Research Article - Diabetes Management (2019) Volume 9, Issue 2
A study investigating the pharmacokinetic properties of insulin degludec/insulin aspart in healthy Chinese subjects
- Corresponding Author:
- Hanne Haahr
Novo Nordisk A/S, Vandtårnsvej 114, 2860 Søborg, Denmark
E-mail: hhaa@novonordisk.com
Abstract
Objective: This study aimed to characterize the pharmacokinetic (PK) properties of the insulin degludec (degludec)/insulin aspart (IAsp) co-formulation (IDegAsp) in healthy Chinese subjects, and compare these with previous findings in Caucasian and Japanese subjects.
Methods: In this single-center, open-label study, 24 healthy Chinese adults received a single dose (SD) of IDegAsp (0.5 U/kg body weight). Regular blood sampling for serum IAsp and degludec measurements was performed for the first 20 hours, and less frequently up to 120 hours post-dose.
Results: Total exposure of IAsp (AUCIAsp,total,SD) was estimated as 541 pmol·h/L (95% confidence interval [CI] 502;582), maximum concentration (Cmax,IAsp,SD) as 217 pmol/L (95%CI 193;244), time to maximum concentration (tmax,IAsp,SD) as 1 hour, and onset of appearance as 14 min. Total exposure of degludec (AUCdegludec,total,SD) was estimated as 88,271 pmol·h/L (95%CI 83,615;93,187), maximum concentration (Cmax,degludec,SD) as 3472 pmol/L (95%CI 3013;4002), and time to maximum degludec concentration (tmax,degludec,SD) as 10.5 hours. Importantly it should be noted that serum concentrations of degludec and IAsp cannot be directly compared and are not additive, as concentrations differ by several orders of magnitude due to the reversible binding of degludec to serum albumin. Six treatment-emergent adverse events occurred in three subjects; none were serious. The PK properties of IAsp and degludec were consistent with those observed in previous trials in Japanese and Caucasian subjects.
Conclusion: The rapid absorption of IAsp and the ultra-long PK profile of degludec were present in healthy Chinese subjects. The clinical benefits of IDegAsp observed in previous IDegAsp studies are expected in Chinese patients, and no specific IDegAsp dosing recommendations should be required. Clinicaltrials.gov identifier: NCT02844790
Keywords
■Chinese ■ insulin degludec/insulin aspart ■pharmacokinetics
Highlights
• The distinct pharmacokinetic profiles of the IDegAsp component insulins are present in healthy Chinese adults, and are consistent with results from previous studies in Japanese and Caucasian populations.
• The clinical benefits of IDegAsp observed in other populations are also expected in Chinese patients.
Introduction
Chinese Diabetes Society (CDS) guidelines recommend the use of either once-daily basal insulin or once- or twice-daily premix insulin for insulin initiation in Chinese patients with type 2 diabetes (T2D), if adequate glycemic control (i.e. HbA1c <7%) cannot be achieved with lifestyle changes plus metformin and ≥1 oral antidiabetic drugs [1]. When further intensification is required, the CDS guidelines recommend multiple daily injections of insulin (basal-bolus regimen or 2-3 injections of premix insulin) or continuous subcutaneous insulin infusion [1]. Premix insulin, which covers both basal and prandial insulin needs in one injection, is a widely used treatment option in China [2,3]. However, overlap between the PK/PD profiles of current premix insulin components (biphasic suspensions with soluble insulin and protaminecrystallized insulin) results in a ‘shoulder effect’ (i.e. a prolonged post-meal glucose-lowering action with inadequate duration of basal action) [4]. The composition of premix insulin also necessitates resuspension prior to use, creating substantial variability in the pharmacological response, and an additional requirement for patient education [5]. IDegAsp (Ryzodeg 70/30, Novo Nordisk A/S, Søborg, Denmark) is a unique co-formulation of two insulin analogues: 70% ultra-long-acting insulin degludec (degludec) and 30% rapid-acting insulin aspart (IAsp), approved for the treatment of diabetes in adults in many countries [6,7]. Unlike premix insulin formulations, degludec and IAsp are present in IDegAsp as separate stable, soluble components; thus, IDegAsp is optimized for use without the need for resuspension. IDegAsp provides a superior glucose-lowering profile versus premix insulins, with fewer daily injections versus basalbolus regimens [8]. The individual components of IDegAsp underpin the mode of action. After subcutaneous injection, IAsp hexamers immediately break down into rapidly absorbed monomers, providing a rapid onset of glucoselowering effect. In contrast, upon injection, degludec di-hexamers form long chains of multi-hexamers in a subcutaneous depot [9]. Monomers are slowly and continuously released from the hexamers into the circulation, where they attach with high selectivity to circulating albumin, producing a flat, long-lasting glucoselowering effect [6,8,10,11]. Race and ethnic background may influence the pharmacological properties of exogenous insulin and could potentially affect dosing recommendations [12,13]. To date, the pharmacological properties of IDegAsp have been described in Japanese and Caucasian subjects with type 1 diabetes (T1D) [11,14-17]. In addition, the PK properties of the IAsp component of IDegAsp have been described in Caucasian subjects with T1D [11,15], and the PK properties of degludec have been described in Japanese and Caucasian subjects with T1D [10,18,19], Caucasian, African American and Hispanic/Latino subjects with T2D [20,21], as well as healthy Chinese adults [22]. The present study aimed to characterize the PK properties of IDegAsp in healthy Chinese adults, enabling comparison with IDegAsp data obtained from previous trials in other populations which used similar methodology.
