Research Article - Interventional Cardiology (2022)
Association of MMP9 Polymorphism with Myocardial Infarction in Pakistani Population
- Corresponding Author:
- Arslan Habib
Laboratory of Molecular Immunology,
School of Life Sciences, Fudan University,
Shanghai,
China,
E-mail: 20110700169@fudan.edu.cn
Received date: 28-Mar-2022, Manuscript No. FMIC-22-58661; Editor assigned: 30-Mar-2022, PreQC No. FMIC-22-58661 (PQ); Reviewed date: 18-Apr-2022, QC No. FMIC-22-58661; Revised date: 25-Apr-2022, Manuscript No. FMIC-22-58661 (R); Published date: 02-May-2022, DOI: 10.37532/1755-5310.2022.14(S10).229
Abstract
Background: Myocardial Infarction (MI) is the most common type of coronary heart disease. The current study was conducted to identify the susceptibility of the MMP-9 gene among families affected by myocardial infarction in the Pakistani population.
Methods: A family clustering study based on 5 families having myocardial infarction patients was conducted. Blood samples from patients and their family members were collected for further genetic analysis. Patients were with a mean BMI (Body Mass Index) of (30.2 ± SD) kg/m2. The mean age for diagnosis of disease was (50 ± SD) years. Genomic DNA was isolated from the blood through manual extraction. Primers were optimized and genotyping was done by Polymerase Chain Reaction (PCR) which was followed by DNA sequencing and Restriction Fragment Length Polymorphism (RFLP).
Results: As a result of polymorphism, A into G and C into T conversions were identified on rs17576 and rs3918242 polymorphic sites on the MMP-9 gene respectively.
Conclusion: In conclusion smoking, hypertension, diabetes and polymorphism of rs17576 and rs3918242 were significantly associated with the onset of MI in the Pakistani population and males were at higher risk. Further studies should be conducted on large scale to evaluate the association of MMP-9 polymorphism with MI.
Keywords
Family clustering •Myocardial infarction• MMP-9 • polymorphism
Introduction
Myocardial Infarction (MI) is the most common type of Coronary Heart Disease (CHD) and a major cause of death all around the world [1]. MI takes place whenever there is a blockage in the coronary artery and other arteries that supply blood to heart tissues that cause a severe reduction in the blood flow and hence become infarcted [2]. The prevalence of the disease approaches 3 million people worldwide [3]. For gender, the prevalence of MI in men was about three times higher than that for women. The incidence of MI in Asian countries including Pakistan is higher in younger with 45 years of age than older with 60 years of age both men and women [4]. Both modifiable and non-modifiable risk factors are associated with MI [5]. The modifiable risk factors include smoking, diabetes, hypertension, air pollution, cholesterol and high- density lipoprotein level [6]. Non-modifiable risk factors include sex, age and positive family history [5]. Genome-wide association studies have found the association of 27 genetic variations with the increased risk of myocardial infarction [7]. Genes reported to be associated with MI are PCSK9, SORT1, MIA3, WDR12, MRAS, PHACTR1, LPA, TCF21, ZC3HC1, CDKN2A, 2B, APOA5, SMG6, LDLR, SLC5A3, MRPS6, KCNE2 and most of these genes are in that region which has not been previously linked with coronary artery disease [8]. Many studies highlighted the importance of MMP9 concerning myocardial infarction. The human MMP9 gene is located in the chromosomal region 20q11.2–q13.1 [9]. Genetic variations in the promoter region enforce allele-specific effects on the gene expression of MMP-9 [10]. Particularly, the -1562C/T polymorphism has been reported to increase the expression of the MMP-9 gene and was associated with the onset of MI [11]. Other than this, R279Q and R668Q polymorphism of the MMP-9 gene is also linked with the onset of MI [12]. With this, our goal was to investigate whether rs17576 (R279Q) and rs3918242 (R668Q) polymorphism were related to the development of MI and the association of risk factors including smoking, hypertension and diabetes with the onset of MI and their linkage in certain families of Pakistani population. Identification of the specific mutations in the MMP9 gene will be helpful to assess the risk of myocardial infarction before the onset of the disease.
