Commentary - Journal of Pharmaceutical Toxicology (2023) Volume 6, Issue 3
Acute and sub-acute toxicity of n-butanol extract from Mitracarpus hirtus leaves L. (DC.)
Ousmane Faye1; Cheikh Sall1; Awa Ndong2; Fatoumata Bah2; Robert Faomowe Foko2; Tonleu Linda Bentefouet1; Mamadou Soumboundou1; Fatou Bintou Sarr1; Guata Yoro Sy3; Mathilde Cabral2
1UMRED, Health Training and Research Unit, University of Iba Der Thiam of Thies, BP 967, Thies, Senegal
2Laboratory of Toxicology and Hydrology, FMPOS, UCAD, PB 5005 Dakar Fann, Senegal
3Laboratory of Pharmacology FMPOS, UCAD, PB 5005 Dakar Fann, Senegal
Received: 01-June-2023, Manuscript No. oajpt-23-102437; Editor assigned: 05- June -2023, PreQC No. oajpt-23-102437 (PQ); Reviewed: 19- June -2023, QC No. oajpt-23-102437; Revised: 21- June -2023, Manuscript No. oajpt-23-102437 (R); Published: 28-June -2023; DOI: 10.37532/ jpt.2023.6(3).96-104
Abstract
Mitracarpus hirtus, grass of the Rubiaceae family, is a seasonal species. Among other things, it is traditionally used to treat skin diseases and diabetes, and as an antibiotic and antidote to insect bites and stings. In order to establish the safety of these treatments, the aim of this study was to assess the effects of the acute and sub-acute toxicity of the n-butanol extract of Mitracarpus hirtus leaves, the most active faction responsible for the anti-diabetic, antioxidant and anti-α-amylase activities. The results of this acute toxicity study on the n-butanol moiety of M. hirtus demonstrated that administration of single dose samples orally resulted in no deaths or behavioral changes across all of the different doses tested. The LD50 being greater than 5000 mg/kg body weight, the extract thus presents a toxicity index of 5 corresponding to almost non-toxic according to the Hodge and Sterner scale. Concerning the study of subacute toxicity or toxicity by repeated administration, the rats’ water and food consumption and the weights of the organs removed from the rats (liver, lung and kidney) did not undergo any significant change (p>0.05) compared to the control groups throughout the experiment. No significant disturbance on biochemical and haematological parameters was noted except a decrease of ASATS in females at high doses, index of a hepatoprotective effect of this extract. Histopathological analysis did not reveal any signs of cytolysis inherent to a possible toxicity of n-butanol extracts of M. hirtus leaves.
Keywords
Mitracarpus hirtus • Wistar Rats • LD50 • Biochemical and Haematological Parameters • Histopathology
Introduction
Toxicity can be defined as all the harmful effects caused by a substance introduced at a relatively high single dose or at small doses repeated for a long time. It is often manifested by morphological and functional lesions in a living organism. The study of the toxicity of a substance is a set of pharmacological tests, which determine the degree of the harmful character for a regulation of its use with less danger. The action of a poisonous substance depends on various factors, including the strength of secondary metabolites, the amount consumed, the exposure time, the different parts of the plant (root, oil, leaves, bark and seeds of the stem), individual body chemistry, climate and soil, and genetic differences within the species [1]. But it was evaluated by analyzing several parameters such as the observed mortality rate, weight change, modification of certain biochemical parameters of the blood (ALT, AST, glucose, creatinine, and urea), haematological and histologic appearance of certain organs [2].
Furthermore, the use of plants has always been part of human culture. In Africa, for example, plants are used in traditional medicine to treat various infectious and non-infectious diseases [3, 4]. Plants provide a wide variety of useful biochemical to mankind. Their uses include foods, dyes, fragrances, and agricultural and pharmaceutical chemicals. In recent years, there has been a resurgence of interest in herbal medicine. Indeed more and more people are using medicinal plants to heal themselves because they are a valuable source of natural products [5]. Nearly 80% of people living in developing countries still depend on traditional herbal medicine for their primary health care [6]. And for dosage problems or misuse, these populations are exposed to the risk of intoxication from these plants. Therefore, assessment of the toxicity parameters of medicinal plant extracts or isolated molecules is a good step forward for better and less risky use.
Mitracarpus hirtus, grass of the Rubiaceae family, is a seasonal species. It spreads easily in gardens, farms and fields in the neo-tropical and tropical regions in the wild during the rainy season [7, 8]. It is among other things traditionally used in the treatment of skin diseases, diabetes, as an antibiotic and antidote to stings and insect bites [7]. Phytochemical studies of the plant have indicated the presence of tannins, flavonoids and alkaloids. A significant presence of flavonoids was noted on the n-butanol fraction which would be at the original of the antioxidant and anti-α-amylase activities observed [9]. Thus, the objective of this study is to evaluate the effects of acute and sub-acute toxicity of n-butanol extract from Mitracarpus hirtus leaves which was the most active faction on the studied diabetes parameters in our previous works.
