Research Article - Pharmaceutical Bioprocessing (2016) Volume 4, Issue 4
Anti-Anxiety Effects of Mercurialis annua Aqueous Extract in the Elevated Plus Maze Test.
- Corresponding Author:
- Zouhra Doukkali
Pharmacodynamy Research Team, ERP, Laboratory of Pharmacology and Toxicology, Faculty of Medicine and Pharmacy, University Mohammed V of Rabat, Morocco
E-mail: d.zouhra@yahoo.fr
Abstract
The most widely prescribed medications for anxiety disorders are the Benzodiazepines; however, they have prominent side effects. Therefore, the development of new pharmacological agents is well justified and interest in alternative medicine that affects the ‘mind’ is growing. Among medicinal plants, Mercurialis annua has been recommended for relief of anxiety in Morocco folk medicine. Method: The present study was undertaken to investigate the effects of methanolic extract of Mercurialis annua in the elevated plus maze (EPM) and Open Field (OF) models of anxiety. The Mercurialis annua extract was administered orally to Balb/c mice, at graded doses and diazepam was given intraperitonealy. Result: In the EPM, methanolic extract at 200-400 mg/kg showed an anxiolytic effect by increasing the time spent on open arms and the percentage of open arm entries compared to control group. In an open field test methanolic extract of M.a (400 mg/kg) increased the central area crossing, the time spent and number of rearing in the center of arena. Conclusion: These data support that the methanolic extract of M.a might possess significant anxiolytic potential to be pursued further for drug development process and provide a scientific evidence for its traditional claim.
Keywords
anxiety, balb/c Mice, eelevated Plus maze, open field, methanolic extract of Mercurialis annua, morocco
Introduction
Anxiety disorders are among the most common psychiatric disorders that affect all age groups of the general population [1]. Anxiety disorders include Agoraphobia, Specific Phobia, Social Anxiety Disorder (Social Phobia), Panic Attack, Separation Anxiety Disorder, and Selective Mutism [2] became a very important area of research interest in psychopharmacology.
Major drug classes for the treatment of anxiety disorders are benzodiazepines and selective serotonin-reuptake inhibitors (SSRIs) [3]. However, these compounds have a number of undesirable effects such as insomnia, muscle relaxation and hepatotoxicity [4]. These considerations implicate the search for new anxiolytic compounds that have a fast onset of action present with less side effects and a wider safety margin. Medicinal plants are a good source to find new remedies for these disorders.
In folk medicine, some species belonging to the family Euphorbiaceae, such as Mercurialis annua, is known to possess anxiolytic action [5]. Researchers in Africa particularly in morocco are exploring the traditional remedies to find a suitable cure for these mind affecting diseases. Moreover, Moroccan climate and favored geographical location have contributed to diversity of medicinal plants.
Mercurialis annua L. (Euphorbiaceae) is a windpollinated annual plant that occupies ruderal and roadside habitats throughout central and western Europe and around the Mediterranean Basin [6]. The species is a winter annual in the Mediterranean region, and has long been known to have tranquillizing effects among the Moroccan people [5,7].
Reaching 10–50 cm in height, M. annua contains large amounts of flavonoids (Aquino et al., 1987 [8]) and of the pyridinone-type alkaloid, hermidin [8]. Ethnobotanical reports attribute purgative, diuretic or antisyphilitic effects to the dried plant.
In the present study we have investigated the anxiolytic activities of aqueous extract of Mercurialis annua by the elevated plus-maze (EPM) and Open field models of anxiety in mice.
Materials and Methods
Animals
Adult Swiss mice (20–30 g) of either sex were used for the study. The animals were acquired from the animal experimental centre of Mohammed V souissi University, Medicine and pharmacy Faculty, Rabat. The animals were maintained in a room with controlled temperature (25 ± 1 °C) and lighting (light/dark 12:12 h in polypropylene cages, with food and water ad libitum. Animals were acclimatized to laboratory conditions at least 1 h prior to initiation of experiments. The animals were divided into four groups, each consisting of six mice, implemented in all sets of experiments.