Methodology
The present study was ethically approved by the Board of Advance Research, Department of Zoology, GCU Lahore. A total of five families (39 individuals) with the age range of 5 to 75 years were included in this study. Written consent was taken from all participants (above 18 years) and minors (under 18 years) from their parents/guardians included in this study. A pre-informed consent was signed by all the subjects. Blood samples for the family clustering study were collected from those families, in which one or two members were affected with myocardial Infarction. Blood samples and demographic data were taken from different Districts of Punjab (Lahore, Bahawalpur and Khanewal) Pakistan. Following World Health Organization (WHO) criteria all the patients were clinically diagnosed by the physician. Demographic data were recorded using a questionnaire that included: BMI, Family History, Blood Pressure, Age, Sex, Gender, Environmental Factors and Cholesterol. A total of five families (39 individuals) with an age range from 5 to 75 years were included in this study. DNA was extracted using the phenol/chloroform manual method. All the DNA samples were quantified (concentration) and qualified (purity) on Nano-drop. Single Nucleotide Polymorphisms (SNPs) in MMP9 gene were identified by using literature searches as well as public SNP databases. For genotyping, the PCR-RFLP method was selected by using a thermocycler instrument. The total reaction mixture of 12 µl with 1 µl of DNA sample, 0.75 µl of forward primer and 0.75 µl of reverse primer, 4 µl of PCR Master Mix and 5 µl of Diethyl Pyrocabonate (DEPC). We used the following two sets of primers, sense: 5′ATGGGTCAAAGAACAGGA-3′, antisense: 5′GGTAGACAGGGTGGAGG-3′ and sense: 5′GCCTGGCACATAGTAGGCCC-3′,antisense: 5′CTTCCTAGCCAGCCGGCATC-3′ [13]. The conditions for PCR cycles were as follows: after heating the lid, Initial denaturation was set for 5 minutes at 95°C, 35 cycles were set for the denaturation step for 45 seconds at 95°C, gradient or annealing temperature for these primers was 57.5°C for 45 seconds, at 72°C elongations were set for 30 seconds, then final elongation was set for 10 minutes at 72°C and finally storage at 4°C. The amplified PCR product was digested by SmaI and SphI restriction endonucleases (thermo-scientific). SmaI yielded fragments of 243 bp, 145 bp and 85 bp. SphI yielded fragments of 369 bp, 209 bp and 131 bp. The online progeny tool was used to draw family pedigrees.
Statistical analysis
The online progeny tool was used to draw family pedigrees. Hardy Weinberg Equilibrium was also applied. The demographic data were presented in percentage form. Genotype and allelic frequencies were calculated by SHEsis available online (http://analysis.bio-x. cn/SHEsisMain.htm). Fisher’s tests were also applied.
Results
Blood samples of five families (39 individuals) with a positive family history of myocardial infarction were collected from the areas of Lahore, Bahawalpur and Khanewal Punjab, Pakistan. Among thirty-nine individuals of the diseased families, there were five probands (affected by MI), one deceased, two parents, three siblings, eight members were outside of families, thirteen first degree relatives and seven-second degree relatives. There were eighteen females and twenty-one males who participated in this study. The ratio of males was high as compared to females. The mean age of probands was (50.83 ± SD) years whereas; the mean BMI of the probands was (30.20 ± SD) Kg/m2. The clinical characteristics of the families are presented in Table 1A and other clinical characteristics of diseased patients which include environmental factors and other complications associated with MI presented in Table 1B.