Methods
■ Study design
This was a single-center (Clinical Trial Centre, Beijing Hospital), open-label, single-dose study investigating the PK properties of IDegAsp in healthy Chinese adults (ClinicalTrials. gov identifier: NCT02844790). The protocol was reviewed and approved according to local regulations by the appropriate health authorities, and the local Ethics Committee of Beijing Hospital (IRB approval number: 2015BJYYEC-094-02). The study was conducted in agreement with the Declaration of Helsinki (World Medical Association 2008). All subjects gave their informed consent prior to inclusion in the study.
■ Subjects
Subjects were healthy male or female Chinese adults aged 18-45 years (both inclusive), with body mass index 19–24 kg/m2 (both inclusive) and fasting plasma glucose ≤6.0 mmol/L. Exclusion criteria included abnormal hematology, biochemistry, lipid, or urinalysis screening test results; systolic blood pressure <90 or >140 mmHg; diastolic blood pressure <60 or >90 mmHg; any potentially confounding illness; pregnancy or intention of becoming pregnant; or use of non-herbal Chinese or local medicine with unknown/unspecified content within the 90 days prior to screening. No concomitant illnesses or medications were recorded in the included trial population at screening. One subject reported using levofloxacin hydrochloride and sodium bicarbonate during the trial period.
■ Procedures
All subjects attended a screening visit, a dosing visit (2-14 days after screening), and a follow-up visit (7-21 days after dosing visit). Subjects were instructed to avoid smoking, strenuous exercise, consumption of alcohol, coffee, tea, or beverages containing methylxanthine for 24 hours prior to the dosing visit. At the dosing visit, all eligible subjects received a single dose of IDegAsp (0.5 U/kg body weight) via subcutaneous injection in the lower abdomen using a 3 mL pre-filled pen (PDS290 [FlexTouch®] 100 U/mL, Novo Nordisk A/S). Subjects were provided with standard meals (breakfast [served after dosing], lunch [~4 hours after breakfast], and dinner [~10 hours after breakfast]). Subjects were permitted water ad libitum throughout; however, tea and coffee were prohibited. If signs of hypoglycemia occurred, subjects were encouraged to have sweet drinks, snacks, or regular food. Intravenous glucose could be administered if deemed necessary by the investigator. Subjects stayed in the clinic for 48 hours post-dose, during which blood samples were taken for PK analysis. Following discharge from the clinic, subjects returned for blood sampling at 72, 96, and 120 hours post-dose. Blood sampling was performed pre-dose (-15 and 0 min) and frequently thereafter until 12 hours for IAsp (every 10 min until 120 min, 135 min, 150 min, 165 min, 180 min, 210 min, and 240 min, then every hour until 12 hours) and until 120 hours for degludec (30 min, 1 hour, 2 hours, 4 hours, 6 hours, then every hour until 20 hours, 22 hours, 24 hours, 30 hours, 36 hours, 48 hours, 72 hours, 96 hours, and 120 hours). Plasma glucose was monitored at regular intervals until 20 hours post-dose, and then concurrently with PK blood sampling until discharge.Serum degludec and IAsp concentrations were quantified using a validated, specific enzyme-linked immunosorbent assay.