Plant material
The aerial part of Mercurialis annua was collected from the north of Morocco near the town of Wazzan (Jaaouna el Basra), with assistance of a traditional medical practitioner. The plant was authenticated by botanists of scientific institute Pr. M. Ibn Tatou and Pr. Halim Khammar. A voucher specimen (N° RAB78984) was deposited in the Herbarium of Botany Department of the Scientific Institute of Rabat.
Preparation of the aqueous extract
The aereal part of Mercurialis annua (100 g) were extracted with 1L distilled water (70°C, 30 min), concentrated and then freeze-dried producing aqueous extract (AE) with a 32.70% yield.
Drugs
The aqueous extract of Mercurialis annua was suspended in distilled water. Diazepam (ampoule 10 mg/2 ml) was diluted with saline to the required concentration before use. It is well known that benzodiazepines act as anxiolytics at low doses and that they induce sedation and muscle relaxant effects at higher doses [9]. Therefore, we used diazepam (1 mg/kg) as a positive control for anxiolytic-like effects.
Treatment schedule
Experimental groups of mice were treated orally (p. o.) with aqueous extract of Mercurialis annua at doses of (100-600 mg/kg), whereas control groups received normal saline by the same routes. Diazepam (1 mg/kg) was administered intraperitoneally (i.p.). All drugs were freshly prepared before each experiment. The doses of extracts were calculated to administer 0.25 ml of the suspension of extracts to the mice of 20 g. The trial was carried out 1 H min after the treatments. The anxiolytic activity was examined by using the light/dark box test and the hole board test, and motor coordination test assessed with the rota rod test.
Acute toxicity study
The procedure was followed as per OECD 423 guidelines [10] (OECD/OCDE. 2002). The extract was administered orally at a dose of 2000 mg/kg body weight. Mice were kept under observed for 14 days to register possible mortality; their weights were registered and study their behavioral neurological toxicity.
Elevated plus maze
The elevated plus maze (EPM) was first proposed as an animal model of anxiety by Handley and Mithani [11] Constructed of black–colored wooden planks consisting of two close arms, 50 cm x 10 cm x 40 cm, and two open arm, 50 cm x 10 cm, connected to a central platform (10cm x 10cm). Covered with a removable lid, such that the open or closed arms were opposite to each other [12].The maze was elevated to a height of 50 cm above the floor. During the experiment each mouse was placed in the central compartment facing one of the open arms. The number of entries and the time spent in open arms were recorded for 5 min. An entry was counted when all four paws of the mouse entered an open or closed arm. An increase in open arms entries and increase in time spent in open arms were interpreted as an index of potential anxiolytic activity. All test sessions were taped by using a video camera.
Open field test
Experimental groups of 6 mice were treated with vehicle saline, aqueous extract of Mercurialis annua (100-600 mg/kg, po) or diazepam 1 mg/ kg, ip). After the treatment -Sixty minutes for aqueous extract and 30 min for Diazepam- the animals were placed individually in the center of the arena and were subjected to a 5-min period to the open field test. The open field apparatus was an opaque plexiglass cage (72 × 72 cm) with walls 35-cm in height where the floor was divided with white lines by 16 squares (18 × 18 cm) of identical dimension. A digital video camera was installed above the cage to record the activity of the mice. The entire room, except the OF was kept dark during the experiment, in this study was evaluated both the general motor activity of the mice to discard hypoactivity, hyperactivity, or no changes associated with treatments, which could interfere with the behavioral activity of the mice in the elevated plus maze also the observed parameters were as follows: (a) total number of crossings, (b) central area crossings, (c) time spent in central area (d) central area rearing [13,14].
After each test session, the elevated plus-maze apparatus and the open field cage were carefully cleaned with water and allowed to dry to remove the scent of the previously evaluated animal, which could otherwise modify the spontaneous behavior of the subsequent evaluated mice [15].
Also this test reflects the conflict between the innate fear that mice have of the central area of a novel open field versus their desire to explore new environments. When anxious, the natural tendency of mice is to prefer staying close to the walls.
Ethical approval
The study was conducted in accordance with the accepted principles outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health and all efforts were made to minimize animal suffering and the number of animals used. Ethics approval was obtained from the central laboratory of animal of Facutly of medecine and pharmacy from the Mohammed V University of Rabat.