| Sr. No | Code | Gender (M/F) | Age (Years) | BMI (Kg/m2) | Category | Diseased (Diagnosed) |
|---|---|---|---|---|---|---|
| MI Family=MI 1 | ||||||
| 1 | F1M1* | M | 54 | 30.3 | Ob | Diseased |
| 2 | F1M2 | F | 50 | 39.1 | Ob | Healthy |
| 3 | F1M3 | M | 22 | 23.8 | N | Healthy |
| 4 | F1M4 | M | 16 | 22.2 | N | Healthy |
| 5 | F1M5 | M | 28 | 30.1 | Ob | Healthy |
| 6 | F1M6 | F | 30 | 28 | Ow | Healthy |
| 7 | F1M7 | M | 35 | 31.4 | Ob | Healthy |
| 8 | F1M8 | F | 11 | 35.9 | Ob | Healthy |
| MI Family=MI 2 | ||||||
| 9 | F2M1 | F | 75 | 28.4 | Ow | Healthy |
| 10 | F2M2* | M | 56 | 29 | Ow | Diseased |
| 11 | F2M3* | M | 52 | 30.9 | Ob | Diseased |
| 12 | F2M4 | F | 45 | 23.1 | N | Healthy |
| 13 | F2M5 | F | 42 | 27.6 | Ow | Healthy |
| 14 | F2M6 | F | 12 | 21.5 | N | Healthy |
| 15 | F2M7 | M | 15 | 22 | N | Healthy |
| 16 | F2M8 | M | 34 | 24.2 | N | Healthy |
| 17 | F2M9 | F | 54 | 23.7 | N | Healthy |
| 18 | F2M10 | F | 24 | 21 | N | Healthy |
| 19 | F2M11 | F | 22 | 19.4 | N | Healthy |
| MI Family=MI 3 | ||||||
| 20 | F3M1 | M | 60 | 30.3 | Ob | Healthy |
| 21 | F3M2* | F | 48 | 26.2 | Ow | Diseased |
| 22 | F3M3 | M | 25 | 28.4 | Ow | Healthy |
| 23 | F3M4 | M | 19 | 33.2 | Ob | Healthy |
| 24 | F3M5 | M | 29 | 33 | Ob | Healthy |
| 25 | F3M6 | F | 28 | 18.3 | N | Healthy |
| 26 | F3M7 | F | 9 | 16.6 | Uw | Healthy |
| 27 | F3M8 | F | 7 | 13.5 | Uw | Healthy |
| MI Family=MI 4 | ||||||
| 28 | F4M1 | F | 70 | 33.6 | Ob | Healthy |
| 29 | F4M2 | M | 44 | 25.7 | Ow | Healthy |
| 30 | F4M3 | M | 34 | 30.5 | Ob | Healthy |
| 31 | F4M4* | F | 40 | 33.6 | Ob | Diseased |
| 32 | F4M5 | M | 60 | 14.9 | Uw | Healthy |
| 33 | F4M6 | F | 70 | 21.5 | N | Healthy |
| MI Family=MI 5 | ||||||
| 34 | F5M1* | M | 55 | 31.2 | Ob | Diseased |
| 35 | F5M2 | F | 42 | 22.4 | N | Healthy |
| 36 | F5M3 | F | 24 | 19.4 | N | Healthy |
| 37 | F5M4 | M | 35 | 21.5 | N | Healthy |
| 38 | F5M5 | M | 5 | 27.5 | Ow | Healthy |
Note: M: Male; F: Female; N: Normal weight; Ob: Obese; Ow: Over weight; Uw: Underweight; (*), diseased patient
Table 1A: Clinical characteristics of the families.
| Sr. No | Code | Environmental factors | Associated complications | ||||
|---|---|---|---|---|---|---|---|
| Smoking | Alcohol | Air pollution | Diabetes | Hypertension | Hyperlipidemia | ||
| 1 | F1M1 | - | - | * | * | * | - |
| 2 | F2M2 | * | - | * | - | * | - |
| 3 | F2M3 | * | * | * | * | * | - |
| 4 | F3M2 | - | - | * | * | - | * |
| 5 | F4M4 | - | - | * | - | * | - |
| 6 | F5M1 | * | - | * | * | * | * |
Note: (*), presence of factors; (-), absence of factors
Table 1B: Clinical characteristic of the diseased patients including environmental factors and other complications associated with MI.
