Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 6
Comparison of changes of fatigue and psycho-physical wellbeing in rheumatoid arthritis patients during add-on treatment with alfacalcidol or prednisolone
- Corresponding Author:
- Katarina Simic Pasalic
Institute of Rheumatolog
University of Belgrade Medical School
Belgrade
Serbia
E-mail: simicpasalickatarina@gmail.com
Abstract
The aim of this study was to investigate and compare effects of vitamin D analogue alfacalcidol (1αD3) or prednisolone add-on treatment to fatigue and psycho-physical well-being in patients with active RA. The study included 67 RA patients with active disease (DAS28 >3,2), despite standard methotrexate (MTX) therapy. We collected data on disease activity based on joints examination and erythrocyte sedimentation rate (DAS28 ESR). Psycho-psychical wellbeing assessed by patient reported outcomes (PRO): health assessment questionnaire (HAQ), short form 36 quality of life (SF-36Qol) and functional assessment of chronic illness therapy (FACIT F) questionnaires. At enrolment, patients were randomly assigned to three-month supplementation with 1 μg (group A1) or 2 μg (group A2) or 3 μg (group A3) of 1αD3 daily or prednisone (group C) 20 mg daily, for the first month and 10 mg afterwards, in addition to MTX. We found significantly improved functional status-HAQ-DI (p<0,05) and physical components of quality of life - PCSQol (p<0,05), in A2 group (N=19). Subscales of quality of life such as vitality, general health (p<0,01), social, pain and mental components of quality of life - MCSQol (p<0,05) significantly improved only in the same, A2 group, while fatigue improved in all 1αD3 treated patients, in contrast to negative change in group C. Upon completed treatment, we found disease activity highly significantly reduced in all four treatment groups (DAS28 p<0,01). Alfacalcidol add-on treatment has positive impact on fatigue and psycho-physical wellbeing in RA patients, particularly 2 μg daily, which was as effective as prednisolone in disease control with significant multifaceted favourable impact on fatigue and wellbeing.
Keywords
rheumatoid arthritis • alfacalcidol • patient reported outcomes
Introduction
Rheumatoid Arthritis (RA) is chronic autoimmune inflammatory disease which may cause joint damage, disability, various comorbidities and affect psycho-physical wellbeing [1]. Nowadays, due to huge advances in development of pharmacological treatment options, rheumatologists can provide almost full control of inflammation, prevention of joint damage and preservation of functional ability to RA patients, using treat to target strategy [2]. Modern rheumatology also appreciates patient`s perspective, so Patient Reported Outcomes (PRO) are implemented as outcome tool to estimate RA treatment effectiveness. Several PRO data indicate that, although inflammation is well treated, RA still has many deleterious consequences, however, patient-reported consequences of disease activity may differ from the assessment made by health professionals [3,4]. Health related quality of life questionnaire- SF-36 and health assessment disability index- HAQ-DI are widely used to assess health status. It can discriminate across many RA severity categories and can detect changes in early disease [5-7]. Quality of life assessment is a multi-dimensional concept which is used to describe individuals` perceptions, satisfaction and evaluation of different areas of life, providing a broader view of daily living activities and subjective wellbeing of any patient [8]. Up to 80% patients with RA report clinically relevant fatigue which may cause severe stress and decrease of quality of life. Few questionnaires validated for assessment of fatigue in RA are independent to impact of comorbidities [9]. Fatigue in RA is described as lack of motivation, energy, as unpredictable overwhelming tiredness [9-11]. This multifaceted health problem encompasses personal and emotional factors as well as clinical factors connected to the disease [12].
The discovery of the immunomodulatory and antitumor properties of D Hormone (DH) prompted researchers to investigate the possibility of its use as therapeutic agent for autoimmune and malignant diseases [13-15]. During the last several decades there were many investigations with an aim to synthesize hormone D analogs (VDR agonists) with the same biologic activity and even stronger anti-inflammatory properties, but lower blood calcium-increasing capacity [16]. One of steroidal VDR agonists, alfacalcidol (1αD3) differs from DH only by lacking 25(OH) group. Recently it has been shown that despite previous beliefs, it acts directly on inflammatory cytokine production without needing to be additionally hydroxylated at the C25 position [17].
D hormone acts within the Central Nervous System (CNS) as a neuro steroid with multiple actions (interacts with the synthesis and degradation of some neurotransmitters, has an important role in the regulation of several neurotrophic factors and supports brain’s antioxidative defence). Changes in DH availability to VDRs may have implications in brain function and mood [18]. Alfacalcidol showed beneficial effects on disease activity and immunoregulation in patients with active rheumatoid arthritis [14,15,19,20].