Statistical analysis
All the results were expressed as mean ± SEM. All statistical analysis was done using one way analysis of variance (ANOVA) followed by the Tukey’s post hoc test. P<0.05 was considered as significant when compared to their respective control group.
Results
Acute toxicity study
Following oral administration aqueous extract of Mercurialis annua at a dose of 2000 mg/kg, P.O., animals were observed for signs of toxicity such as convulsions, hypothermia, hyperactivity, and grooming continuously for 2 h and for mortality up to 24 h after administration of the doses. No toxicity and no significant changes in the body weight were observed between the treated and control group.
Elevated plus maze
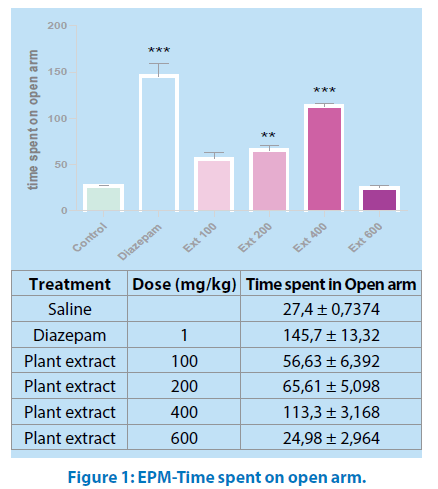
ANOVA of the times spent by mice in the open arms of elevated plus maze test showing significant (P˂0.05) differences between the groups (Figure 1).
All values are mean ± SEM (n=6); *p<0.05, ***p<0.001 when compared to control. Oneway ANOVA, Tukey’s Multiple Comparison post hoc tests. ##p<0.01, ###p<0.001 when compared Ext 100 and Ext 200 to Ext 400.
Post hoc comparisons made with Tukey’s test revealed animals which received MeOH –extract of Mercurialis annua ( 100-400 mg/kg, po.) and intraperitoneal diazepam 1mg/kg increased the time spent on the open arms compared to the control group (P˂0.01 and P˂0.001, respectively).
The values of the group treated with Mercurialis annua at 400mg/kg body weight were higher than that of the treatment with lower doses (100,200 mg/kg).
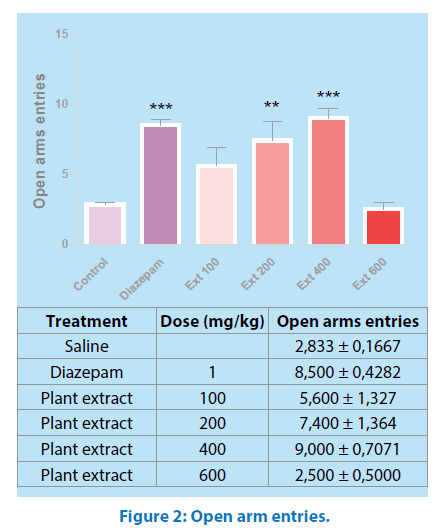
Similarly, ANOVA of the number of entries into open arms of the elevated plus maze showing significant (P˂0.05) differences between the groups. Further post hoc comparisons made with Tukey test revealed that animals receiving aqueous extract of Mercurialis annua (100-600 mg/kg) made significantly more entries into open arms of the maze than control (P˂0.05 and P˂0.001, respectively) Figure 2.
All values are mean ± SEM (n=6); *p<0.05, ***p<0.001 when compared to control. Oneway ANOVA, Tukey’s Multiple Comparison post hoc tests. ##p<0.01, ###p<0.001 when compared Ext 100 and Ext 200 to Ext 400.
However, 400mg/kg body weight treated groups showed higher number of entries and more time spent in open arms comparable to that of standard drug diazepam 1mg/kg.