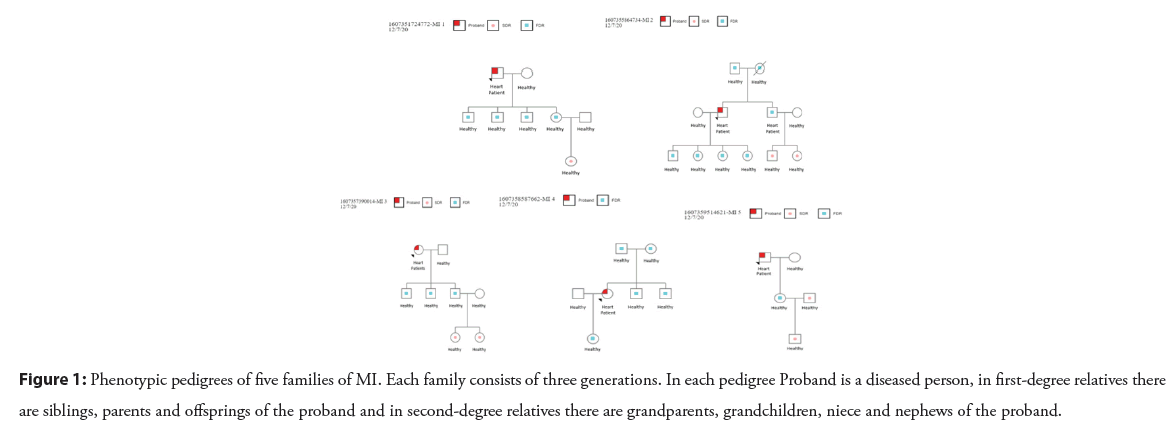
Family no. 1 consisted of eight members. Blood samples and demographic data were collected from each member for genetic analysis. The proband (father) was diagnosed with MI at the age of 54 yrs while other members of the family were healthy. Family no. 2 consists of eleven members. Proband and his brother were affected by MI. At the age of 56 yrs and 52 yrs, the proband and his brother were affected with MI, respectively. The remaining nine members of the family were healthy. The family no. 3 consists of eight members. The proband (mother) had MI at the age of 48 yrs while other remaining members of the family were healthy. Family no. 4 consists of seven members. The proband (daughter) was diagnosed with MI at the age of 40 yrs having a BMI value of 36.6 kg/m2 which shows that the proband was obese while other members of the family were healthy. Family no. 5 consists of five members (Figure 1). The proband (father) was diagnosed with MI at the age of 55 yrs while other members of the family were healthy. Blood samples of all the members with demographic data were collected.
Figure 1: Phenotypic pedigrees of five families of MI. Each family consists of three generations. In each pedigree Proband is a diseased person, in first-degree relatives there are siblings, parents and offsprings of the proband and in second-degree relatives there are grandparents, grandchildren, niece and nephews of the proband.
PCR amplification was performed on DNA samples of all the diseased families for both SNPs (rs17576 and rs3918242). The PCR products of 243bp and 369bp of both rs17576 and rs3918242 were obtained by using gradient PCR. The amplicons were confirmed by running 2% agarose gel electrophoresis, observed under the gel documentation system. The PCR amplicons of a single-family were observed (Figures 2A and 2B).
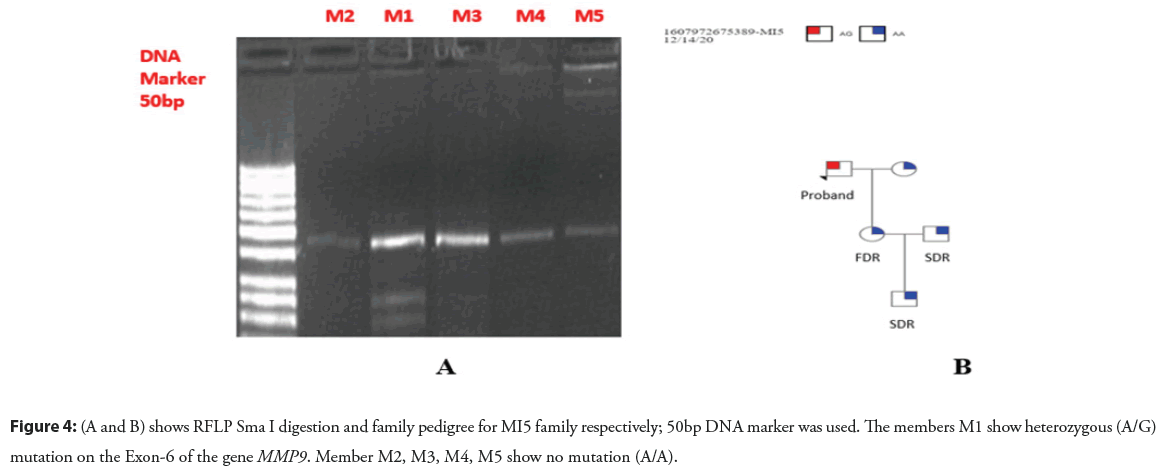
As a result of Sma I enzyme digestion, DNA fragments of 85bp, 145bp and 243bp were produced. For homozygous GG alleles 130bp and 243bp, for homozygous AA alleles 243bp and heterozygous AG alleles 85bp, 145bp and 243bp fragments were yielded (Figures 3A and 3B) (Figures 4A and 4B).