Aim of this study was to investigate influence of vitamin D analogue (alfacalcidol), as addon treatment, on fatigue and psycho-physical wellbeing in active rheumatoid arthritis patients, in comparison to prednisolone add-on treatment.
Materials and methods
Study population and protocol
The open label intervention prospective study was approved by the Ethics Committee (decision No 29/1-7) and the Medicines and Medical Devices Agency (decision No 515-04-0544-12- 2). Written informed consent was obtained from all patients prior to enrolment.
The study population consisted of 67 RA patients (46 females) on stable methotrexate (MTX 10- 25 mg/weekly) therapy of longer than 3 months’ duration prior to enrolment. RA diagnosis was established at least six months before the study, using ACR/EULAR 2010 criteria [21]. Patients were eligible for participation if RA was active (ESR-DAS28 was >3.2 on maximal tolerated stable MTX dose [22]. No other DMARD or CS treatment, nor D3 or calcium supplements use was allowed. Patients were advised to maintain a daily fluid intake of at least 1.5 l throughout the duration of the study. Patients were randomly assigned to administration of 1 μg/daily 1αD3 (group A1, N=17), 2 μg/daily 1αD3 (group A2, N=19), 3 μg/daily 1αD3 (group A3, N=16) treatment for three months or 20 mg prednisone daily for one month, followed by 10 mg prednisone daily for two months (group C, N=15), with an unchanged MTX dosing. Alfacalcidol was provided by investigator as gelatine capsules for oral administration (Alpha D3RTEVA, Serbia). Prednisone was provided by physician`s prescription. Alfacalcidol dosing was modified only in case of toxicity, i.e. disturbances in calcium levels in blood or urine (more than 2.65 mmol/l in blood or more than 0.3 g/DU in urine measured in two consecutive samples).
Study outcome and safety parameters
Demographic and clinical data were collected, blood samples taken, functional status, quality of life, fatigue and disease activity were assessed in all patients at study entry and after 3 months of treatment. Disease activity was assessed by ESR DAS 28 score, calculated from erythrocyte sedimentation rate (ESR), number of tender (TJC) and swollen joints (SJC) and the patients` assessment of disease activity (PVAS) based on answer to the question “"Given the overall impact of your arthritis to you, how are you feeling today?" Rated on a scale of 100 mm, answer may vary from 0 mm - very good, to 100 mm - very poor, i.e. lower score reflected better state [23]. Functional status was assessed by HAQ-DI self-reported measurement of disability in RA, containing questions about ability of patients to perform 20 activities of daily living, classified in 8 categories, provided in Serbian language, with answers offered range from 0-no difficulty to 3-unable to perform [5]. Quality of life was assessed using SF-36Qol questionnaire in Serbian language which was self-administered. Items in SF-36Qol consists of 36 questions in 10 sections which were constructed using the Likert method of summated ratings. Answers to each question were scored, then these scores were summed to produce raw scores to each health concept which were then transformed to 0-100 scale. Scoring algorithms could then be applied to produce the result, given in 8 subscales: physical functioning (PF), role-physical (RP), bodily pain (BP), general health (GH), vitality (V), social functioning (SF), role-emotional (RE) and mental health (MH) and 2 summary measures which aggregated all 8 subscales: Physical Health (PCS) and Mental Health (MCS) domains of quality of life. For quality assessment of SF-36 one need to respond to least 50% of questions in each section. Offered estimates vary 1-5. The numbers represent estimates correspond to different variables offered, depending on the question being asked. Answers to some questions are reversed. Larger summation means a better quality of life [6,23]. Fatigue was measured using functional assessment of chronic illness therapy fatigue scale FACIT-F, a questionnaire in Serbian language, which consists of 13 questions (general, physical, mental, vigour domains), one should answer at least 7/13 questions in order to obtain good quality assessment, scores range from 0-52, with greater score reflecting less fatigue [9]. Safety follow-up visits were conducted monthly. Patients were assessed for any relevant change in clinical status, weight, arterial pressure, electrocardiogram, haematology (Coulter HmX haematology analyser) and biochemistry tests glucose, creatinine, uric acid, alkaline phosphatase, albumin, AST, ALT, calcium, ionized calcium and daily urine calcium excretion (spectrophotometry-Ilab 300+).
At the end of the treatment period, clinical and laboratory data obtained were compared to baseline to assess efficacy and safety of the four different treatments in term of changes in fatigue and psycho-physical wellbeing pre/post treatment and to each other.