■ Outcomes
The primary endpoint was to assess the total PK exposure after a single dose of IDegAsp in terms of degludec and IAsp, determined using area under the serum IAsp concentration–time curve from 0 to 12 hours (AUCIAsp,total,SD), and area under the serum degludec concentration–time curve from 0 to 120 hours (AUCdegludec,total,SD). Secondary endpoints included additional PK properties and assessment of the safety and tolerability of IDegAsp. Safety data included treatment-emergent adverse events (AEs), electrocardiogram, physical examination, vital signs, and laboratory assessments.
■ Statistical analyses
Endpoints were analyzed from data obtained from all subjects dosed with IDegAsp. Primary endpoints were derived from individual PK profiles using the linear trapezoidal technique based on observed values. Missing data were imputed using linear interpolation. Secondary PK endpoints were derived from insulin concentration–time curves. All PK endpoints and safety data were summarized using descriptive statistics. Statistical operations were performed using Statistical Analysis System (SAS) version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
■ Subjects
Of the 65 subjects screened, 24 were eligible for the study. The majority of subjects (32) failed screening due to violation of inclusion or exclusion criteria. Nine subjects failed screening due to withdrawal of consent. All eligible subjects were exposed, completed the study, and contributed to the PK and safety analyses. There were no withdrawals due to safety concerns or protocol deviation. Baseline demographics are shown in Table 1.
| Characteristic | Mean (SD) | Median |
|---|---|---|
| Age, years | 28.6 (5.2) | 26.5 |
| Weight, kg | 62.7 (6.0) | 63.5 |
| BMI, kg/m2 | 22.0 (1.3) | 22 |
| Male, n (%) | 15 (62.5) | |
| Female, n (%) | 9 (37.5) | |
| Race, Asian non-Indian (Chinese), n (%) | 24 (100) |
BMI: body mass index; SD: standard deviation
Table 1. Baseline characteristics
■ IAsp PK endpoints in healthy Chinese subjects following a single dose of IDegAsp
The estimated mean total IAsp exposure following a single dose of IDegAsp (AUCIAsp, total, SD) was 541 pmol·h/L (95% confidence interval [CI] 502;582). The estimated mean maximum IAsp concentration (Cmax,IAsp,SD) was 217 pmol/L (95% CI 193;244). The estimated median onset of IAsp appearance (time from administration until concentration >30 pmol/L) was 14 min, and the median time to maximum IAsp concentration (tmax,IAsp,SD) was 1.0 hour.
■ IAsp PK characteristics obtained in healthy Chinese subjects and other populations following a single dose of IDegAsp
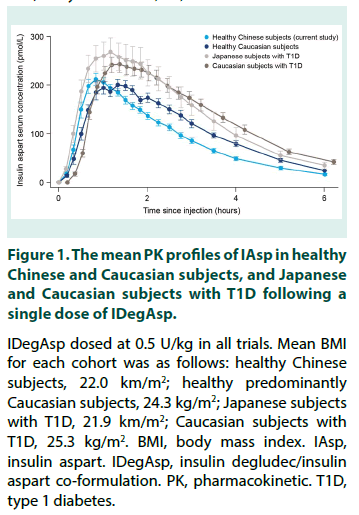
To facilitate comparison of IAsp PK characteristics following a single dose of IDegAsp in healthy Chinese subjects versus those in other populations, the IAsp PK profiles in healthy Chinese subjects, heathy Caucasian subjects, and Caucasian and Japanese subjects with T1D are shown in Figure 1 [17,23,24]. In addition, selected IAsp PK characteristics following singledose IDegAsp derived from the present study and other PK trials with similar methodology are provided in Table 2 [17,23,24].
Figure 1: The mean PK profiles of IAsp in healthy Chinese and Caucasian subjects, and Japanese and Caucasian subjects with T1D following a single dose of IDegAsp.