Materials and Methods
Preparation of n-butanol extract
Samples of M. hirtus were collected in October 2020 from various areas in the village of Sagne in the region of Fatick, located in western Senegal with a dry and hot semi-arid climate, and are identified at the botanical laboratory of the Faculty of Medicine, Pharmacy and Odontology of Cheikh Anta Diop University. Then they were dried in the dark for a week and then reduced to powder with an electric grinder. Preparation of n-butanol extract was done following the method used by [8]. A mass of 100 g of M. hirtus leaves powder obtained was subsequently macerated over 48 hours with pure methanol. The filtrates obtained were made dry at low pressure and at 40° C, with a rotary evaporator of the Buchi R-100 type. After a methanolic maceration of the crude powder of M. hirtus leaves, a fractionation (repartition of secondary metabolites according to their polarities) of the crude extract was carried out with solvents of different polarities. We used successively cyclohexane (for the recovery of apolar metabolites such as: oils, fats...), then dichloromethane (for the less polar ones), ethyl acetate (for polar compounds) and n-butanol (for the more polar ones). Then all the obtained fractions were reduced to dryness with rotary evaporator at 40°C and kept cold before use. Among the obtened fractions, n-butanol was the most active in studies of hyperglycaemia and normal glycaemia in rats, and consequently was used in this experiment. The volume of extract solution administered to laboratory animals (rats) for chronic and subchronic toxicity depended on dose and body weight. It was calculated according to the following formula:
V= (D*P)/C
D: dose in mg/kg body weight; P: weight of the animal in kg; C: Concentration of the solution to be administered (mg/ml)
Animals
Male and female rats were used for acute and sub chronic toxicology studies. The rats were obtained from the animal facility of the Toxicology and Hydrology Laboratory of the Faculty of Medicine, Odontology and Pharmacy of UCAD. Animals were acclimatized to laboratory condition prior to experiments. The rats were kept at room temperature, with a light/ dark cycle. They were kept in cages lined with wood shavings and had free access to water and food. Twelve female rats divided into four groups of 3 rats were used for the acute toxicity test. For the sub-acute test, 24 male and female rats were used in 4 groups of 6 rats (3 females and 3 males). Their weights were recorded just before the treatments.
Acute toxicity
The acute toxicity test is conducted according to the method of the OECD guideline 211 [10]. Briefly, three doses were tested for acute toxicity: 500, 2000 and 5000 mg/Kg. For this test, twelve rats with an average weight of 192,5 g were selected and randomly divided into 4 groups of 3 rats each. Group 1 was the control group and it received only distilled water. Groups 2, 3, and 4 have respectively received doses of 5000 mg/ kg, 2000 mg/kg and 500 mg/kg of body weight of the plant extract. The animals were fasted (deprived of food but with access to water) during the night preceding the experiment. They were then weighed before administration of the test substance (extract) by gavage (intra-oesophageal route). After administration, animals were observed regularly every hour for 6 hours and then daily for 14 days in search of signs of acute intoxication (tremors, sleep, lethargy, aspect of the stools, diarrhea, modification of the skin and body hair, salivation and death) [1].
The method makes it possible to exercise a judgment concerning the classification of the test substance in a toxicity class delimited by previously fixed values of LD50. In the event that toxic effects are noted, the LD50 corresponds to the dose which causes the death of 50% of the rats exposed to a single dose of the extracts.
Sub-chronic oral toxicity study
Treatment of animals
The sub-acute toxicity test was performed according to the method described by OECD guideline 423 (OECD 2018). Rats were divided into 4 groups with 6 rats per group (3 males and 3 females) and their weights were recorded before the start of treatment. Group 1 (control) received distilled water at 1 ml/100 g body weight. Groups 2, 3 and 4 received 50, 100 and 200 mg/kg body weight of the extract respectively. The average weight of the rats used was 112g for this study. All doses were administered orally once a day for 28 days. During this experimental period, the rats were fed, hydrated, and visual observations for mortality, behavioral patterns (Salivation, fur, lethargy, and sleep), changes in physical appearance, injury, pain and signs of illness were conducted once daily during that period [11]. Every week, body weight of rats was taken, as well as a collection of urine with the metabolism cages from 6 p.m. to 8 a.m. in bottles for urine parameters analyses.
At the end of the experiment, all animals were anesthetized through intraperitoneal injection of a cocktail containing ketamine (60 mg/ kg) and xylazine (7.5 mg/kg). Blood samples were collected via cardiac puncture into nonheparinized and EDTA-containing tubes for biochemical and haematological parameters analyses, respectively. After cardiac puncture, the rats were dissected and the organs were excised, weighed, and examined microscopically. The relative organ weights have been calculated. These weights by organs were calculated using the following formula:

Pr: relative weight of the organ (g/100 g); Po: organ weight (g); Pa: rat body weight (g).
After sacrificing the rats, principal vital organs collected (liver, kidney and lung) were preserved in solution of 10% solution of buffered formalin for the histopathological study.