Figure 3: (A and B) shows RFLP Sma I digestion and family pedigree for MI1 family respectively; 100bp DNA marker was used. The members M1 show heterozygous (A/G) mutation on the Exon-6 of the gene MMP9. Member M2, M3, M4, M5, M6, M7, M8 show no mutation (A/A).
Figure 4: (A and B) shows RFLP Sma I digestion and family pedigree for MI5 family respectively; 50bp DNA marker was used. The members M1 show heterozygous (A/G) mutation on the Exon-6 of the gene MMP9. Member M2, M3, M4, M5 show no mutation (A/A).
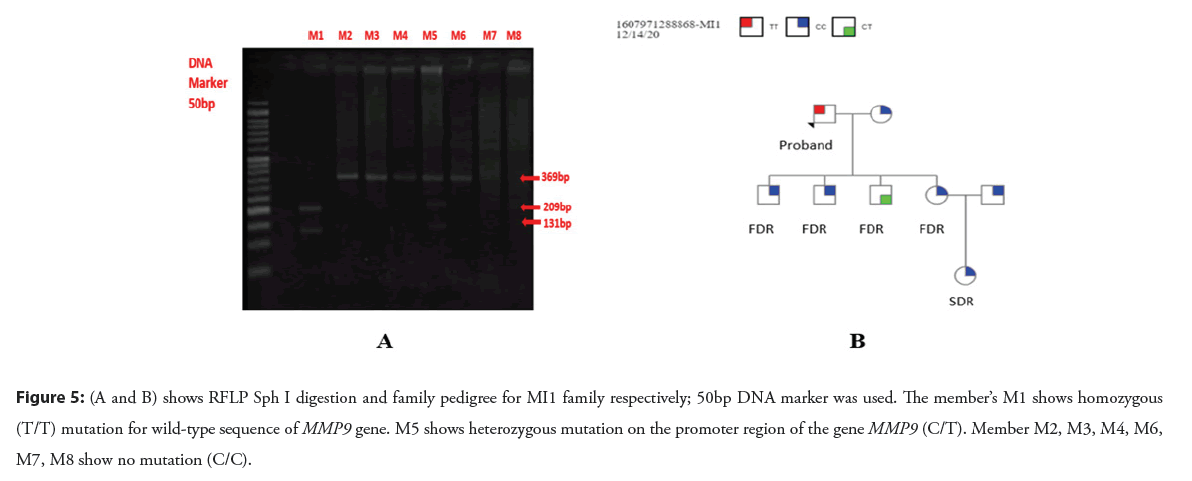
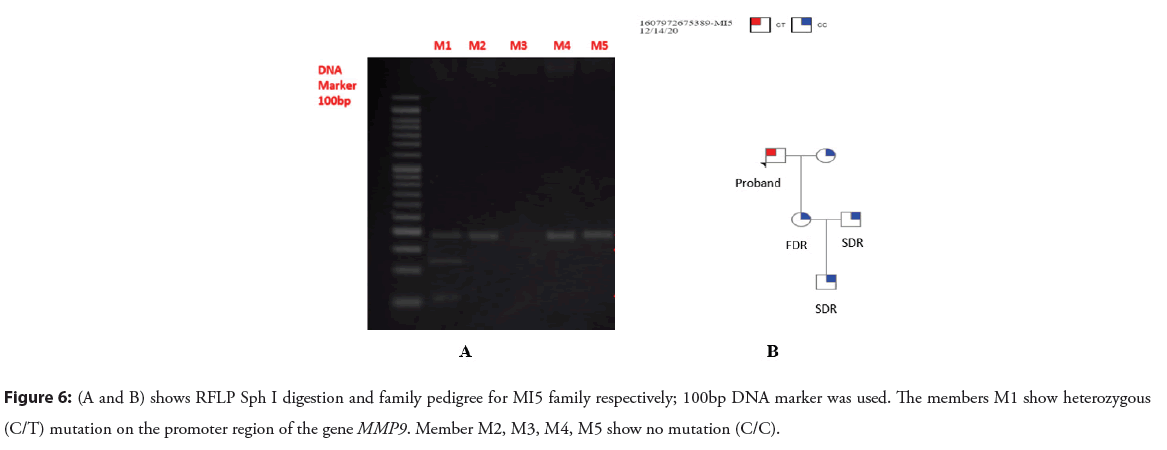
As a result of Shp I enzyme digestion, DNA fragments of 131bp, 209bp and 369bp were produced. For homozygous TT alleles 131bp and 209 bp, for homozygous CC alleles 369bp and heterozygous TC alleles 131bp, 209bp and 369 bp fragments were yielded (Figures 5A and 5B) (Figures 6A and 6B).