IDegAsp dosed at 0.5 U/kg in all trials. Mean BMI for each cohort was as follows: healthy Chinese subjects, 22.0 km/m2; healthy predominantly Caucasian subjects, 24.3 kg/m2; Japanese subjects with T1D, 21.9 km/m2; Caucasian subjects with T1D, 25.3 kg/m2. BMI, body mass index. IAsp, insulin aspart. IDegAsp, insulin degludec/insulin aspart co-formulation. PK, pharmacokinetic. T1D, type 1 diabetes.
| Population | AUCIAsp,total,SD (pmol·h/L), mean (CV) | Cmax,IAsp,SD (pmol/L), mean (CV) | tmax,IAsp,SD (hours), median (min;max)(min;max) |
Onset of appearance† (minutes), median (min;max) |
|---|---|---|---|---|
| Healthy, Chinese (n = 24) (current study) |
541 (18) | 217 (27) | 1.0 (0.7;1.3) |
14 (7;28) |
| Healthy, predominantly Caucasian (n = 26) | 645 (22) | 218 (27) | 1.3 (0.7;2.5) |
20 (6;34) |
| T1D, Japanese (n = 21) |
813 (53) | 280 (49) | 1.2 (0.5;2.8) |
12 (2;23) |
| T1D, Caucasian (n = 27) |
833 (33) | 252 (30) | 1.3 (0.5;2.8) |
N/A |
AUCIAsp,total,SD, total IAsp exposure (area under the serum aspart concentration–time curve) following an SD of IDegAsp. Cmax,IAsp,SD, maximum IAsp concentration following an SD of IDegAsp. CV, coefficient of variation. IAsp, insulin aspart. IDegAsp, insulin degludec/insulin aspart co-formulation. N/A, not applicable. tmax,IAsp,SD, time to maximum IAsp concentration following IDegAsp SD. SD, single dose. T1D, type 1 diabetes.
Table 2. PK properties of IAsp in different populations following a single dose of IDegAsp
■ Degludec PK endpoints in healthy Chinese subjects
The estimated mean total degludec exposure following single-dose IDegAsp (AUCdegludec, total, SD) was 88,271 pmol·h/L (95% CI 83,615;93,187). The estimated mean maximum degludec concentration (Cmax,degludec,SD) was 3472 pmol/L (95% CI 3013;4002), and the median time to maximum degludec concentration (tmax,degludec,SD) was 10.5 hours.
■ Degludec PK characteristics in different populations following IDegAsp or degludec after a single dose, and degludec at steady state
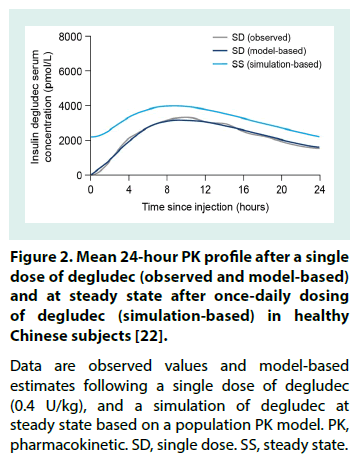
The degludec PK profile, observed following a single dose (0.4 U/kg) of degludec in healthy Chinese subjects, is displayed in Figure 2 alongside PK profiles derived from a population PK model (non-linear mixed-effects method) applied to simulate degludec at steady state in these subjects [22]. To facilitate comparisons across populations, overviews of selected PK properties are provided in Table 3 (single-dose IDegAsp or degludec) [17,22-24] and Table 4 (at degludec steady state) [10,19,22,25-28].
Figure 2: Mean 24-hour PK profile after a single dose of degludec (observed and model-based) and at steady state after once-daily dosing of degludec (simulation-based) in healthy Chinese subjects [22].
Data are observed values and model-based estimates following a single dose of degludec (0.4 U/kg), and a simulation of degludec at steady state based on a population PK model. PK, pharmacokinetic. SD, single dose. SS, steady state.