Statistical analysisStatistical analysis was performed using the SPSS 20 package, data are presented as mean±SD (min-max). Subgroup changes of variables pre and post treatment were analysed by paired t-test or Wilcoxon test. The subgroup differences were assessed by independent t-test, ANOVA or chi square test, as appropriate and least significant difference (LSD) method, post hoc. Correlations were assessed by Spearman’s test; p<0,05 was considered statistically significant in all analyses.
Results
Demographic characteristics of patient population
All patients completed three months’ study period. Out of 67 RA patients included, 46 (68,65%) were females, average age was 56,24 ± 12,423 (23-83), disease duration 7,71 ± 6,68 (1-33) years, MTX dose 15,41 ± 3,28 (10-25) mg/weekly and MTX use of 5,67 ± 5,899 (0,5- 15) years, mean disease activity ESRDAS28 was 5,58 ± 0,905 (3,22-7,55) at the baseline. At enrolment DAS28 was >5,1 in 47 (70,14%) and DAS28>3,2 in 20 (29,85%) patients. Average HAQ-DI was 0,57 ± 0,516 (0-1,85) while average score of physical components of quality of life was: PCSQol 36,54 ± 8,033 (12,1-53,1), mental components of quality of life MCSQol 47,04 ± 9,8536 (20,5-63,8), FACIT-F 17,47 ± 8,842 (5-26). The patients randomized in four different treatment arms for three months, were fully comparable and their demographic and clinical characteristics are presented in Table 1.
Table 1. Comparison of demographic, disease, clinical and patient reported outcomes characteristics among study subgroups (A1, A2, A3 and C).
Variable |
A1 | A2 | A3 | C | p |
|---|---|---|---|---|---|
| N(%M) | 17/29.4% | 19/31.5% | 16/31.2% | 15/33.3% | 0.881 |
| M/F ratio | 5/12 | 7/12 | 5/11 | 4/11 | 0.926 |
| Age(y) | 57.94 ± 12.279 | 53.79 ± 12.012 | 53.06 ± 10.909 | 60.67 ± 13.751 | 0.257 |
| RA (y) | 9.82 ± 8.149 | 7.63 ± 7.12 | 5.63 ± 4.334 | 7.53 ± 6.791 | 0.374 |
| MTX(y) | 7.82 ± 2.112 | 6.71 ± 1.567 | 5.31 ± 2.223 | 6.89 ± 4.114 | 0.232 |
| MTX (mg/w) | 14.09 ± 1.988 | 14.45 ± 3.533 | 16.87 ± 3.476 | 16.66 ± 2.937 | 0.061 |
| Comorb.(n%+/N) | 6(35.29)/17 | 9(47.36)/19 | 6(37.5)/16 | 7(46.66)/15 | 0.690 |
| PsDMARD(n%+/N) | 8(47.05)/17 | 12(63.15)/19 | 11(68.75)/16 | 9(60)/15 | 0.978 |
| PbDMARD(n%+/N) | 1(5.88)/17 | 1(5.26)/19 | 1(6.25)/16 | 1(6.66)/15 | 0.998 |
| BMI (kg/m2) | 22.2 ± 1.87 | 21.9 ± 2.34 | 23.1 ± 2.76 | 22.7 ± 2.98 | 0.542 |
| RF (n%+/N) | 15(88.23)/17 | 13(68.42)/19 | 14(87.5)/16 | 12(80)/15 | 0.567 |
| ACPA(n%+/N) | 3(17.64)/17 | 7(36.84)/19 | 4(25)/16 | 4(26.66)/15 | 0.588 |
| CRP (mg/l) | 8.25 ± 14.876 | 28.41 ± 28.162 | 22.42 ± 26.322 | 23.55 ± 24.92 | 0.093 |
| ESR (mm/h) | 28.18 ± 20 | 39.84 ± 23.735 | 42.88 ± 28.98 | 43.73 ± 15.895 | 0.188 |