Analysis of biochemical parameters
Blood from the rats was collected by cardiac puncture at the end of the experiment into dry tubes for biochemical assay. Then it was centrifuged at 3000 rpm for 10 min using the Biosystems BTS 350 machine and the serum collected was used to determine the biochemical parameters such as the level of glucose, creatinine and the ALT and AST enzymes. Creatinine was assayed by Jaffé, colorimetric method, using the creatinine kit. Blood glucose was measured by the method of glucose oxidase, glutamic oxaloacetic and pyruvic transaminases [12]. ALT and AST were determined by the optimized UV kinetic method using the GPT-ALT LR GSMitalia and GOT-AST LR GSMitalia kits respectively [13,14]. The urinary biochemical parameters (glucose, proteins, blood, ketone bodies, leukocytes, nitrile, etc.) were then searched for in the urine using U-AQS-3GK® multipara metric reactive strips.
Assays of haematological parameters
Blood was collected in a tube containing an anticoagulant (EDTA) and kept at a temperature between 2 and 8°C until analysis. The analysis of the formed elements of the blood (red blood cells, white blood cells and platelets) and the hemoglobin level were carried out at the Coulter. It identifies the number of pulses by which the number of cells and the amplitude of the pulse produced are determined. The pulse produced is in this case proportional to the volume of the cell. This method allows the construction of erythrocyte, leukocyte and platelet histograms and the determination of the associated parameters [15].
Histopathological study
Histopathological study was performed according to the method described by [16] and repeated by [17] and [18]. Histological evaluation was carried out using the hematoxylin and eosin (H&E) staining technique. The organs, fixed in 10% buffered formalin solution, were cut before being dehydrated in various degrees of alcohol and embedded in paraffin. The organ was then processed and sections were made using a 3-5 μm a spinning microtome before being mounted on glass slides and stained with hematoxylin and eosin. The stained tissue sections were then observed under a light microscope to determine the presence or absence of histopathological lesions.
Statistical analyzes
Statistical analysis was performed using SPSS software. Values were expressed as mean ± SEM and differences between groups were considered significant if p < 0.05.
Results and Discussion
Acute toxicity
Mitracarpus hirtus is a plant species that has many bioactive compounds with different pharmacological effects, either beneficial and/or toxic to human health. According to [19], consumer apprehension due to the lack of scientifically valid evidence has favored the carrying out of studies concerning the competence of plant species that are used by the public as natural medicines.
The results of this acute toxicity study on the n-butanol moiety of M. hirtus demonstrated that administration of single dose samples orally resulted in no deaths or behavioral changes across all of the different doses tested. Furthermore, no significant changes were observed in the body weight and diet of the rats, even at the maximum dose. The LD50 being greater than 5000 mg/ kg body weight, the extract thus presents a toxicity index of 5 corresponding to almost nontoxic according to the Hodge and Sterner scale. Moreover, on the histological level, the macroscopic observation does not reveal any lesion on the liver, the lungs and the kidneys. The corresponding results are in conformity with those of the research carried out by [2], on the crude ethanolic extract of the plant of M. hirtus. They reported that the extract delivered no mortality over the 24 hours of administration and therefore an LD50 > 5000 mg/ kg. In addition, they showed the absence of serious clinical symptoms at lower doses of 625 to 1500 mg/kg. But at higher doses (2500 to 5000 mg/ kg), some clinical symptoms such as a change in breathing and an increase in urine production were observed over the 10 hours of administration in their studies whereas they were absent in ours.
Sub-acute toxicity testing
Effects related to the repeated dose administration of the n-butanol extract of M. hirtus were studied by the evaluation the behavior of the rats, haematological, biochemical and histopathological parameters. Throughout the treatment period, the rats did not show any abnormal behavior.
Effects of extract on body weight and the food of rat
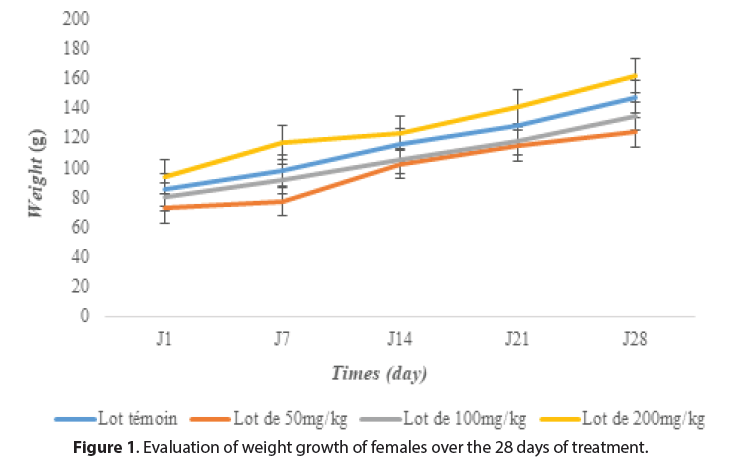
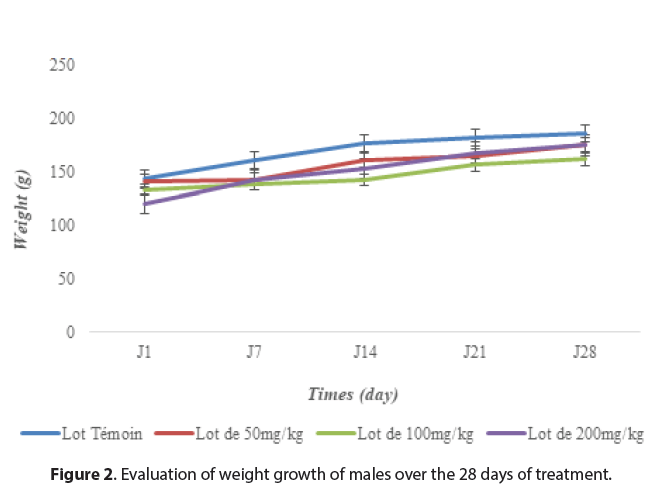
The rats, water and food consumption did not undergo any significant change (p>0.05) compared to the control groups throughout the experiment. However, the body weight of the rats increased during the experiment but did not differ significantly from the control rats (Figures 1and 2). This indicates that the plant extract of M. hirtus has insignificant quality levels on the growth of rats. Several researches confirm this, notably those of [20] and [21], which showed that the extract of this plant had no disturbance in the metabolism of proteins, carbohydrates or fats (Figures 1 and 2).