| Population | AUCdegludec,total,SD (pmol·h/L), mean (CV) |
Cmax,degludec,SD (pmol/L), mean (CV) |
tmax,degludec,SD (hours), median (min; max) |
|---|---|---|---|
| Healthy, Chinese† (n = 24) (current study) |
88,271 (13) | 3472 (35) | 10.5 (7.0; 20.0) |
| Healthy, Chinese‡ (n = 24) |
78,192 (11) | 3489 (26) | 11.0 (4.0; 15.0) |
| Healthy, mainly Caucasian† (n = 26)* |
76,398 (15) | 2400 (20) | 13.0 (7.0; 19.0) |
| T1D, Japanese† (n = 21) |
66,178 (45) | 2068 (36) | 12.0 (6.0; 24.0) |
| T1D, Caucasian† (n = 27) |
81,363 (23) | 2498 (22) | 13.0 (8.0; 22.0) |
Table 3. PK properties of degludec in different populations following a single dose of IDegAsp or degludec
| Population | AUCdegludec,T,SS (pmol·h/L), mean |
AUCdegludec,total,SS/ AUCdegludec,τ,SS, mean |
Cmax,degludec,SS (pmol/L), mean |
tmax,degludec,SS (hours), median |
|---|---|---|---|---|
| Healthy, Chinese (n = 24) |
76,609 | 0.53 | 3996 | 9.5 |
| Healthy, Caucasian (n = 19) |
74,353 | 0.51 | 3367 | 10.5 |
| T1D, Japanese (n = 21) |
81,270 | 0.53 | 4311 | 8.0 |
| T1D, Caucasian (n = 21) |
82,612 | 0.53 | 4363 | 9.0 |
Table 4. PK properties of degludec at steady state in different populations
■ Safety
IDegAsp was well tolerated in all subjects. A total of six treatment-emergent AEs (otitis externa, mouth ulceration, chest discomfort, and diarrhea, dyspnea, and heart palpitations) were observed in three subjects; no AEs were serious and all were mild in severity. No AEs were assessed by the investigator as being probably related to the study drug. No safety concerns were raised following electrocardiogram, physical examination, or laboratory assessments.
Discussion
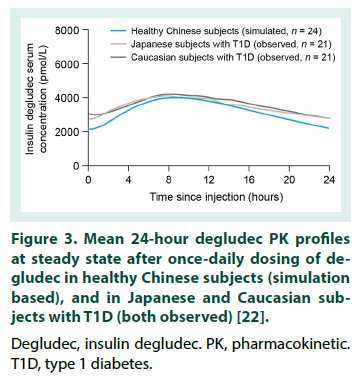
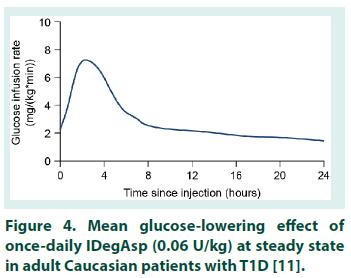
This is the first study to investigate the PK properties of IDegAsp in healthy Chinese adults. However, before the results are considered in detail, it is important to note that serum concentrations of degludec and IAsp are not additive and should not be directly compared. Serum concentrations of degludec and IAsp differ by several orders of magnitude due to the reversible binding of degludec to albumin in the circulation, thus the translation of PK data for the individual degludec and IAsp Data are observed values and model-based estimates following a single dose of degludec (0.4 U/kg), and a simulation of degludec at steady state based on a population PK model. PK, pharmacokinetic. SD, single dose. SS, steady state. components into PD results for IDegAsp is complex [8]. The degludec component has a half-life of >25 hours, and clinically meaningful steady state serum concentrations are only achieved after 2-3 days of once-daily IDegAsp administration. Therefore, given the singledose design, interpretation of the present study is necessarily focused on ascertaining whether the IDegAsp PK profile confirms separation of the basal and bolus glucose-lowering actions in a Chinese population (i.e. confirms the absence of the ‘shoulder effect’, reported with premix insulins [4]), rather than serving as a broader investigation into the clinical efficacy of IDegAsp. The shape of the mean IAsp PK profile following a single dose of IDegAsp in healthy Chinese adults was similar to that obtained in other populations (Table 1) [17,23,24]. In addition, the AUCIAsp,total,SD observed in healthy Chinese adults was comparable with the exposure levels in other populations, and appears to be lower in healthy individuals compared with people with T1D [17,23,24]. The Cmax,IAsp,SD, tmax,IAsp,SD, and time to onset of IAsp appearance in healthy Chinese adults were also comparable with the same endpoints in healthy Caucasian subjects, and Caucasian and Japanese subjects with T1D [17,23,24]. Taking normal inter-subject variation into consideration, these data suggest similar PK properties for IAsp when administered as IDegAsp in all populations investigated to date. For the degludec component of IDegAsp, the AUCdegludec,total,SD in healthy Chinese subjects was comparable with that in other populations following single-dose IDegAsp or degludec (Table 3) [17,22-24].The tmax,degludec,SD, however, was of marginally shorter duration in healthy Chinese subjects versus other populations [17,22-24]. The maximum concentration of degludec (Cmax,degludec,SD ) obtained in healthy Chinese subjects following a single dose of IDegAsp (at 0.5 U/kg, equal to 0.35 U/kg degludec, geometric