| 25(OH)D3 (ng/ml) | 31.88 ± 13.573 | 28.97 ± 9.914 | 28.02 ± 14.118 | 34.02 ± 15.741 | 0.237 |
| DAS28 | 5.28 ± 0.874 | 5.81 ± 0.891 | 5.73 ± 0.869 | 5.86 ± 0.789 | 0.058 |
| PVAS (mm) | 52.51 ± 16.070 | 51.89 ± 17.129 | 51.73 ± 14.992 | 50.40 ± 15.624 | 0.98 |
| HAQ DI | 0.42 ± 0.405 | 0.65 ± 0.599 | 0.60 ± 0.47 | 0.69 ± 0.571 | 0.389 |
| PCS Qol | 40.66 ± 6.889 | 34.44 ± 7.881 | 37 ± 8.498 | 34.47 ± 7.526 | 0.06 |
| MCS Qol | 47.69 ± 7.309 | 47.03 ± 11.287 | 49.75 ± 8.257 | 43.04 ± 11.363 | 0.290 |
| FACIT F | 17.24 ± 1.492 | 15.65 ± 3.603 | 18.9 ± 3.198 | 18 ± 2.579 | 0.633 |
A1: group treated with 1µg 1αD3; A2: group treated with 2µg 1αD3; A3: group treated with 3µg 1αD3; C: group treated with prednisone; N:number of patients; M: men; F: women; y: years; RA: Rheumatoid Arthritis; MTX: Methotrexate weekly dose ; MTX (y): duration of MTX treatment; DAS28: RA activity; ESR: Erythrocyte Sedimentation Rate; CRP: C Reactive Protein; 25(OH)D3: serum level of vitamin D; RF: Rheumatoid Factor positive; ACPA: Anti-Citrullinated Protein Antibodies positive; psDMARD: previous synthetic DMARD treatment; pbDMARD: previous biologic DMARD treatment; BMI: Body Mass Index; comorb: additional chronic disease present; pVAS: patient assessment of RA activity; HAQ-DI: functional ability; PCSQol: physical component summary quality of life; MCSQol: mental component summary quality of life; FACIT-F: Functional Assessment of Chronic Illness Therapy-Fatigue
Influence on fatigue and psycho-physical wellbeing
After three months of study treatment, all patients reported overall statistically significant improvement as assessed by patient VAS assessment of disease activity (PVAS), in group A1 (52,58 ± 16,07 vs. 25,06 ± 8,4, p<0,01), A2 (51,84 ± 17,129 vs. 24,84 ± 10,205, p<0,01), A3 (51,73 ± 14,992 vs. 24,6 ± 9,545, p<0,01), as well as in the group C (50,43 ± 15,624 vs. 27,53 ± 10,569, p<0,01, paired t-test), respectively. Exploring PVAS, we found no difference in PVAS change between four different treatments (27,52 ± 9,361 vs. 27,05 ± 14,35 vs. 27,3 ± 8,311 vs. 22,86 ± 7,989, p=0,586, ANOVA).
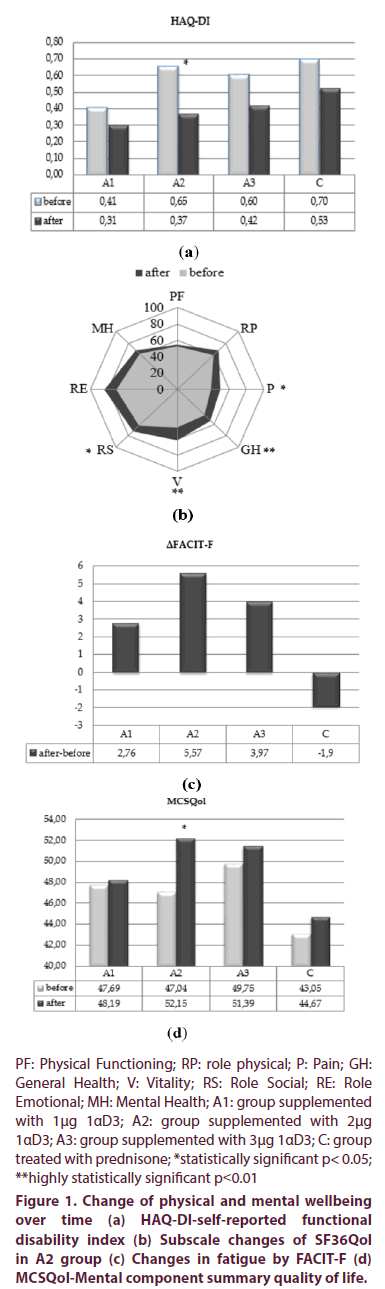
Functional ability assessed by HAQ-DI improved in all treatment’s regimens after three months’ treatment as shown in Figure 1a. Only in A2 group this improvement was statistically significant (0,65±0,589 vs. 0,37±1,032, p<0,05). Clinically significant reduction of HAQ-DI of >0,22 was observed in the same therapeutic group.
Physical components score of quality of life raised to significantly higher values in patients treated with 2 μg 1αD3 and prednisone: in A2 (34,45 ± 7,881 vs. 37,89 ± 7,957, p<0,05, paired-test), and in group C (33,47 ± 7,526 vs. 36,9 ± 8,903, p<0,05, paired t-test), while A3 group improved PCSQol score, but not significantly.