Figure 5: (A and B) shows RFLP Sph I digestion and family pedigree for MI1 family respectively; 50bp DNA marker was used. The member’s M1 shows homozygous (T/T) mutation for wild-type sequence of MMP9 gene. M5 shows heterozygous mutation on the promoter region of the gene MMP9 (C/T). Member M2, M3, M4, M6, M7, M8 show no mutation (C/C).
Figure 6: (A and B) shows RFLP Sph I digestion and family pedigree for MI5 family respectively; 100bp DNA marker was used. The members M1 show heterozygous (C/T) mutation on the promoter region of the gene MMP9. Member M2, M3, M4, M5 show no mutation (C/C).
Genotyping of families was done and most of the members showed no mutation having genotypes AA and CC for rs17576 and rs3918424 respectively. In the family MI1 (diseased) show heterozygous mutation (AG) with rs17576 and homozygous mutation (TT) with rs3918424. While in the same family, one member M5(healthy) was also having a CT mutation for rs3918242. In another family MI 4, the proband was having mutation AG and CT for both SNPs respectively. In the family, MI 5 member 1 (proband) shows the heterozygous mutation (CT) with rs3918242 and A/G for rs17576 (Table 2).
| Code | Genotyping | |
|---|---|---|
| rs17576 | rs3918424 | |
| MI Family=MI 1 | ||
| M1(proband) | AG* | TT* |
| M2 | AA | CC |
| M3 | AA | CC |
| M4 | AA | CC |
| M5 | AA | CT* |
| M6 | AA | CC |
| M7 | AA | CC |
| M8 | AA | CC |
| MI Family=MI 4 | ||
| M1 | AA | CC |
| M2 | AA | CC |
| M3 | AA | CC |
| M4 (proband) | AG* | CT* |
| M5 | AA | CC |
| M6 | AA | CC |
| MI Family=MI 5 | ||
| M1(proband) | AG* | CT* |
| M2 | AA | CC |
| M3 | AA | CC |
| M4 | AA | CC |
| M5 | AA | CC |
Note: (*) shows genotypes of diseased and carrier persons
Table 2: Genotyping of MI families for the SNPs rs17576 and rs3918424.
The allelic and genotypic frequencies analysis was done of all patients and control members whose data was collected from five families. The genotype and allelic frequencies of all 38 subjects were analyzed and represented in Tables 3 and 4 respectively. MMP9 SNP identified T/T and C/T genotypes rs3918242 in patients and C/C in controls while A/G genotype rs17576 in patients and A/A in controls. The SNPs followed the Hardy Weinberg equilibrium and rs17576 and rs3918242 were significantly associated with the onset of MI in the Pakistani population at genotypic as well as the allelic level (p<0.05). We found the T allele as a risk allele having a significant association (p<0.05) of variant rs3918242 with MI because it’s allelic frequency is higher among patients than in control. Moreover, allele G of rs17576 was found as a risk allele having a significant association (p<0.05) with MI because of its higher allelic frequency among patients than in control. The p values were calculated from Fisher’s test.
| Sr. No | SNP ID | Allele | Frequency in patients | Frequency in controls | Fisher's p value |
|---|---|---|---|---|---|
| 1 | Rs17576 | A | 64(1.000) | 12 (1.000) | - |
| G | - | - | |||
| 2 | Rs3918242 | C | 0(0.000) | 62(0.969) | 0.00e+000 |
| T | 12(1.000) | 2(0.031) |
Table 3: Allelic frequency of patients and controls.
| Sr. No | SNP ID | Genotype | Frequency in patients | Frequency in controls | Fisher's p value |
|---|---|---|---|---|---|
| 1 | Rs17576 | AA | 0(1.000) | 38(1.000) | 6.02e-09 |
| AG | 5 | 0 | |||
| GG | 0 | 0 | |||
| 2 | Rs3918242 | CC | 0(0.000) | 30(0.938) | |
| CT | 2(0.000) | 2(0.062) | |||
| TT | 1(1.000) | 0(0.000) | |||
Table 4: Genotypic frequency of patients and controls.