Effects of extract on the urine and relative organ weight
With regard to the weights of the organs removed from the rats (liver, lung and kidney), no statistically significant differences were noted compared to the control group in either males or females (Tables 1 and 2). According to [22] and[23], an increase in the relative weight of the liver or the reins is a true indicator relatively sensitive to nephrotoxicity or hepatotoxicity unlike its decrease. On this basis, we can say that the n-butanol fraction of M. hirtus does not induce any toxic effect on the kidneys and the liver, in accordance with the work of [2]. In addition, the qualitative analysis of the urinaries didn’t show the presence of Glucose, Blood, Protein, Ketone, Bilirubin, Nitrile, etc. at all doses tested.
| Groups | Kidneys | Liver | Lungs |
|---|---|---|---|
| Control | 1,54±0,30 | 7,16±1,20 | 1,73±0,20 |
| 50 mg/ml | 1,31±0,20 | 5,78±0,60 | 1,34±0,30 |
| 100 mg/ml | 1,14±0,10 | 5,31±0,14 | 1,31±0,20 |
| 200 mg/ml | 1,22±0,05 | 5,91±0,62 | 1,19±0,10 |
Table 1. Effects of butan-1-ol on relative organ weights in g of female rats.
| Groups | Kidneys | Liver | Lungs |
|---|---|---|---|
| Control | 1,81±0,30 | 4,16±1,20 | 1,82±0,20 |
| 50 mg/ml | 1,03±0,20 | 4,78±0,60 | 1,89±0,30 |
| 100 mg/ml | 1,92±0,10 | 3,31±0,14 | 1,84±0,20 |
| 200 mg/ml | 1,85±0,05 | 3,91±0,62 | 1,79±0,10 |
Table 2. Effects of butan-1-ol on relative organ weights in g of male rats.
Effects of extract on the biochemical parameters
The assay data for biochemical parameters show for creatinine and glucose, a non-significant modification in both males and females rats (Tables 3 and 4). In addition, the results on AST and ALT revealed no significant variation compared to control rats, except in females, where a significant decrease was noted at the dose of 100 mg/kg and 200 mg/kg. A decrease in AST and/or ALT hepatic enzymes could indicate a hepatoprotective effect of this extract [5, 24]. More, according to studies conducted by [25], these enzymes increase in case of myopathy, rhabdomyolysis or myocardial infarction or in case of hemolysis [26]. ALT are more specific for liver damage, but AST are somewhat more sensitive [27]. Therefore, according to these results obtained, it is very likely that the n-butanol fraction has a hepatoprotective action from a dose of 100 mg/kg in females. Therefore, we can say that there is no damage of hepatic origin. In addition, the genus of the species can also play a role in the evolution of certain parameters, which could explain the conformity of these results with those of [28] on the extracts of the aerial part of Mitracarpus frigidus. Because they had observed no significant variation in ALT and AST at doses below 1000 mg/kg (D=200 and 300 mg/kg).
| Groups | Glucose (mg/dl) | Creatinine (mg/dl) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| Control | 262,00±18,35 | 22,56±9,37 | 1,33±0,58 | 2,33±0,57 |
| 50 mg/ml | 182,00±2,65 | 14,16±3,75 | 1,00±0,00 | 2,11±0,57 |
| 100 mg/ml | 199,33±30,80 | 13,33±0,35 | 1,66±0,58 | 2,33±0,57 |
| 200 mg/ml | 131,66±61,37 | 12,20±0,82 | 1,00±0,00 | 2,33±0,57 |
Table 3. Effect of n-butanol extract on biochemical parameters of males rats.
Effects of extract on the haematological parameters
| Groups | Glucose (mg/dl) | Creatinine (mg/dl) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| Control | 153,00±10,20 | 18,16±3,70 | 55,33±11,50 | 63,00±18,38 |
| 50 mg/ml | 185,33±5,52 | 21,26±7,60 | 45,00±1,73 | 66,33±2,31 |
| 100 mg/ml | 204,33±21,46 | 21,00±5,20 | 30,66±8,50 | 4,33±3,21* |
| 200 mg/ml | 283,66±12,34 | 25,37±8,50 | 2,00±0,00* | 3,00±1,00* |
Table 4. Effect of n-butanol extract on biochemical parameters of female rats.