Subscale SF36Qol score changes were also analyzed. Neither one subscale item changed significantly in A1 group. Group of patients treated with 2 μg 1αD3 (A2) for three months, significantly improved general health (44,38 ± 13,25 vs. 54,32 ± 16,075, p<0,01), pain (39,4 ± 13,755 vs50,0 ± 16,5, p<0,05), vitality (46 ± 17,838 vs. 61,85 ± 18,044, p<0,01) and role social (62,61 ± 25,224 vs72,35 ± 22,661, p<0,05, paired t test) shown in Figure 1b. In the same group, subscale item of mental health pre-post treatment change was near to statistical significance (61,57 ± 21,093 vs. 68,21 ± 21,017, p=0,052, paired t-test). The only statistical significant improvement in group A3 was found in subscale role physical (48,63 ± 30,772 vs. 64,6 ± 22,302, p<0,05, paired t test) and in group C only subscale pain (35,75 ± 25,729 vs. 49 ± 27,828, p<0,01, paired t test).
Fatigue assessed by FACIT-F, improved in all three alfacalcidol treatment regiments, while worsened in prednisone treated group, yet, neither one difference was statistically significant pre-post treatment, nor the opposite changes, compared to each other (ΔFACIT-F). Data is shown in Figure 1c.
Mental components score got better in all study groups, yet, significantly improved in A2 group only (47,04 ± 11,287 vs. 52,15 ± 10,672, p<0,05, paired t-test) as showed in Figure 1d.
We examined correlations between activity of RA and indices of quality of life. At the end of treatment period significant negative correlation was found between DAS28 and MCSQol, also GH and MH in group of patients treated with 1 μg 1αD3 (p<0,01, Spearman test), also between DAS28 and PCSQol in group treated with 1αD3 2 μg. Significant negative correlation was found in patients treated with prednisolone, between DAS28 and MCSQol, DAS28 and PCSQol (p<0,01, both, Spearman test).
Influence on disease activity
Clinical efficacy indices showed marked improvement in term of ESR DAS28 in all therapeutic regimens, with highly statistically significant reduction achieved in all treatment groups (p<0,01, paired t-test). In order to further explore the efficacy of different treatment regimens in term of disease activity, we used EULAR DAS28 response model [24]. Change in disease activity during 12- week study, in four treatment regimens was A1 vs. A2 vs. A3 vs. C, p<0,05, ANOVA, yet there was no difference in A2 vs. C (p=0,437, post hoc LSD). Also, there was no significant difference in the frequency of patients with good DAS28 EULAR response in subgroups A2, A3 and C (p=0,532, χ2).
Influence on safety parameters
Patients reported overall good tolerability of study treatments, no serious adverse events or any laboratory nor clinical adverse event was observed.
Serum calcium and ionized calcium follow up were particularly of interest, both remained unchanged, in all treatment groups, while daily urine calcium excretion was significantly raised in group treated with alfacalcidol 2 μg and 3 μg, in the latter exceeding slightly upper range normal (ULN) of 0,3 g/DU (in A2 0,179 ± 0,0687 vs. 0,265 ± 0,090, p<0,01, in A3 0,133 ± 0,0466 vs. 0,321 ± 0,1093, p<0,01, paired t-test). Alfacalcidol daily dose was decreased to 1 μg daily, for a week in four patients, due to increase in daily calciuria, registered in two patients in A3 group, two weeks, and eight weeks apart from the start of study. After daily dose reduction, calciuria normalized, study treatment was continued as before.
Serum glucose levels raised pre-post treatment in C group by 1,7 mmol/l (about 40% compared to baseline), yet not above ULN, in contrast to 1αD3 treated patients, who had about 2% lower glucose levels.
Discussion
Several studies have confirmed the therapeutic potential of 1αD3 in autoimmune diseases, chronic arthritis, osteoporosis, sarcopenia, over the past decades [14,20,25-29]. Data from our study additionally support the evidence of alfacalcidol treatment efficacy in RA, as we got highly significant improvement of disease activity assessed by DAS28 ESR, in all alfacalcidol treated patients.