mean: 3472 pmol/L), or, as recently reported by Hu et al. following a single dose of 0.4 U/ kg degludec (geometric mean: 3489 pmol/L) [22], was higher than that in other populations (range of geometric mean values reported in healthy Caucasian subjects, and Caucasian and Japanese subjects with T1D: 2068-2498 pmol/L) [17,23,24]. In order to reflect clinical practice (whereby degludec exposure following a single dose of IDegAsp corresponds to the first dosing interval in a once-daily regimen, with exposure progressing to steady state over the first few days), Cmax,degludec,SD must be considered in the steady state context. At steady state, degludec exposure over 24 hours reflects absorption of the previous 2-3 days of injections, producing a flatter, more consistent PK profile compared with similar observations after a single dose [8]. For both IAsp and degludec, the between-subject variability in insulin absorption was low as the observed coefficient of variation in PK endpoints was small. Recent data confirmed the ability of a population PK model to accurately predict the degludec PK profile following a single dose of degludec in healthy Chinese subjects (note the similarity of the model-based and observed single-dose profiles, Figure 2) [22]. The model was subsequently employed to simulate a steady state degludec PK profile. Additional modelling data demonstrated the similarity of the degludec PK profiles that have been simulated with IDegAsp at steady state in healthy Chineseo subjects, healthy Caucasian adults, and Caucasian and Japanese subjects with T1D (Figure 3) [10,19,22]. Across the wider literature, degludec PK endpoints reported in healthy Chinese subjects with steady state degludec (including the Cmax,degludec,SS and tmax,degludec,SS) are comparable with the same endpoints reported in other populations (Table 4) [10,19,25-28]. Given that the PK properties of IAsp after a single dose of IDegAsp, and degludec at steady state in healthy Chinese subjects, were comparable with other populations, it is reasonable to infer that IDegAsp PD data from Caucasian and Japanese adults are applicable to Chinese adults (see Haahr et al. 2017 for an overview).The glucoselowering profile of IDegAsp at steady state over 24 hours (administered once-daily in patients with T1D) shows a clear separation between the bolus and basal components; the rapid increase and distinct peak of IAsp, and the flat, consistent, ultra-long duration of degludec (Figure 4) [11]. Throughout the IDegAsp clinical development program, IDegAsp has demonstrated effective fasting and postprandial glucose control, with a relatively low risk of hypoglycemia (including nocturnal episodes), in both insulin-naive and insulin-experienced patients with diabetes (refer to Haahr et al. 2017 for an overview). The low within-subject day-to-day variability in the glucose-lowering effect of the basal component should allow for IDegAsp to be titrated to low fasting plasma glucose targets without unacceptable rates of nocturnal hypoglycemia [29,30]. A treat-to-target trial with intervals of 8-40 hours between injections demonstrates how the protracted, flat, glucose-lowering action of the degludec basal component allows flexibility in the timing of IDegAsp administration [31]. It has also been argued that the ultra-long duration of action of the IDegAsp basal component may reduce the impact of missed injections in Japanese patients [19]. Given that we demonstrate the applicability of the Japanese and Caucasian PD data, this would also be expected for Chinese patients.
Figure 3: Mean 24-hour degludec PK profiles at steady state after once‑daily dosing of degludec in healthy Chinese subjects (simulation based), and in Japanese and Caucasian subjects with T1D (both observed) [22].
Degludec, insulin degludec. PK, pharmacokinetic. T1D, type 1 diabetes.
Figure 4: Mean glucose-lowering effect of once-daily IDegAsp (0.06 U/kg) at steady state in adult Caucasian patients with T1D [11].
Limitations
The main limitations of the present study were the single-dose design (being representative of the first day of IDegAsp once-daily dosing only), and that no direct PD measurements were performed. However, the study fulfilled the intention of demonstrating that, following a single dose of IDegAsp, the PK properties of the component insulins in Chinese adults were comparable with those obtained in other populations.