In the study of haematological parameters, no significant disturbance was observed in male and female rats. A slight increase in the number of red blood cells (RBC), white blood cells (WBC) and platelets (PLA) was obtained at the normal (100 mg/kg) and maximum dose (200 mg/kg) administered. Contrary to the work of [2], which showed significant decrease of WBC, MCV and platelet obtained at doses (500mg/kg) and (1000mg/kg) compared with the mean control group of ethanolic extracts of M. hirtus. The difference could be related to the doses administered which were higher than ours. Furthermore, our results corroborate those of [28] which showed that M. frigidus did not significantly affect the number of basophils, neutrophils, lymphocytes or eosinophils treated mice to increasing concentrations (100, 200, and 400 μg/mL), compared to the respective control. So we can say that the extract does not content substances that have a haematotoxic effect up to the dose of 200 mg/kg (Tables 5 and 6).
| Settings haematological | Control | D=50mg/kg | D=100mg/kg | D=200mg/kg |
|---|---|---|---|---|
| Lymphocyte (%) | 83,23±3,40 | 86,83±4,58 | 85,60±0,20 | 87,97±0,28 |
| Monocyte (%) | 9,47±2,30 | 7,10±0,40 | 7,77±1,61 | 8,47±0,70 |
| Hemoglobin (g/L) | 11,40±2,08 | 13,37±0,58 | 13,00±0,43 | 13,90±0,10 |
| Hematocrites (%) | 0,20±0,00 | 0,20±0,00 | 0,40±0,00 | 0,60±0,00 |
| GRA (%) | 9,60±0,52 | 8,40±0,30 | 7,30±0,87 | 4,40±0,56 |
| GB ( 103/mm3) | 10,40±1,173 | 7,80±1,67 | 8,27±1,01 | 15,40±0,36 |
| GR (103/mm3) | 0,04±0,00 | 0,013±0,00 | 0,86±0,03 | 1,17±0,07 |
| PLA (103/mm3) | 208±15,55 | 198±18,90 | 2444±206,47 | 3184,67±606,58 |
| TGMH | 64,10±0,00 | 62,00±0,00 | 62,00±0,00 | 84,83±5,98 |
| CCMH | 74,10±0,00 | 74,17±0,08 | 77,30±2,59 | 76,33±0,77 |
| VGM(µm3) | 55,33±1,15 | 55,00±2,01 | 44,67±1,07 | 40,33±1,60 |
| VMP(µm) | 2,40±0,00 | 2,37±0,06 | 2,60±1,70 | 4,37±0,06 |
Table 5. The haematological parameters of female rats.
| Settings haematological | Control | D=50mg/kg | D=100mg/kg | D=200mg/kg |
|---|---|---|---|---|
| Lymphocyte (%) | 73,50±0,00 | 71,40±0,00 | 86,23±0,05 | 83,30±0,14 |
| Monocyte (%) | 11,37±0,23 | 13,60±0,00 | 09,40±1,55 | 09,17±0,70 |
| Hemoglobin (g/L) | 12,40±1,04 | 12,70±1,07 | 11,37±1,13 | 11,87±0,07 |
| Hematocrites (%) | 23,20±0,42 | 19,67±0,64 | 16,37±0,21 | 17,20±0,60 |
| GRA (%) | 18,35±0,00 | 15,00±0,00 | 7,03±0,80 | 7,50±0,90 |
| GB ( 103/mm3) | 9,27±0,78 | 11,73±0,63 | 6,93±0,28 | 7,33±0,92 |
| GR (103/mm3) | 2,64±0,90 | 2,03±0,46 | 1,36±0,25 | 1,39±0,23 |
| PLA (103/mm3) | 1232,33±123,74 | 457,67±22,63 | 299,67±0,71 | 653,33±95,46 |
| TGMH | 92,67±0,00 | 99,33±1,41 | 121,33±4,95 | 118,00±1,00 |
| CCMH | 7,43±0,50 | 7,13±0,51 | 7,07±0,90 | 7,37±0,15 |
| VGM(µm3) | 51,76±1,69 | 65,27±9,62 | 83,43±3,39 | 81,20±3,41 |
| VMP(µm) | 55,80±5,30 | 66,13±4,52 | 68,97±0,92 | 68,70±1,41 |
Table 6. The haematological parameters of male rats..