Nowadays, lowering of disease activity should not satisfy as an only treatment goal, nor outcome, in approach to RA treatment [2]. Treatment of RA is effective not only if provides reduction of disease activity, but also improvement of functional status and quality of life [30-32]. Disease modifying drugs, both synthetic and biologic, significantly reduce HAQ-DI and the reduction is maintained for 2-5 years [3]. In our study, improvement of functional ability occurred in all treatment groups, the most prominent in A2 treatment group (ΔHAQDI=- 0,28), similar to such achieved in combined MTX and adalimumab treatment in RA patients (ΔHAQ-DI=-0,34), in whom some of patients failed to respond to previous antiTNF [33]. Majority of investigations suggest an inverse correlation between serum concentrations of 25(OH)D3 and quantitative measures of disease activity, pain and disability in RA patient: the lower serum vitamin D status, the more active disease with worse pain and disability is [34- 37]. Seasonal vitamin D elevation influence on RA activity is present in northern and in southern Europe [34]. There was low prevalence of vitamin D insufficiency in our group of patients (14% with 25(OH)D3<20 ng/ml) and no correlation between serum 25(OH)D3 and change of disease activity, functional ability, nor with quality of life. Corticosteroid monotherapy was associated with trend to lower vitamin D levels than in patients treated with DMARD or in patients without RA treatment [36]. Threemonth prednisolone treatment in our study resulted in highly significant reduction of serum level of 25(OH)D3, from 34,02 to 21,93 ng/ ml, which is not desirable result, as it is closely negatively related to at least RA disease activity, according to some studies [36]. On the contrary, anti-inflammatory effects of all three alfacalcidol treatment regimens resulted in increased levels of serum 25(OH)D3. This is probably due to reduced need for serum substrate for D hormone synthesis in target tissues, because of effective ongoing anti-inflammatory treatment [29].
A very important work in area of quality of life was published in 2012, which investigated changes of individual components of quality of life in RA, psoriatic arthritis (PSA) and psoriasis under MTX therapy and/or anti-TNF-α drugs [38]. It was shown that the components with the lowest values, had the most expressive improvement under the influence of successful treatment of the underlying disease. The results of our study showed abundant favourable influence of three month alfacalcidol treatment (2 μg exceptionally) on global physical and mental quality of life, also on subscale general health, pain, vitality and role social in contrast to group of RA patients receiving prednisolone (mean 13,3 mg daily) treatment, in whom at the same time disease activity reduction was accompanied by significant improvement of subscale SF36Qol item pain, only, with some improvement in physical functioning and worsening of fatigue.
A very important work in area of quality of life was published in 2012, which investigated changes of individual components of quality of life in RA, psoriatic arthritis (PSA) and psoriasis under MTX therapy and/or anti-TNF-α drugs [38]. It was shown that the components with the lowest values, had the most expressive improvement under the influence of successful treatment of the underlying disease. The results of our study showed abundant favourable influence of three month alfacalcidol treatment (2 μg exceptionally) on global physical and mental quality of life, also on subscale general health, pain, vitality and role social in contrast to group of RA patients receiving prednisolone (mean 13,3 mg daily) treatment, in whom at the same time disease activity reduction was accompanied by significant improvement of subscale SF36Qol item pain, only, with some improvement in physical functioning and worsening of fatigue.
Recent study based on the data from BSRBR registry (N=2652), reported that reduction of pain, inflammation and improvement in fatigue were not associated, even in anti TNFα treated RA patients [42]. They found that improvement of fatigue under anti TNFα treatment was associated with good mental health, no history of depression, no use of steroids, low disability and female sex. They used subscale SF36Qol vitality as a measure of fatigue, considering change of >10 U as clinically significant. We also found such clinically significant change pre-post treatment in our A2 group (Δvitality=16 U), supporting alfacalcidol`s capacity for improvement of wellbeing. Also, in order to achieve downstream improvement of fatigue in RA patients, it was suggested to modulate pain, functional disability and mental health, all of which observed to be positively influenced by alfacalcidol treatment in our investigation (Figure 1).
All the benefits in fatigue, psycho-physical wellbeing and disease activity of alfacalcidol supplementation in active RA, did not resulted from correcting vitamin D deficiency, since the patients were already vitamin D replete (Table 1). We assume that direct effect of alfacalcidol on the nuclear VDRs in different tissues (muscles, immune cells) produced such effects. Alfacalcidol also can act directly on the VDRs in the central nervous system to increase the level of expression of genes for tryptophan synthetase, choline acetyl transferase, thereby increase availability of dopamine, noradrenaline, adrenaline and acetylcholine [17,43]. The lack of these neurotransmitters lies in the pathophysiologic basis of many psychological and psychiatric disorders [43,44]. One on the recent studies of barriers and facilitators of overcoming fatigue in RA showed importance of mental health being active, making exercise easy, reaching for balance, receiving support to be physically active and dealing with RA [45-47]. Mental health should be routinely judged and managed alongside chronic disease as RA, to optimize health outcomes as mental health is responsive to vitamin D supplementation, also VDR analogue alfacalcidol supplementation could be used, as supported by our findings.
