).
- D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology 131: 1611–1624 (2006).
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol 44: 217-231 (2006).
- Zipprich A, Garcia-Tsao G, Rogowski S, Fleig WE, Seufferlein T, Dollinger MM. Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int 32: 1407-1414 (2012).
- Giannini EG, Savarino V, Farinati F, Ciccarese F, Rapaccini G, Marco MD, et al. Influence of clinically significant portal hypertension on survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Liver Int 33: 1594–1600 (2013).
- Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, et al. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 18: 1209-1216 (2012).
- Hiraoka A, Aibiki T, Okudaira T, Toshimori A, Kawamura T, Nakahara H, et al. Muscle atrophy as pre-sarcopenia in Japanese patients with chronic liver disease: computed tomography is useful for evaluation. J Gastroenterol 50: 1206–1213 (2015).
- Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eqhtesad B, et al. Post-liver transplantation sarcopenia in cirrhosis: A prospective evaluation. J Gastroenterol Hepatol 29: 1250–1257 (2014).
- Irisawa A, Obara K, Bhutani MS, Saito A, Shishido H, Shibukawa G, et al. Role of para-esophageal collateral veins in patients with portal hypertension based on the results of endoscopic ultrasonography and liver scintigraphy analysis. J Gastroenterol Hepatol 18: 309-314 (2003).
- Nakamura S, Murata Y, Mitsunaga A, Oi I, Hayashi N, Suzuki S. Hemodynamics of esophageal varices on three-dimensional endoscopic ultrasonography and indication of endoscopic variceal ligation. Digest Endosc 15: 289-297 (2003).
- Kuramochi A, Imazu H, Kakutani H, Uchiyama Y, Hino S, Urashima M. Color Doppler endoscopic ultrasonography in identifying groups at a high-risk of recurrence of esophageal varices after endoscopic treatment. J Gastroenterol 42: 219-224 (2007).