Histopathological organs examination
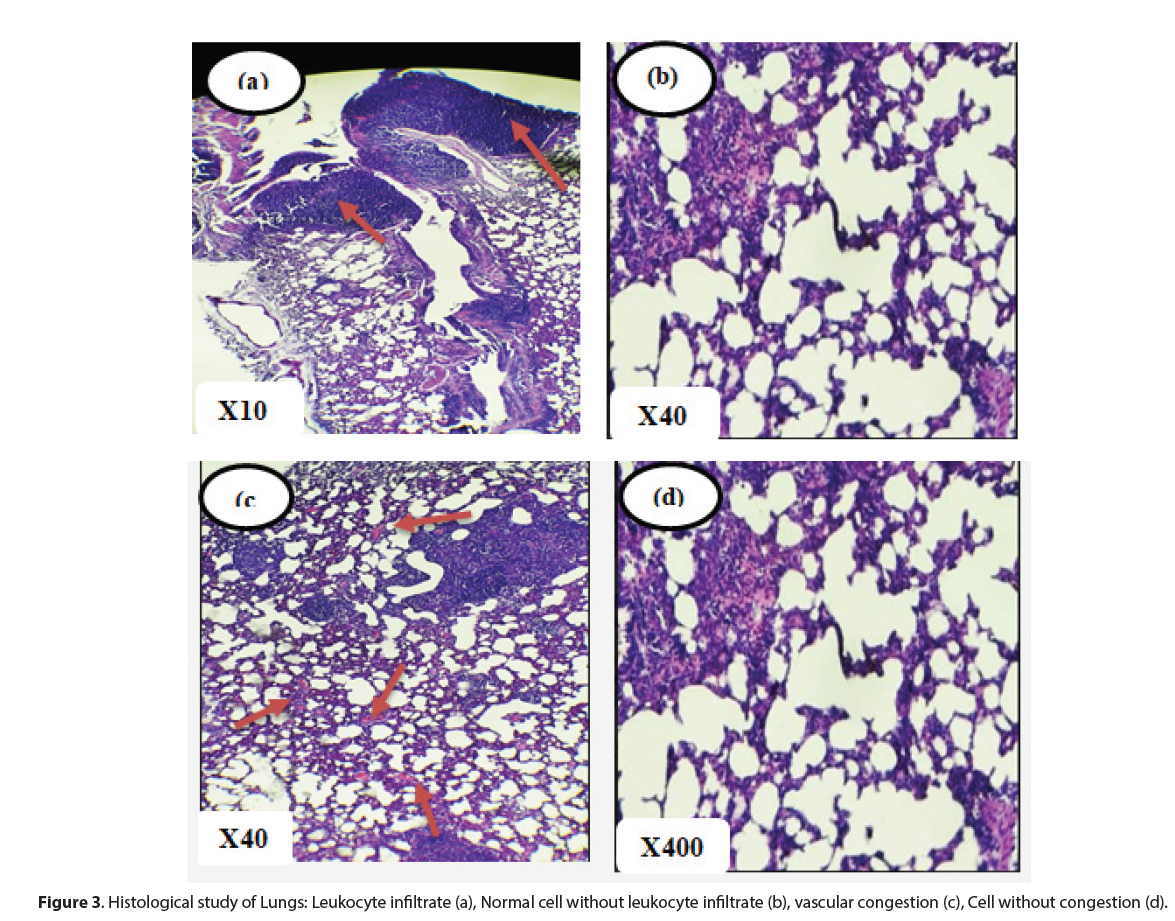
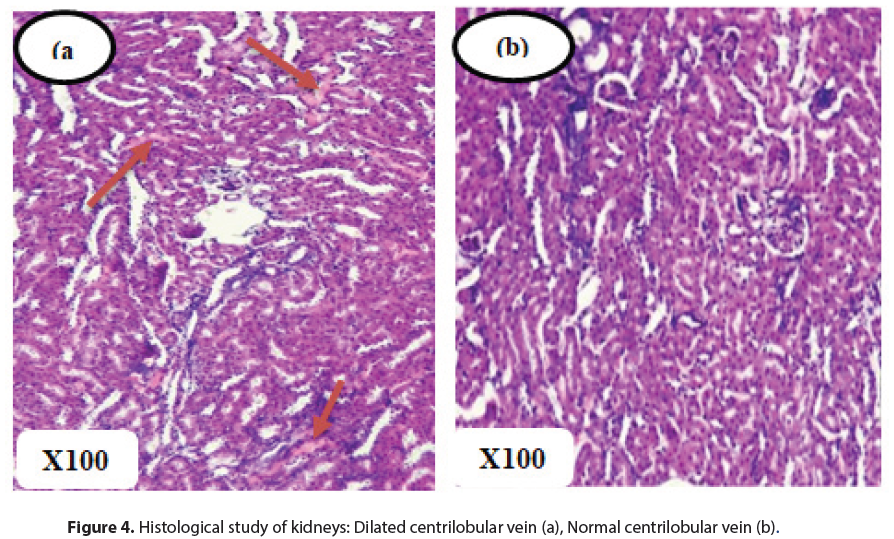
Histopathological analysis did not reveal any signs of cytolysis inherent to a possible toxicity of n-butanol extracts of M. hirtus leaves. Indeed, at the renal level, no organic lesions were observed in the glomeruli and tubules. However, interstitial vascular congestion was noted in both control and treated rats. In the lungs, signs of congestion were also observed, accompanied by diffuse lymphocytic inflammatory infiltration of the interalveolar septa and bronchioles in all groups of rats. These symptoms would therefore be independent of the effects of the n-butanol extract and the vascular congestion could be related to the sacrifice of the rats [29, 30].