Discussion
Myocardial infarction is a life-threatening disease that involved a very complex and multifactorial process. It is the basic cause of sudden death at a young age. Most cardiac arrests run in families. In Pakistan, cousin marriages are very common and increase the risk of transmission of diseases just like myocardial infarction to the next generation [14,15]. Therefore, the present study was conducted to evaluate the genetic susceptibility of MI in Pakistani families.
In the current study, the recruited mean BMI of all the probands (diseased) was observed (30.20 ± SD) Kg/m2. The individuals with high BMI were also at higher risk of cardiovascular diseases [16]. In the present finding, it was revealed that the risk of MI in males was higher as compared to females. It was reported that the occurrence rate of MI in males was twice as compared to females but its causes were not clear [17]. The mean age for diagnosis of MI in males and females was (50.83 ± SD) years. In contrast to the current study, it was found that in the late fifties of life MI was more prevalent [18]. Through this study, it was also revealed that diabetes and hypertension were both associated with the susceptibility to MI. Smoking and exposure to air pollution play an important role in the onset of MI and smoking is an independent and major risk factor for MI [19,20]. Similar to the current finding it was also demonstrated that exposure to air pollutants like CO and SO2 plays an important role as risk factors for MI [21].
Here we investigated the role of MMP-9 gene polymorphism with susceptibility to MI in the Pakistani Population. We studied two SNPs in the MMP-9 gene at location R279Q A/G (rs17576) and C-1562T C/T (rs3918242). Both SNPs reside at the promoter region and exon-6, causing the substitution of adenine into guanine and cytosine into thymine, respectively. The result of the current study showed the association of both SNPs of the MMP-9 gene with MI. After genotyping of families, it was seen that all patients (probands) were having heterozygous mutation i.e., genotype AG for promoter region R279Q A/G (rs17576). While genetic analysis for other SNP rs3918242 (rs1562 C/T) represents TT and CT mutations. It was peculiar to see that all healthy members were having wild-type homozygous CC genotypes for the same SNP. It was also seen in family number, one healthy family member which was not proband was having mutation CT for rs3918242 instead of CC. It might be the early prediction of this member as it could be affected by cardiac arrest in near future. In addition, to find the genetic polymorphism of the MMP-9 genes by PCR-RFLP analysis, family clustering analysis was also performed. Family history makes evident in preventing, diagnosing and treating chronic diseases [16]. Such family studies can be applied for personal diagnosis before the onset of the disease. There is a powerful way to study the association of a particular gene between disease and genetic marker, that shows a linkage in families. In conclusion, the current study demonstrated that rs17576 and rs3918242 polymorphism of the MMP-9 gene has a considerable role in the development of MI. AG and TT genotypes increased the risk of MI development. Genotype CT indicated that the person is a carrier and MI might have occurred at a later age. AA and CC genotypes for both SNPs showed protective effects against the disease development while obesity, diabetes, hypertension, smoking, exposure to the sun and positive family history also increased the risk of MI development.
Conclusion
The current study demonstrated that rs17576 and rs3918242 polymorphism of the MMP-9 gene has a considerable role in the development of myocardial infarction. AG genotypes of the rs17576 variant increased the risk of a heart attack. While TT and CT genotypes of rs3918242 in family members indicated that the person is a carrier and MI might occur at a later age. AA and CC genotypes for both SNPs showed protective effects against the disease development while obesity, diabetes, hypertension, smoking, exposure to the sun and positive family history also increased the risk of MI development.
Limitations of the Study
The members who are carriers and showing different mutations should be further evaluated for their cardiac health and should be analyzed through ECG and cardiac enzyme analysis.