Open field
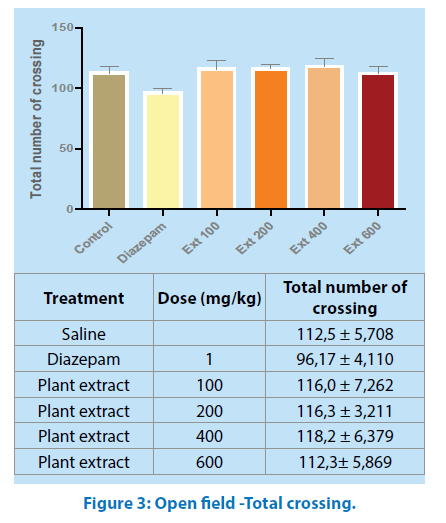
Total crossing: In the open field test, no difference was observed in locomotor activity (Total number of crossing) between groups (Figure 3).
All values are mean ± SEM (n=6). One- way ANOVA, Tukey’s Multiple Comparison post hoc tests.
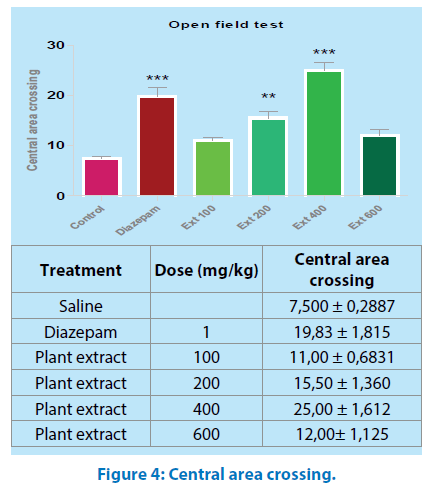
Central area crossing: All values are mean ± SEM (n=6); *p<0.05, **p<0.01 when compared to control. One- way ANOVA, “Tukey’s Multiple Comparison post hoc tests.
The post –hoc test revealed that central area crossing was only augmented in 40 mg/kg of Mercurialis annua and diazepam respectively (P˂0.001, P˂0.05, respectively) as compared with the control and remaining groups (Figure 4).
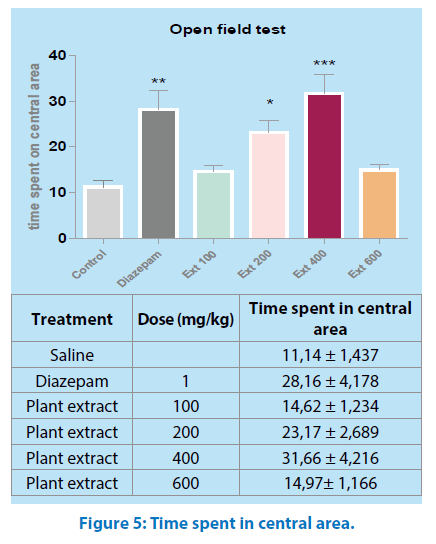
Time spent in central area: Mice treated with either 400mg/kg or diazepam spent significantly more time in the central area (P˂0.01, P˂ 0.001, respectively) Figure 5.
All values are mean ± SEM (n=6); **p<0.01, ***p<0.001 when compared to control. Oneway ANOVA, “Tukey’s Multiple Comparison post hoc tests.
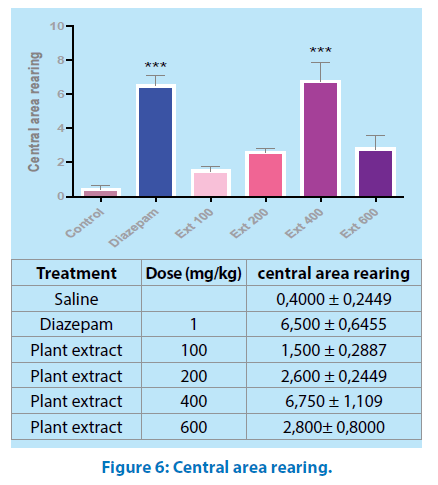
Central area rearing: Central area rearing was increased by aqueous extract of Mercurialis annua 400 mg/kg and diazepam 1mg/kg (P˂0.001, P˂0.05, respectively) Figure 6.