Conclusion
The potential of alfacalcidol for disease and psycho-physical modulation in active RA, derived from our study, can serve as a starting point for further work in this field, through conduction of double-blind placebo-controlled trial, to get more accurate data. The main drawbacks of our study are the absence of a placebo group, short lasting follow up, which were based on the ethical factor in the design of it. However, in vitamin D replete patients with active RA, on a stable dose of MTX, it was shown that alfacalcidol, particularly either 2 μg daily, can improve disease activity as well as indices of fatigue, psychophysical wellbeing, altogether with good safety profile, in contrast to effects of prednisolone use.
Acknowledgments
We are grateful to all patients who accepted to participate in this study.
Disclosures of conflict of interest
None
Source of funding
None
References
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 376(9746), 1094–1108 (2010).
- Smolen JS, Aletaha D, Bijlsma JW et al. Treating rheumatoid arthritis to target: recommendations of international task force. Ann. Rheum. Dis. 69(3), 631–637 (2010).
- Pollard L, Choy EH, Scott DL. The consequences of rheumatoid arthritis:quality of life measures in the individual patient. Clin. Exp. Rheumatol. 23, S43–S52 (2005).
- Studenic P, Radner H, Smolen JS et al. Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis. Rheum. 64(9), 2184–2123 (2012).
- Bruce B, Fries JF. Stanford Health Assessment Questionnaire:a review of its history, issues, progress and documentation. J. Rheumatol. 30(1), 167–178 (2003).
- Ware JE, Kosinski M. Interpreting SF-36 Summary Health Measures: A Response. Quality of Life Research. 10(5), 405–413 (2001).
- Marra CA, Woolcott, JC, Kopec JA et al. A comparison of generic, indirect utility measures (the HU12, HU13, SF-6D and EQ-5D) and disease specific instruments (the RAQoL and the HAQ) in rheumatoid arthritis. Soc. Sci. Med. 60(7), 1571–1582 (2005).
- Ruta DA, Hurst N, Kind P et al. Measuring health status in British patients with rheumatoid arthritis: reliability, validity and responsiveness of the short form 36-item health survey (SF-36) Br. J. Rheumatol. 37(4), 425–436 (1998).
- Cella D, Yount S, Sorensen M et al. Validation of the functional assessment of chronic illness therapy fatigue scale relative to other instrumentation in patients with rheumatoid arthritis. J. Rheumatol. 32, 811–819 (2005).
- Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J. Rheumatol. 23, 1407–1417 (1996).
- Feldthusen C, Björk M, Forsblad-d'Elia H, Mannerkorpi K. Perception, consequences, communication, and strategies for handling fatigue in persons with rheumatoid arthritis of working age-a focus group study. Clin. Rheumatol. 32(5), 557–566 (2013).
- Rinke HS, Gjesdal CBG, Markussen H et al. Patient reported fatigue in patients with rheumatoid arthritis who commence biologic therapy: a longitudinal study. Peer. J. 7, e6771 (2019).
- De Luca HF. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80(6), 1689S-1696S (2004).
- De Luca HF. Vitamin D: The vitamin and the hormone. Fed. Proc. 2211–2219 (1974).
- Andjelkovic Z, Vojinovic J, Pejnovic N et al. Disease modifying and immunoregulatory effects of high oral dose1α(OH)D3in rheumatoid arthritis patients. Clin. Exp. Rheumatol. 17, 59–62 (1999).
- Antico A, Tampoia M, Tozzoli R et al. Can supplementation with vitamin D reduce the risk of modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 12(2), 127–136 (2012).
- Eduardo-Canosa S, Fraga R, Sigueiro R et al. Design and synthesis of active vitamin D analogs. J. Steroid. Biochem. Mol. Biol. 121(1-2), 7–12 (2010).
- Vojinovic J, Zivanovic-Ranic T, Sefik-Bukilica M et al. Alphacalcidol (D hormone analog) modulate inflammatory cytokine production without conversion to calcitriol. Ann. Rheum. Dis. 71(suppl 3), 672 (2012).
- Kiraly SJ, Kiraly MA, Hawe RD et al. Vitamin D as neuroactive substance: review Scientific. World. J. 6, 125–139 (2006).
- Hein G, Oelzner P, Vitamin D metabolites in rheumatoid arthritis: findings- hypothesis-consequencies. J. Rheumatol. 59(S1), 28–32 (2000).
- Yamauchi Y. A double blind trial of alfacalcidol on patients with rheumatoid arthritis (RA). Ryumachi. 29, 11–24 (1989).
- Aletaha D, Neogi T, Silman J A et al. 2010 Rheumatoid Arthritis Classification Criteria. Ann. Rheum. Dis. 69, 1580–1588 (2010).