Ethical Consideration
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
All the authors are supported in the manuscript formation, data analysis and reviewing of the final data. All of them also supported the technical issues and approved the final version of the manuscript.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- Alwan A. Global status report on non-communicable diseases 2010: WHO. (2011).
- Goldstein JA, Demetriou D, Grines CL, et al. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 343(13): 915-22 (2000).
[CrossRef] [Google Scholar] [PubMed]
- Alaour B, Liew F, Kaier TE. Cardiac troponin-diagnostic problems and impact on cardiovascular disease. Ann Med. 50(8): 655-65 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Mansur AdP. Current cardiovascular disease death rate in Rio De Janeiro State: More than Only a Dream in Rio. SciELO Brasil, p. 116(4): 772-773 (2021).
[CrossRef] [Google Scholar] [PubMed]
- A Ebada Elsayed R, M Mohamed Y. Effect of multimodal cardiac rehabilitation program on patients after acute myocardial infarction: Nursing sensitive outcomes. Egyptian J Nurs Health Sci. 2(2): 54-80 (2021).
- Mechanic OJ, Gavin M, Grossman SA, et al. Acute myocardial infarction (Nursing). StatPearls (Internet): StatPearls Publishing. (2021).
- Nikpay M, Goel A, Won H-H, et al. A comprehensive 1000 Genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 47(10): 1121 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Wang X-Y, Zhang F, Zhang C, et al. The biomarkers for acute myocardial infarction and heart failure. Biomed Res Int. (2020).
[CrossRef] [Google Scholar] [PubMed]
- Jean PS, Zhang X, Hart B, et al. Characterization of a dinucleotide repeat in the 92 kDa type IV collagenase gene (CLG4B), localization of CLG4B to chromosome 20 and the role of CLG4B in aortic aneurysmal disease. Annal Hum Genet. 59(1): 17-24 (1995).
[CrossRef] [Google Scholar] [PubMed]
- Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: A review of their structure and role in acute coronary syndrome. Cardiovasc Res. 59(4): 812-23 (2003).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Koh YS, Chang K, Kim PJ, et al. A close relationship between functional polymorphism in the promoter region of matrix metalloproteinase-9 and acute myocardial infarction. Int J Cardiol. 127(3): 430-2 (2008).
[CrossRef] [Google Scholar] [PubMed]
- Rodius S, Mulliert G, Azuaje F, et al. Matrix metalloproteinase 9 polymorphism and outcome after myocardial infarction. Cardiogenet. 1(1):13-9 (2011).
[Google Scholar] (All versions)
- Wang L, Ma YT, Xie X, et al. Interaction between MMP-9 gene polymorphisms and smoking in relation to myocardial infarction in a Uighur population. Clin Appl Thromb Hemost. 18(1): 72-8 (2012).
[CrossRef] [Google Scholar] [PubMed]
- Bhatnagar P, Wickramasinghe K, Williams J, et al. The epidemiology of cardiovascular disease in the UK 2014. Heart. 101(15): 1182-9 (2015).
[CrossRef] [Google Scholar] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 130(25): 2354-94 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Zhu J, Su X, Li G, et al. The incidence of acute myocardial infarction in relation to overweight and obesity: A meta-analysis. Arch Med Sci. 10(5): 855 (2014).
[CrossRef] [Google Scholar] [PubMed]
- Albrektsen G, Heuch I, Løchen M-L, et al. Risk of incident myocardial infarction by gender: Interactions with serum lipids, blood pressure and smoking. The Tromsø Study 1979–2012. Atherosclerosis. 261: 52-9 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Jefferis BJ, Whincup P, Welsh P, et al. Prospective study of matrix metalloproteinase-9 and risk of myocardial infarction and stroke in older men and women. Atherosclerosis, 208(2): 557-63 (2010).
[CrossRef] [Google Scholar] [PubMed]
- Yusuf S, Hawken S, Ôunpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 364(9438): 937-52 (2004).
[CrossRef] [Google Scholar] (All versions) [PubMed]
- Lu Y, Wang Z, Georgakis MK, et al. Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. J Am Heart Assoc. 10(1): e017986 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Prabhakaran D, Jeemon P. Should your family history of coronary heart disease scare you? Mt Sinai J Med. 79(6): 721-32 (2012).
[CrossRef] [Google Scholar] [PubMed]