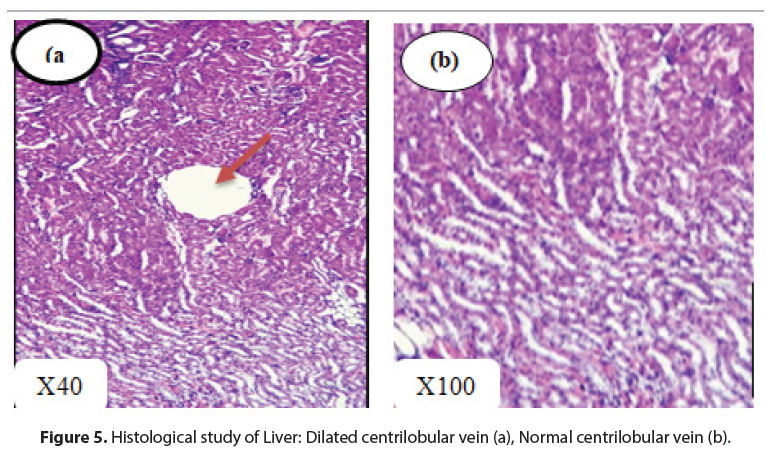
The livers had an overall normal histological structure. There was no evidence of necrosis, fibrosis or leukocytic infiltrate (Figures 3 and 4). These observations remain consistent with those of[31], who found no significant changes in the liver with the extract of Mitracarpus frigidus species. However, in this study, dilated centrilobular veins were observed in both treatment and control rats, which may be related to the oxygen requirements of the organ leading to dilation of vessels for better blood supply [32, 33].
As all these abnormalities were noted in all groups (even in control rats), the conclusion would be to exclude doubts about a possible correlation between the highlighted disturbances and the proper actions of the M hirtus n-butanol extract [34]. Furthermore, the results of the analysis of biochemical parameters, in particular ALT and AST, which did not vary significantly, constitute a confirmation of the absence of toxicity or serious lesions (Figure 5).
Conclusion
The n-butanol extract of Mitracarpus hirtus leaves, causing antioxidant and anti-α-amylase activities, did not show acute and sub-acute toxicity in Wistar rats by oral route. Indeed, the LD50 was higher than 5000 mg/kg body weight and no significant disturbance on biochemical and haematological parameters except a decrease of ASATS in females at high doses, index of a hepatoprotective effect of this extract. Histopathological analysis did not reveal any signs of cytolysis inherent to a possible toxicity of n-butanol extracts of M. hirtus leaves. These results confirm the traditional use of this plant in the management of diabetes.
Competing Interest
Authors have declared that no competing interests exist concerning this manuscript.
References
- Singhal KG, Ghanshyam DG. Hepatoprotective and Antioxidant Activity of Methanolic Extract of Flowers of Nerium Oleander against CCl 4-Induced Liver Injury in Rats. Asian Pac J Trop Med. 5, 677-685 (2012).
- Bkudu, Aminu A, Joseph O et al. Acute and Sub-Acute Toxicity Study of Ethanolic Crude Extract of Mitracarpus Hirtus Plant on Wistar Rats. Asian J of P Science. 2, 1-11 (2013).
- Sokar Z, Gadhi CA, Benharref A et al. Toxic Effect of Herniaria Cinerea DC. on the Stomach, Intestine, Lung, and Kidney of Rats. J Ethnopharmacol. 88, 149-153 (2003).
- Fabri, Rodrigo L, Roberta AG, Jonatas RF et al. Pentacyclic Triterpenoids from Mitracarpus Frigidus (Willd. Ex Roem. & Schult.) K. Shum: In Vitro Cytotoxic and Leishmanicidal and in Vivo Anti-Inflammatory and Antioxidative Activities. Medicinal Chemistry Research. 23, 5294-5304 (2014).
- Celia, Ouahchia, Cherif Hamida-saida et al. Toxicite Aigue et Subaigue Des Extraits Méthanoliques Acute and Subacute Toxicity of Inula Viscosa L. (Dittrichia Viscosa L.). Revue Agrobiologia. 7, 562-573 (2017).
- Ukwuani k, Angela N, Abubakar MG et al. Toxicological Studies of Hydromethanolic Leaves Extract of Grewia Crenata Toxicological Studies of Hydromethanolic Leaves Extract of Grewia Crenata. Int J of Pharma Sci Drug Res. (2012).
- Etienne, Ouattara K, Stefano D et al. Chemical Characterization, Antioxidant and Enzyme Inhibitory Effects of Mitracarpus Hirtus Extracts. J Pharm Biomed Anal. (2021).
- Faye, Ousmane, Cheikh S et al. Antioxidant and Anti -Amylase Activities of Polar Extracts of Mitracarpus Hirtus and Saba Senegalensis and the Combinason of Their Butanolic Extracts. International Research J. 22, 1-8 (2021).
- Faye, Ousmane, Cheikh S et al. Evaluation of Antioxidant and Anti α -Amylase Activity of Flavonoids and Alkaloids Extracted from Mitracarpus Hirtus Leaves. 10, 59-64 (2022).
- OECD. OECD Ligne Directrice 211 Guielines for the Testing of Chemicals Daphnie. (2008).
- Bensakhria, Ayoub. Relation dose-effet et optimisation de la dosimétrie en radiothérapie interne sélective du carcinome hépatocellulaire. (2018).
- Junge, Wolfgang, Baerbel W et al. Determination of Reference Intervals for Serum Creatinine, Creatinine Excretion and Creatinine Clearance with an Enzymatic and a Modified Jaffé Method. Clinica Chimica Acta. 344,137-148 (2004).