All values are mean ± SEM (n=6); *p<0.05, ***p<0.001 when compared to control. One way ANOVA, “Tukey’s Multiple Comparison post hoc tests
Discussion
In the present work, we examined the anxiolytic properties of M.a. We have chosen to evaluate the aqueous extract of M.a treatment behavioral anxiolytic effect in tow of the most validated models for researching anxiety like behavior in animals, namely the Elevated plus maze and the Open field are a well-accepted, experimental animal model typically used to test the effectiveness of anxiolytics drugs [16-18].
The elevated plus maze test is based on the observation that the natural behavior of rats or mice to display an aversion to novel open spaces and fear of balancing on a relatively narrow, raised platform that can induce anxiety in humans [18]; therefore, avoidance of the open arms is interpreted as anxiogenic behavior [17,19]. An anxiolytic agent increases the exploration of the open arms (time and entries into open arms). Diazepam increases the percentage of open arms entries and the time spent in the open arms [19], confirming its anxiolytic effects. Finding of this study show that animals treated with graded doses of Urtica urens (100-400 mg/kg) increased the entries into and time spent in the open arms, indicating an anxiolytic like effect, in addition to the fact that this reduction an anxiety like behavior was similar to the one observed following the conventional treatment with the diazepam.
The open field is a very popular animal model of anxiety-like behavior. Since the creation of the open field test, conceived in 1934 to provide objective measures of emotionality in rats [20], the procedure consists of subjecting an animal, usually a rodent, to an unknown environment from which escape is prevented by surrounding walls [21].
In fact, anxiety behavior in the open field is triggered by two factors: individual testing (the animal is separated from its social group) and agoraphobia (as the arena is very large relative to the animal’s breeding or natural environment).
The open field test is designed to study exploratory activity [22]; in this test, M.a did not alter the crossing number (Figure 3), aqueous extract of M.a treatment with 400 mg/ kg body weight increased the preference for the central area enhancing the crossing number, the time spent and the rearing in the central area of the apparatus (Figures 4, 5 and 6); an increase in these parameters could be indicative of anxiolytic-like effects [23].
The efficacy of most herbal remedies is attributed to various active principles in combination, in the present study aqueous extract from areal part of M.a containing significant amounts of flavonoid composants [8] which are probably more responsible for the anxiolytic activity of M.a. Recently some natural and synthetic flavonoids have been found to bind specifically and competitively to benzodiazepine receptors and to possess anxiolytic effects [24-26].
Conclusion
In conclusion, the data presented hereby reinforce the traditional use and our previous study of methanolic extract of Mercurialis annua [17] and indicate that the aqueous extract of the aerial parts of M.a has anxiolytic-like effects. Thus, M.a may be a promising candidate for future development as a new anxiolytic drug. Further pharmacological investigations are underway to identify the active constituents of the plant extract responsible for the showed activity. Knowing the precise mechanism of how these extracts function and at what molecular targets they act should give us insights into how to develop better mood aids based on the natural products.
Competing interest
The authors declare that they have no competing interest.
Authors’ contribution
DZ, I carried out all the studies and drafted the manuscript with the help of the above authors, as regards TK participated in this work and drafted with me the manuscript. EHB helped us in the chemistry part and NM carried out the behavioral tests with me, and CY is the director of the laboratory he advises me and guides me always in my work, after my PhD supervisor KA she corrects the manuscript, guides me and advises me. All authors read and approved the final manuscript.
Acknowledgment
Authors are grateful to Pr. Amina Zellou, Dr. Hamid Khammar, botanist of scientific institute, Rabat, Hanae Hosni, Amina Bounihi and Ghizlane Hajjaj, for their encouragement and interest in the research work.
References
- Jung YH, Ha RR, Kwon SH, Hong SI, Lee KH. Anxiolyticeffects of Julibroside C1 isolatedfrom Albizzia julibrissin in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry . 44, 184-192 (2013).
- The Diagnostic and StatisticalManual of Mental Disorders (5th Edn) American Psychiatric Association, Washington, DC (2013).
- Kunovac JL, Stahl SM. Future directions in anxiolyticpharmacotherapy. Psychiatr. Clin. North. Am. 18, 895-909 (1995).
- Lader M, Morton S. Benzodiazepineproblems. Br J. Addict. 86, 823-828 (1991).