Conclusion
This study demonstrates that the distinct PK profiles of the IDegAsp component insulins are present in healthy Chinese adults, and are consistent with similar observations in Japanese and Caucasian populations. Although insulin doses are adjusted on an individual basis, this study suggests that no specific IDegAsp dosing recommendations are required for Chinese patients. The clinical benefits of IDegAsp observed in other populations, including basal and prandial insulin coverage with one injection and low rates of hypoglycemia, are also expected in Chinese adults.
Acknowledgements
The authors would like to thank Xu Beibei, Ting Jia, Edmond Gabriel Fita, Jing Lu, Jing Yang and Catherine Jing Zhu (Novo Nordisk A/S) for their contributions to the trial and preparation of the manuscript. Medical writing and editing assistance were provided by James Currie, Helen Parker and Erin Slobodian of Watermeadow Medical, an Ashfield Company, part of UDG Healthcare plc, funded by Novo Nordisk. This study was supported by Novo Nordisk.
Funding
This study was supported by Novo Nordisk.
Data sharing
The subject level analysis data sets presented in the publication are available from the corresponding author on reasonable request.
Disclosures
Shi Aixin, Li Yang, Xie Panpan, Qi Wenyuan and Yang Lei have no conflicts of interest to declare. Zhang Ran, Hanne Haahr and Hongfei Xu are employees of Novo Nordisk. Hanne Haahr and Hongfei Xu are also shareholders of Novo Nordisk.
Contributions
Shi Aixin contributed to the concept/design of the protocol, subject recruitment, data collection, data interpretation, and statistical analysis. Li Yang contributed to the concept/ design of the protocol, subject recruitment, data collection, data interpretation, and statistical analysis. Xie Panpan contributed to subject recruitment, data collection, data interpretation, and statistical analysis. Qi Wenyuan contributed to subject recruitment, data collection, data interpretation, and statistical analysis. Yang Lei contributed to subject recruitment, data collection, data interpretation, and statistical analysis. Xu Hongfei contributed to the concept/ design of the protocol, medical support to trial execution and data interpretation. Zhang Ran contributed to the statistical analysis. Hanne Haahr was responsible for trial design and data interpretation. All authors contributed to drafting and critical revision of the manuscript.
References
- Chinese Diabetes Society. Guidelines for the prevention and treatment of type 2 diabetes in China (2017 edition). Chin. J. Diabetes. Mellitus. 10, 4-67 (2018).
- He X, Chen L, Wang K et al. Insulin adherence and persistence among Chinese patients with type 2 diabetes: a retrospective database analysis. Patient. Prefer. Adherence. 11, 237-245 (2017).
- Polinski JM, Kim SC, Jiang D et al. Geographic patterns in patient demographics and insulin use in 18 countries, a global perspective from the multinational observational study assessing insulin use: understanding the challenges associated with progression of therapy (MOSAIc). BMC. Endocr. Disord. 15(1), 46 (2015).
- Cengiz E, Swan KL, Tamborlane WV et al. The alteration of aspart insulin pharmacodynamics when mixed with detemir insulin. Diabetes. Care. 35(4), 690-692 (2012).
- Suzuki K, Aoki C, Kato K et al. Evaluation of a premixed insulin analog suspension in Japanese people with type 2 diabetes and the clinical importance of improved injection techniques: a cross-sectional pilot study. Diabetes. Ther. 8(2), 445-449 (2017).
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002499/WC500139011.pdf
- https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/203313Orig1s000ltr.pdf
- Haahr H, Fita EG, Heise T. A review of insulin degludec/insulin aspart: pharmacokinetic and pharmacodynamic properties and their implications in clinical use. Clin. Pharmacokinet. 56(4), 339-354 (2017).
- Jonassen I, Havelund S, Hoeg-Jensen T et al. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm. Res. 29(8), 2104-2114 (2012).
- Heise T, Hovelmann U, Nosek L et al. Comparison of the pharmacokinetic and pharmacodynamic profiles of insulin degludec and insulin glargine. Expert. Opin. Drug. Metab. Toxicol. 11(8), 1193-201 (2015).
- Heise T, Nosek L, Roepstorff C et al. Distinct prandial and basal glucose-lowering effects of insulin degludec/insulin aspart (IDegAsp) at steady state in subjects with type 1 diabetes mellitus. Diabetes. Ther. 5(1), 255-265 (2014).
- Morello CM. Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus. Int. J. Gen. Med. 4, 827-835 (2011).
- Chen ML. Ethnic or racial differences revisited: impact of dosage regimen and dosage form on pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 45(10), 957-964 (2006).
- Heise T, Nosek L, Klein O et al. Insulin degludec/insulin aspart produces a dose-proportional glucose-lowering effect in subjects with type 1 diabetes mellitus. Diabetes. Obes. Metab. 17(7), 659-664 (2015).
- Biester T, Danne T, Blasig S et al. Pharmacokinetic and prandial pharmacodynamic properties of insulin degludec/insulin aspart in children, adolescents, and adults with type 1 diabetes. Pediatr. Diabetes. 17(8), 642-649 (2016).
- Haahr H, Sasaki T, Bardtrum L et al. Insulin degludec/insulin aspart in Japanese patients with type 1 diabetes mellitus: distinct prandial and basal glucose-lowering effects. J. Diabetes. Investig. 7(4), 574-580 (2016).
- Novo Nordisk A/S. Clinical Study Report, trial ID NN5401-1983. Effect of NN5401 in Japanese subjects with type 1 diabetes (2011).
- Korsatko S, Deller S, Mader JK et al. Ultra-long pharmacokinetic properties of insulin degludec are comparable in elderly subjects and younger adults with type 1 diabetes mellitus. Drugs. Aging. 31(1), 47-53 (2014).
- Ikushima I, Kaku K, Hirao K et al. Pharmacokinetic and pharmacodynamic properties of insulin degludec in Japanese patients with type 1 diabetes mellitus reflect similarities with Caucasian patients. J. Diabetes. Investig. 7(2), 270-275 (2016).
- Heise T, Nosek L, Bottcher SG et al. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes. Obes. Metab. 14(10), 944-950 (2012).
- Hompesch M, Morrow L, Watkins E et al. Pharmacokinetic and pharmacodynamic responses of insulin degludec in African American, white, and Hispanic/Latino patients with type 2 diabetes mellitus. Clin. Ther. 36(4), 507-515 (2014).
- Hu P, Jiang J, Bardtrum L et al. Pharmacokinetic properties of insulin degludec in healthy Chinese subjects. Diabetes. Manag. 9, 20-27 (2019).
- Novo Nordisk A/S. Clinical Study Report, trial ID NN5401-1980. A comparison between two formulations of NN5401 in healthy subjects (2011).
- Novo Nordisk A/S. Clinical Study Report, trial ID NN5401-3857. Comparison of the effect of NN5401 with the effect of NN1250 and insulin aspart in subjects with type 1 diabetes (2011).
- Nosek L, Coester HV, Roepstorff C et al. Glucose-lowering effect of insulin degludec is independent of subcutaneous injection region. Clin. Drug. Investig. 34(9), 673–679 (2014).
- Haahr H, Ikushima I, Hirao K et al. Similar pharmacokinetic and pharmacodynamic properties of insulin degludec in Japanese and Caucasian subjects with T1DM. J. Diabetes. Investig. 3, 197 (2012).
- Novo Nordisk A/S. Clinical Study Report, trial ID NN1250-1993. A trial evaluating the effect of NN1250 at steady state conditions in subjects with type 1 diabetes (2011).
- Novo Nordisk A/S. Clinical Study Report, trial ID NN1250-1996. A trial investigating the effect of NN1250 in Japanese subjects with type 1 diabetes (2011).
- Fulcher GR, Christiansen JS, Bantwal G et al. Comparison of insulin degludec/insulin aspart and biphasic insulin aspart 30 in uncontrolled, insulin-treated type 2 diabetes: a phase 3a, randomized, treat-to-target trial. Diabetes. Care. 37(8), 2084-2090 (2014).
- Franek E, Haluzik M, Canecki Varzic S et al. Twice-daily insulin degludec/insulin aspart provides superior fasting plasma glucose control and a reduced rate of hypoglycaemia compared with biphasic insulin aspart 30 in insulin-naive adults with type 2 diabetes. Diabet. Med. 33(4), 497-505 (2016).
- Meneghini L, Atkin SL, Gough SC et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes. Care. 36(4), 858-864 (2013).