- Smolen JS, Breedveld FC, Schiff MH et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford). 42(2), 244–257 (2003).
- Revecki DA, Leidy KN. Questionnaire scaling: models and issues. In: Staquet MJ, Haus RD, Fayers PM ed. Quality of life assessment in clinical trials, methods and practice. New York: Oxford University Press: pp, 157–168 (1998).
- Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Rheum. Dis. Clin. North. Am. 35(4), 745–viii (2009).
- Vojinovic J. Vitamin D receptor agonists' anti-inflammatory properties. Annals of the New York Academy of Sciences. 1317(1), 47–56 (2014).
- Zold E, Szodolay P, Gaal J et al. Vitamin D deficiency in undifferentiated connective tissue disease Arthritis. Res. Ther. 10(5), R123 (2008).
- Ringe J.D, Dorst A, Faber H et al. Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid induced osteoporosis. Rheumatol. Int. 24(2), 63–70 (2004).
- Gaal J, Lakos G, Szodoray P et al. Immunological and clinical effect s of alphacalcidol in patients with psoriatic arthropathy: results of an open follow up pilot study. Acta. Derm. Venereol. 89(3), 140–144 (2009).
- Schacht E. Rationale for treatment of involutional osteoporosis and for prevention and treatment of corticosteroid induced osteoporosis with alfacalcidol. Calcif. Tissue. Int. 65(4), 317–327 (1999).
- Kirwan JR, Buttgereit F. Symptom control with low-dose glucocorticoid therapy for rheumatoid arthritis. Rheumatology (Oxford). 51(Suppl 4), iv14–iv20 (2012).
- Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 70(2), 121–145 (2010).
- Grigor C, Capell H, Stirling A et al. Effect of treatment strategy of tight control for rheumatoid arthritis (the TICORA study): A single blind randomised controlled trial. Lancet. 364(9430), 263–269 (2004).
- Bennet AN, Peterson, P, Zain A et al. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients including comparison of patients with and without previous anti-TNF exposure. Rheumatology (Oxford). 44(8), 1026–1031 (2005).
- Cutolo M, Otsa K, Laas K et al. Circannual vitamin D serum levels and disease activity in rheumatoid arthritis: Northern versus Southern Europe. Clin. Exp. Rheumatol. 24, 702–704 (2006).
- Rossini M, Maddali Bongi S, La Montagna G et al. Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis. Res. Ther. 12(6), R216 (2010).
- Raczkiewicz A, Kisiel B, Kulig M et al. Vitamin D status and its association with quality of life, physical activity and disease activity in rheumatoid arthritis patients. J. Clin. Rheumatol. 21(3), 126–130 (2015).
- Vojinovic J, Tincani A, Sulli A et al. European multicenter pilot survey to assess vitamin D status in rheumatoid arthritis patients and early development of a new Patient Reported Outcome questionnaire (D-PRO). Autoimmun. Rev. 16, 548–554 (2015).
- Strand V. Singh JA. Newer biological agents in rheumatoid arthritis:impact on health-related quality of life and productivity. Drugs. 70(2), 121–145 (2010).
- Hewlet S, Cockshott Z, Byron M et al. Patients perceptions of fatigue in rheumatoid arthritis: overwhelming, uncontrollable, ignored. Arthritis. Rheum. 53(5), 697–702 (2005).
- Smolen JS, Beaulieu A, Rubbert-Roth A et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomized trial. Lancet. 371(9617), 987–997 (2008).
- Yount S, Sorensen MV, Cella D et al. Adalimumab plus methotrexate or standard therapy is more effective than methotrexate or standard therapy alone in the treatment of fatigue in patients with active, inadequately treated rheumatoid arthritis. Clin. Exp. Rheumatol. 25, 838–846 (2007).
- Druce KL, Jones GT, Macfarlane GJ et al. Patients receiving anti TNF therapies experience clinically important improvements in RA related fatigue: results from the British Society for Rheumatoid Arthritis. Rheumatology. 54(6), 964–971 (2015).
- Kalueff A.V, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr. Opin. Clin. Nutr. Metab. Care. 10(1), 12–19 (2007).
- Strumpf WE, Sar SA, Clark M et al. Brain target sites for 1,25-dihydroxyvitamin D3. Science. 215(4538), 1403–1405 (1982).
- Feldthusen C, Mannerkorpi K. Factors of importance for reducing fatigue in persons with rheumatoid arthritis: a qualitative interview study. BMJ. Open. 9(5), e028719 (2019).
- Matcham F, Norton S, Scott DL et al. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis:secondary analysis of a randomized controlled trial. Rheumatology. 55(2), 268–227 (2016).