- Doukkali Z, Bouidida H, Srifi A, et al. Anxiolytic plants in Morocco: Ethnobotanical and ethno-pharmacological study. Phytothérapie (2014).
- Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM. Flora Europaea. Cambridge: Cambridge UniversityPress.Durand B. Le complexe Mercurialisannua L. s.l. une étude biosystématique. Annales. Des. Sciences. Naturelles. Botanique. 12, 579-736 (1964).
- Krahenbuhl M, Yuan YM, Kupfer P. Chromosome and breeding system evolution of the genusMercurialis (Euphorbiaceae): implications of ITS molecularphylogeny. Plant Systematic and Evolution. 234, 155-170 (2002).
- Aquino R, Behar I, D’Agostino M, De Simone F, Schettino O. Phytochemical investigation on Mercurialisannua. Biochem. System. Ecol. 15, 667-669 (1987).
- Novas ML, Wolfman C, JH, De Robertis E. Proconvulsantand ‘anxiogenic’ effects of n-butyl β carboline-3 carboxylate, an endogenousbenzodiazepinebindinginhibitorfrombrain. Pharmacol. Biochem. Behav. 30, 331-336 (1988).
- OECD/OCDE. Guidelines for the testing of chemicals, reviseddraft guidelines 423; acute oral toxicity-acute toxic class method, OECD publishing (2002).
- Sharma V, Gilhotra R, Dhingra D, Gilhotra N. Possible underlying influence of p38MAPK and NF- B in the diminishedantianxietyeffect of diazepam in stressedmice. J. Pharmacol. Sci. 116, 257-263 (2011).
- Pellow S, Chopin P, File SE, Briley M. Validation of open closed arm entries in an elevated plus maze as a measure of anxiety in the rat. J. Neurosci. Methods. 14, 149-167 (1985).
- Archer J. Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205-235 (1973).
- Siegel PS. A simple electronicdevice for the measurement of grossbodilyactivity of smallanimals. Journal of Psychology. 21, 227-236 (1946).
- Gutiérrez-García AG, Contreras CM, Mendoza-López MR, Cruz-Sánchez S, García-Barradas O. A single session of emotional stress producesanxiety in Wistar rats. BehaviouralBrain Research. 167, 30-35 (2006).
- Lister RG. The use of plus-maze to measureanxiety in the mouse. Psychopharmacology. 92, 180-185 (1987).
- Belzung C, Griebel G. Measuring normal and pathologicalanxiety-likebehavior in mice: a review. Behav. Brain. Res. 125, 141-149 (2001).
- Walf AA, Frye CA. (2007) The use of the elevated plus maze as an assay of anxiety-relatedbehavior in rodents. Nature Protocols. 2, 322-328.
- Crawley J, Goodwin FK. Preliminary report of a simple animal behaviour for the anxiolyticeffects of benzodiazepines. Pharmacol. Biochem. Behav. 13, 167-170 (1980).
- Hall CS. Drive and emotionality: Factorsassociated with adjustment in the rat. Journal of Comparative Psychology. 17, 89-108 (1934).
- Walsh RN, Cummins RA. The open-field test A critical review. Psychol. Bull. 83, 482-504 (1976).
- Crawley JN. Exploratorybehaviourmodels of anxiety in mice. Neurosci. Biobehav. 9, 37-44 (1985).
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-likebehaviors: a review. Eur. J. Pharmacol. 463, 30-33 (2003).
- Marder M, Viola H, Wasowski C, Wolfman C, Waterman PG. 6-Bromoflavone, a high affinity ligand for the central benzodiazepinereceptors is amember of a family of active flavonoids. Biochemical and Biophysical Research Communications. 223, 384-389 (1996).
- Viola H, Wasowski C, Levi de Stein M, Wolfman C, Silveira R. A pigenin, a component of Matricariarecutitaflowers, is a central benzodiazepinereceptors–ligand with anxiolyticeffects. Planta. Medica. 61, 213-216 (1995).
- Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P. Anxioselectiveproperties of 6,3’-dinitroflavone, a high affinitybenzodiazepinereceptor ligand. Eur. J. Pharmacol. 318, 23-30 (1996).