- Sanghavin NM, Jivani NG. A Colorimetric Method for the Determination of Nitrazepam. Talanta. 26, 63-64 (1979).
- Akter, Shamima, Hossain Uddin Shekhar et al. Application of Biochemical Tests and Machine Learning Techniques to Diagnose and Evaluate Liver Disease. Advances in Bioscience and Biotechnology. 12, 154-172 (2021).
- Ndong Awa. Acute and Subacute Toxicity of Annona Senegalensis Pers . and Annona Muricata L . ( Annonaceae ) and Their Effects on Biochemical and Haematological Parameters on Wistar Rats. Journal of Toxicology and Pharmacology. (2022).
- Lamb. Manual of Veterinary Laboratory Techniques in Kenya. Ministry of Livestock Development (1981).
- Gome, MB, Kouakou K, Toure A et al. Etude de La Toxicité Aiguë et Subchronique de l’extrait Aqueux de <em>Passiflora Foetida</Em> Linn. (Passifloraceae) Chez Les Rats et Les Souris. International J of Biological and Chemical Sci. (2012).
- Suriyavadhana M, Pakutharivu T. Evaluation of Acute and Sub-Acute Toxicity of Ethanol Extracts of Entada Pursaetha , Toddalia Aculeata , and Ziziphus Mauritiana. World J Life Sci and Med Res. 1, 43-47 (2011).
- Saad B, Hassan A, Ghassan A et al. Safety of Traditional Arab Herbal Medicine. Evid Based Complement Alternat Med. 3, 433-439 (2006).
- Mir AH, Sexena M, Malla MY. An Acute Oral Toxicity Study of Methanolic Extract from Tridex Procumbens in Sprague Dawley’s Rats as per OECD Guidelines 423. 18, 1-14 (2013).
- Rajalakshmi, Narayanaswamy. Pharmacology & Toxicology Toxicity Analysis of Different Medicinal Plant Extracts in Swiss Albino Mice. Pharm & Toxicology Research. (2018).
- William MK. Renal Function Tests as Indicators of Kidney in Subacute Toxicity Studies. Toxicology and Applied Pharma. 57, 414-424 (1981).
- Manda P. Etude des Toxicities Aigue et Subaiguë Du Remède Nature. Rev Ivoir Sci Technol. 145-158 (2017).
- Musila, Michael N, David N et al. Acute and Sub-Chronic Oral Toxicity Study of Methanolic Extract of Caesalpinia Volkensii (Harms). J of Drug Metabolism & Toxicology. 08, 1-8 (2017).
- Jacques. Etude de La Toxicité Aigue et Subaigüe de l’ Extrait Au Vin Des Graines de Carica Papaya Linn. J. Appl. Biosci. (2017).
- Guessan, Amenan G, Kouakou EE et al. La Toxicité Subaiguë de l ’ é Corce de Racines de Dichrostachys Cinerea ( L .) Wight Subacute Toxicity of Dichrostachys Cinerea ( L .) Wight & Arn . ( Fabaceae ). Root Bark. 13, 836-48 (2019).
- Mukinda JT, Syce JA. Acute and Chronic Toxicity of the Aqueous Extract of Artemisia Afra in Rodents. J Ethnopharmacol. 112, 138-144 (2007).
- Fabri, Rodrigo Luiz, Danielle Maria et al. In-Vivo Laxative and Toxicological Evaluation and in-Vitro Antitumour Effects of Mitracarpus Frigidus Aerial Parts. J Pharm Pharmacol. 64, 439-448 (2012).
- Sun H, Lynda F, Leslie Z et al. Effects of Renal Failure on Drug Transport and Metabolism. Pharmacol Ther. 1109, 1-11 (2006).
- Nana M, Ngon RA. Ngane JR. Acute and Sub-Acute Toxicity of the Methanolic Extract of Pteleopsis Hylodendron Stem Bark. J Ethnopharmacol. 137, 70-76 (2011).
- Fabri RL, Nogueira MS, Braga FG et al. Mitracarpus Frigidus Aerial Parts Exhibited Potent Antimicrobial, Antileishmanial, and Antioxidant Effects. Bioresour Technol. 100, 428-433 (2009).
- Moussa, Gbogbo, Oussou N et al. Acuminata ( Rubiaceae ) Chez Le Rat Wistar [ Acute Toxicity Study of an Ethanolic Extract of Massularia Ac ... Etude de La Toxicité Aigüe d ’ Un Extrait Éthanolique Des Tiges de Massularia Acuminata ( Rubiaceae ) Chez Le Rat Wistar. Acute Toxicity Study. 35, 260-267 (2022).
- Ferreira, Carpejane, Ana C et al. Acute and Sub-Chronic Toxicity Study of the Extract and Powder of Operculina Macrocarpa ( L .) Urb . in Mice. Afr J Biotechnol Ataxia. 15, 2776-2783 (2016).
- Toyoda k, Shibutani M, Tamura T et al. Repeated Dose 28-Day Oral Toxicity Study in Rodents (OECD TG 407). Arch Toxicol. 74,127-132 (2000).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref