Research Article - Clinical Investigation (2020) Volume 10, Issue 2
A phase 1 study on midostaurin plus panobinostat in unfit acute myeloid leukemia and high-risk myelodysplastic syndrome
- Corresponding Author:
- Chin-Hin NG
National University Cancer Institute, Singapore
E-mail: Chin_Hin_NG@nuhs.edu.sg
Submitted: 6 February 2020; Accepted: 14 February 2020; Published online: 25 February 2020
Abstract
Introduction: Midostaurin is a non-specific FLT3 inhibitor that showed anti-leukemic activity against both FLT3 mutated as well as unmutated Acute Myeloid Leukemia (AML) in early phase studies. Panobinostat, a potent oral pan-acetylase inhibitor had also shown significant anti-leukemic signals in early phase studies. In the preclinical study, our group had previously reported synergistic anti-leukemic activity when an FLT3 inhibitor was used in combination with an HDAC inhibitor in vitro as well as in vivo animal study. The current study is our first bench-to-bedside study to further evaluate this combination therapy in AML patients. Method: Elderly AML who were either newly diagnosed and declined Hypomethylating Agent (HMA) or who failed HMA, MDS-RAEB-2, and relapsed refractory AML who were unfit for intensive chemotherapy or have no other treatment option were eligible regardless of FLT3 mutation status. The classical 3+3 dose escalation method was applied. Dose level 1 for Midostaurin was 50 mg bd and Panobinostat was 10 mg 3 times per week. Dose escalations were planned with only one drug escalation at a time on every level. Midostaurin was administered daily as a continuous cycle while Panobinostat was given for the first 3 weeks of a 4 weeks cycle. Dose-limiting toxicities were defined as grade 3 or more non-haematologic toxicities occurring within the first cycle. Chronic toxicity is defined as recurrent or persistent adverse events that are possibly related to investigational products. Response assessments were done at post cycle 2 and cycle 4. Concomitant treatment with hydroxyurea was allowed up to cycle 1 day 14 to control leukocytosis if needed. Results: A total of 7 patients have been recruited with a median age of 71 years old (range: 47 to 82 years). 2 patients had MDS-RAEB2, one with Acute myelomonocytic leukemia, 3 had AML with myelodysplasia-related changes (AML-MRC) and one with de novo FLT3-ITD AML. One of the AML-MRC had FLT3-ITD mutation and another had FLT3-TKD mutation. 4 out of 7 had prior HMA therapy, the remaining three were treatment naïve. 2 patients were not evaluable for DLT and response assessment due to progressive disease during cycle 1. 2 completed 2 cycles, the other 3 completed 4, 5 and 9 cycles respectively. No DLT was observed within cycle 1. Significant grade 1-2 treatment emerging adverse events were anorexia (4/5), fatigue (3/5), nausea (2/5), and dysgeusia (2/5). These toxicities persist beyond cycle 1 with increasing frequency. Grade 2 fatigue was observed in 4 patients, grade 2 anorexia in 4 patients, grade 1 dysgeusia in 4 patients, and grade 3 lipase elevation in one patient. 4 out of 5 patients developed severe grade 4 thrombocytopenia requiring regular platelet transfusion. Two had baseline grade 4 thrombocytopenia but the severity worsen after 1 cycle of treatment. These were thought to be chronic toxicities because the symptoms were largely resolved or improved during dose interruptions. In view of significant chronic toxicities, no further dose escalation was done and dose level 1 would be further evaluated in the expansion cohort. 5 out 7 had post cycle 2 response assessment. One patient with FLT3-TKD achieved CRi, one AML-MRC showed 50% blast reduction, and the remaining 3 had stable disease. The one who achieved CRi eventually withdrew from the study after completing 6 cycles due to fatigue, anorexia, severe grade 4 thrombocytopenia with gastrointestinal bleeding. His transfusion requirement has also reduced. He remained in stable disease without further anti-leukemic therapy until February 2019 (16 months after withdrawal from the study) when he suffered a frank relapse. One with stable disease who demised during cycle 10 due to complicated acute cholecystitis and sepsis. The remaining 5 demised due to progressive disease. Conclusion: Even though no DLT was observed within cycle 1, significant chronic toxicities such as fatigue and anorexia were observed. The limited anti-leukemic activity was observed in 5 evaluable patients. Further exploration with different dosing schedules or used in combination with other anti-leukemic agents is required to improve the tolerability profile and efficacy.
Keywords
Midostaurin • Panobinostat • Myeloid leukemia • Myelodysplastic syndrome
Introduction
The median age of patients diagnosed with acute myeloid leukemia (AML) was reported to be around 60-65 years. Intensive chemotherapy, typically the “3+7” regimen could induce remission in 60%-70% of AML but this regimen is too toxic for older AML patients. In our own experience, the induction of death was as high as 20% in patients older than 60 years old. To address this gap, many novel agents with more tolerable toxicity profiles have been in various stages of the developmental phase over the past 10 years. At least 5 new novel anti-leukemic agents have been approved by FDA over the past 2 years, which are relatively less toxic with significant anti-leukemic therapy when used alone or in combination with other low-intensity therapy [1-7].
In our pre-clinical work with ABT-869, an FLT3 inhibitor showed remarkable ability in inducing apoptosis in vitro and in vivo and synergized with chemotherapy in a sequence-dependent manner [8]. We had demonstrated that combining ABT-869 with SAHA led to the synergistic killing of AML cells with FLT3 mutations through the downregulation of PRL-3, a metastasis-associated phosphatase [9]. Furthermore, on-going work suggests this synergism is a class effect and not limited to ABT-869 and SAHA only.
Both Midostaurin and Panobinostat had been evaluated as monotherapy in the early phase studies. In a phase 2 study, patients with FLT3-mutant were treated with Midostaurin at a dose of 75 mg orally three times daily. It was well tolerated and demonstrated ≥ 50% reduction in peripheral blasts and/or bone marrow blast count in 14 (70%) of 20 patients [10]. In another phase 2 study, 95 patients with AML or MDS with either wild-type (n=60) or mutated (n=35) FLT3 were randomly assigned to receive Midostaurin at 50 or 100 mg twice daily [11]. 71% of AML with FLT3 mutant showed significant blast reduction while 42% of wild-type FLT3 AML had also demonstrated significant blast reduction. Both doses were well tolerated. Panobinostat, a potent class I/II pan-HDAC inhibitor (pan-HDACi) that has shown anti-tumor activity in both preclinical models and cancer patients [12]. In a phase 1 study conducted by a Japanese group, Panobinostat administered 3 times a week is well tolerated with minimal toxicities up to the dose of 20 mg [13]. In another phase 1 study, Panobinostat was administered as 28-day treatment cycles according to one of the two dosing schedules: once a day three days per week every week or every other week with a starting of 20 mg and 30 mg respectively [14]. The study population consisted of a range of hematological malignancies including 83 patients with AML. The Maximum Tolerated Dose (MTD) was 60 mg for both arms and the recommended phase 2 dose (RP2D) was 40 mg. Some grade 3 and 4 adverse events included thrombocytopenia (41.5%), fatigue (21%) and neutropenia (21%) were observed.
A combination of therapy with Midostaurin and Panobinostat has not been studied. Though the toxicity profiles of the respective monotherapy were acceptable, the safety of this combination is unknown. This phase 1a firstin- human combination study would evaluate the safety and tolerability of the combination therapy besides exploring for the signal of efficacy and prognostic biomarker.
Methods
Study design
This is a single-center Phase 1, open-label, doseescalation study to evaluate the safety and tolerability of Panobinostat and Midostaurin combination therapy and to determine the RP2D in patients with AML and high-risk MDS. Both Investigational Products (IP) were supplied from Novartis. The study was co-funded by Novartis and the National University Health System (NUHS) of Singapore.
The classical “3+3” design was applied. A minimum of 3 patients and a maximum of 6 patients would be enrolled per dose level. The Dose Limiting Toxicity (DLT) was defined as any grade 3 or more non-haematologic toxicities that required dose interruption of more than 7 days or recurrent grade 3 or more non-haematologic toxicities that required dose reduction or prolonged neutropenia (<0.1 × 109/L>10 days) or thrombocytopenia (<10 × 109/L >14 days) that was not related to persistent disease in the first 28 days (1st cycle) and considered related to one of the IP. The MTD would be exceeded when 2 DLTs were observed within the dose level. Further 3 patients would be treated at one dose level lower to confirm the MTD. All adverse events would be graded according to CTCAEv4.0.
Panobinostat was given 3 times weekly approximately 48 hours apart orally at a starting dose of 10 mg for the first 3 weeks of a 4 weeks cycle, while Midostaurin was taken daily orally at a starting dose of 50 mg bd without a break in the 4 weeks cycle. The treatment would be continued until the Progression of Disease (PD) or the development of unacceptable toxicities. Dose escalation schedule is shown on the table (Table 1).
| Dose level | Midostaurin | Panobinostat | Number of subjects |
|---|---|---|---|
| -1 | 25 mg bd | 10 mg 3x/week | 6-Mar |
| 1 | 50 mg bd | 10 mg 3x/week | 6-Mar |
| 2 | 50 mg bd | 15 mg 3x/week | 6-Mar |
| 3 | 50 mg bd | 20 mg 3x/week | 6-Mar |
| 4 | 75 mg bd | 20 mg 3x/week | 6-Mar |
| 5 | 100 mg bd | 20 mg 3x/week | 6-Mar |
| Midostaurin is administered twice daily continuously in a 28 days cycle | |||
| Panobinostat is administered 3 times a week (Monday, Wednesday, Friday) for the first 3 weeks of 28 days cycle | |||
Table 1. Dose escalation schedule
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines, and the protocol was approved by the National Health Group Disease-Specific Research Board and Health Science Authority of Singapore. This study was co-funded by NUHS Clinician Research Grant and Novartis who supplied the investigational products.
Study population
The study population includes AML or secondary AML or high-risk MDS (mainly MDS with excess blast>10%) irrespective of Flt3 status. Patients aged 21 years or older who were not fit for intensive but with ECOG performance status of 0-2 were enrolled. Both treatment naïve and relapsed refractory cases were enrolled. For those who were treatment naïve, only those who had been offered hypomethylating agents but declined the HMA therapy would be accepted. All patients enrolled should have an acceptable organ function as per protocol and have no active or uncontrolled infection. Patient on other HDAC inhibitor such as valproate was excluded. Acute promyelocytic leukemia was not included in this study so did patients who are pregnant or breastfeeding.
Response assessment
Bone marrow aspiration and trephine biopsy were performed at baseline, at the completion of cycle 2 and cycle 4 to assess the disease response to this combination therapy. The response criteria are based on the European Leukemia Network criteria [15]. Complete remission is defined as a bone marrow blast count of less than 5% of the total nucleated cell in the marrow with the recovery of the neutrophil count of more than 1.0 × 109/L and platelet count of more than 80 × 109/L. The focus will be on the hematological response (in terms of hemoglobin and platelet recovery) and blast percentage reduction. Cytogenetic study and flow cytometry analysis will also be performed to monitor for clonal evolution and minimal residual disease respectively.
Results
Patients were recruited from a single institution-the National University Hospital of Singapore. A total of 7 patients were recruited over a period of 22 months (June 2016 to April 2018). The study was closed in December 2018 due to poor recruitment. Table 2 showed the baseline patient’s characteristics of the study. All patients recruited were 70 years and above except one. Most patients had poor-risk AML or high-risk MDS.4 subjects failed prior to hypomethylating agent therapy including one with relapsed refractory AML who failed 3 lines of therapies. The other 3 subjects were treatment naïve. The details of baseline disease and prior therapy of the 7 patients are described in Table 3.
| Total number of subjects | N=7 |
|---|---|
| Age (yr), median (range) | 71 (47-82) |
| Male:Female | 4:03 |
| Disease characteristic | |
| De novo AML | 2 |
| Secondary AML | 3 |
| MDS RAEB-2 | 2 |
| Cytogenetics: | |
| Complex | 1 |
| Monosomy 7 | 1 |
| Normal | 3 |
| Trisomy 8 | 1 |
| Inv (9) (p11q13) | 1 |
| Molecular genetics: | |
| FLT3-ITD | 2 |
| FLT3-TKD | 1 |
| FLT3 wild-type | 4 |
| NPM1 mutant | 1 |
| PRL3 expression | |
| Positive (2-3+) | 5 |
| Focal (1-2+) | 1 |
| Negative | 1 |
| Baseline marrow blast (%), median (range) | 32 (5-95) |
| White blood count (× 109/L), median (range) | 2.3 (1.0-4.4) |
| Platelet (× 109/L), median (range) | 45 (11-124) |
| Hemoglobin (g/L) | 7.3 (7.0-8.0) |
| Treatment line | |
| First | 3 |
| Second | 3 |
| Third or more | 1 |
Table 2. Baseline characteristic
| No. | Age (years) | Diagnosis | Disease status | Cytogenetic profiles | Molecular genetic profile | Prior therapy |
|---|---|---|---|---|---|---|
| 1 | 70 | AML-MRC | Progression from chronic myelomonocytic leukemia | Normal | FLT3-t/NPM1-wt | Azacitidine |
| 2 | 71 | AML-MRC | Newly diagnosed | Monosomy 7 | FLT3-t/NPM1-wt | Treatment naïve |
| 3 | 71 | AML-MRC | Progression from MDS-RAEB1 | Complex | FLT3-t/NPM1-wt | Azacitidine |
| 4 | 77 | MDS-RAEB2 | Newly diagnosed | Normal | FLT3-TKD/NPM1-wt | Treatment naïve |
| 5 | 82 | MDS-RAEB2 | Newly diagnosed | Inv (9)(p11q13) | FLT3-wt/NPM1-mut | Treatment naïve |
| 6 | 73 | AML-MRC | Progression from MDS-RAEB2 | Normal | FLT3-ITD/NPM1-wt | Azacitidine |
| 7 | 47 | AML | Relapsed refractory | Trisomy 8, t (11;12)(p12-14;p13) | FLT3-ITD/NPM1-wt | Standard induction Azacitidine+Sorafenib, FLAG |
Table 3. Details baseline disease characteristic and prior therapy
5 subjects completed at least one cycle of treatment and eligible for DLT assessment. 2 patients withdrew from the study during cycle 1 due to disease progression. All patients were in dose level 1. A total of 22 cycles completed among the 5 subjects. Details of Treatment-Emergent Adverse Events (TEAE) during cycle 1 are presented in Table 4. No DLT was observed during cycle 1. No death occurred during cycle 1 (Table 4).
| Event | Grade 1/2 | Grade 3/4 | Total |
|---|---|---|---|
| Anorexia | 4 | 0 | 4 |
| Anemia | 0 | 2 | 2 |
| Fatigue | 3 | 0 | 3 |
| Constipation | 2 | 0 | 2 |
| Dysgeusia | 2 | 0 | 2 |
| Thrombocytopenia | 0 | 1 | 1 |
| Lipase increased | 0 | 1 | 1 |
| Neutropenia | 0 | 1 | 1 |
| Nausea | 2 | 0 | 2 |
| Hyperbilirubinemia | 1 | 0 | 1 |
| Diarrhea | 1 | 0 | 1 |
| Amylase increased | 1 | 0 | 1 |
Table 4. TEAEs during cycle 1
2 episodes of Serious Adverse Event (SAE) were reported during cycle 1. One was due to chest infection and the other one was E. Coli sepsis that resulted in hospital admission. Both survived the episode. Notably, some chronic toxicities were observed in the majority of the subjects (beyond cycle 2). These include 4 out of 5 subjects with grade 2 fatigue, grade 2 anorexia, and grade 1 dysgeusia. The adverse events abated when dose interruption occurred and recurred with the resumption of investigational products. One subject decided to withdraw from the study during cycle 6 of treatment due to dysgeusia, anorexia, and fatigue, which significantly impacted his quality of life. We have also noted grade 4 thrombocytopenia developed in 5 patients beyond cycle 2 of treatment. Table 5 shows the comparison of baseline and post cycle 2 platelet count. In view of these chronic toxicities, the decision was made by the medical review board to remain at dose level one, and proceed with an expansion cohort (Table 5).
| Subject | Baseline (× 109/L) | Post 2 cycles (worst count) (×109/L) |
|---|---|---|
| 2 | 24 | 5 |
| 3 | 11 | 2 |
| 4 | 31 | 5 |
| 5 | 119 | 13 |
| 6 | 64 | 29 |
Table 5. Comparison of baseline and beyond cycle 2 platelet count
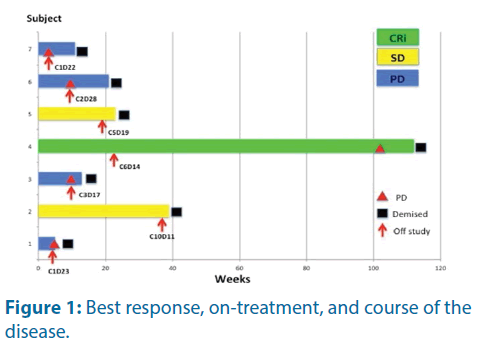
Five subjects had post cycle 2 Bone Marrow Assessment (BMA). The other two had disease progression during cycle 1 and withdrew from the study without BMA. Among the five evaluable subjects, one showed complete remission without count recovery, two had stable disease and two had progression of the disease. The details of the individual response, duration On-Treatment and subsequent progression are shown in the swimmer’s plot below (Figure 1).
Subject 4 achieved complete remission with incomplete count (CRi) recovery after 2 cycles of treatment. His bone marrow blast was 3%, and further reduced to 0% after 4 cycles of treatment. He withdrew from the study during cycle 6 due to chronic toxicities. He eventually relapsed 16 months later. Subject 2 had stable disease after cycle 2 treatment but he declined further BMA. Nevertheless, he showed significant Peripheral Blast (PB) reduction but not fulfilling criteria for complete remission or partial remission. His disease was under control throughout the treatment cycles but unfortunately, he demised during cycle 10 due to complicated acute cholecystitis and sepsis.
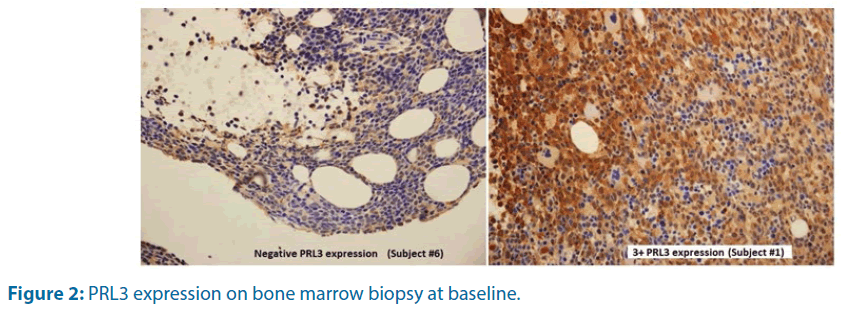
PRL3 expression on leukemia blast was examined on baseline bone marrow biopsy and correlated with response to treatment. Table 6 shows the relative expression of PRL3 protein and its corresponding response. PRL3 expression by immunochemistry on trephine biopsy is shown in Figure 2. There was no correlation of baseline PRL3 expression with clinical outcome (Table 6 and Figure 2).
| Subject | PRL3 expression | Best response |
|---|---|---|
| 1 | 3+ | PD |
| 2 | 2+ | SD |
| 3 | 2+ | PD |
| 4 | 2+ | CRi |
| 5 | 2+ | SD |
| 6 | Negative | PD |
| 7 | 1-2+ | PD |
Table 6. Baseline PRL3 expression and best response
Discussion
This is the first-in-human combination study of Midostaurin and Panobinostat for AML and high-risk MDS. The recruitment was poor due to emerging competing treatment options such as Venetoclax and azacitidine and frailty of the patient population that excluded patients from participation. Most referring physicians would consider a phase 1 study as the last treatment option and very often the patients were in poor performance status after the failure of 1-2 lines prior to anti-leukemic therapy.
DLTs are often evaluated only during cycle 1 in most phase 1 studies. In this study, no DLT was observed for the first dose level. After the first safety monitoring meeting, the plan was to escalate to dose level 2. However, due to the poor recruitment rate, we were able to follow up on the patients beyond cycle 1 in the first 3 evaluable subjects before enrolling the first subject into the next dose level. We noticed some consistent chronic toxicities emerged after cycle 2 in all three cases. These were grade 2 fatigue and anorexia, and grade 1 dysgeusia. We have also noticed worsening thrombocytopenia in all patients who were treated beyond cycle 2 (Table 5). The safety monitoring committee reconvened and decided not to escalate to the next dose level in view of these emerging chronic toxicities. The longest surviving patient (Subject #4) withdrew from the study during cycle 6 due to these chronic toxicities. After stopping the IPs, his fatigue, anorexia, and dysgeusia resolved. His platelet count had also improved from the transfusion-dependent single-digit value to transfusion independent grade 3 (30-40 × 109/L). This clearly confirmed the relatedness of adverse events. We are of the opinion that in defining the DLT, we should evaluate beyond cycle 1 to identify chronic toxicity, which may significantly impact the quality of life. This is especially true in the era of novel agents, which are likely to have a cumulative effect on chronic administration.
In terms of anti-leukemic activity, subject #4 with MDSRAEB- 2 achieved complete remission with incomplete count recovery (neutropenia and thrombocytopenia) after 2 cycles. His transfusion requirement was significantly reduced after withdrawal from the study due to chronic toxicities. He required only 2 units of red cells over the subsequent 12 months, compared to 2-3 units every month prior to enrollment. He remained in stable disease without further anti-leukemic therapy until February 2019 (16 months after withdrawal from the study) when he suffered a frank relapse. He had a normal karyotype and FLTTKD mutant at baseline. Midostaurin has been shown to have significant activity in FLT3 mutated AML in early phase study [10], and more recently it has been used in combination with intensive chemotherapy in a phase 3 study (RATIFY Trial) [3], of which it showed significant improvement in survival. However, subject #6 and #7 with relapsed refractory disease, who harbored FLT3-ITD did not show any response and progressed during cycle 2 and cycle 1 respectively. Subject #2 completed 9 cycles and showed a stable disease throughout with a low level of peripheral blasts (1%-2%). Interestingly, during dose interruption due to intercurrent infection, his peripheral blast would rise above 80% and then reduced to baseline when the IPs were re-introduced. The patient eventually passed on during cycle 10 due to severe sepsis. The two cases had demonstrated a potential anti-leukemic activity of this combination.
Increased PRL3 expression is common in many solid tumors such stomach [16], breast [17], liver [18], ovarian [19] and colon [20]. PRL3 overexpression has also been reported in haematologic malignancies [9,21-24]. It has been associated with metastasis and poor prognosis in solid tumors. In this study, we performed anti-PRL3 immunohistochemistry staining on baseline trephine biopsy on all subjects. 6 out of 7 cases showed at least 2+ expression of PRL3 protein but we couldn’t demonstrate any association with clinical outcome (Table 6 and Figure 2). Further biomarker exploration is required to identify the subgroup of AML that may benefit from this combination therapy.
Conclusion
The combination therapy brought about significant chronic toxicities with limited anti-leukemic activities. Further exploration with different dosing schedules or used in combination with other anti-leukemic agents is required to improve the tolerability profile and efficacy.
References
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133: 7-17 (2019).
- Wei A, Strickland SA, Hou JZ, et al. Venetoclax with low dose cytarabine induces rapid, deep and durable responses in previously untreated older adults with AML ineligible for intensive chemotherapy. Blood 132: 284 (2018).
- Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377: 454-464 (2017).
- Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130: 722-731 (2017).
- DiNardo CD, Stein EM, Roboz GJ, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med 378: 2386-2398 (2018).
- Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 33: 379-389 (2019).
- Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood 123: 3239-3246 (2014).
- Zhou J, Pan M, Xie Z, et al. Synergistic antileukemic effects between ABT-869 and chemotherapy involve the downregulation of cell cycle-regulated genes and c-Mos-mediated MAPK pathway. Leukemia 22: 138-146 (2008).
- Zhou J, Bi C, Chng WJ, et al. PRL-3, a metastasis-associated tyrosine phosphatase, is involved in FLT3-ITD signaling and implicated in anti-AML therapy. PLoS One 6: e19798 (2011).
- Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 105: 54-60 (2005).
- Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol 28: 4339-4345 (2010).
- Khot A, Dickinson M, Prince HM, et al. Panobinostat in lymphoid and myeloid malignancies. Expert Opin Investig Drugs 22: 1211-1223 (2013).
- Fukutomi A, Hatake K, Matsui K, et al. A phase I study of oral panobinostat (LBH589) in Japanese patients with advanced solid tumors. Invest New Drugs 30: 1096-1106 (2012).
- Deangelo DJ, Spencer A, Bhalla KN, et al. Phase Ia/II, two-arm, open-label, dose-escalation study of oral panobinostat administered via two dosing schedules in patients with advanced hematologic malignancies. Leukemia 27: 1628-1636 (2013).
- Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115: 453-474 (2010).
- Bilici A, Ustaalioglu BB, Yavuzer D, et al. Prognostic significance of high phosphatase of regenerating liver-3 expression in patients with gastric cancer who underwent curative gastrectomy. Dig Dis Sci 57: 1568-1575 (2012).
- Hao RT, Zhang XH, Pan YF, et al. Prognostic and metastatic value of phosphatase of regenerating liver-3 in invasive breast cancer. J Cancer Res Clin Oncol 136: 1349-1357 (2010).
- Mayinuer A, Yasen M, Mogushi K, et al. Upregulation of protein tyrosine phosphatase type IVA member 3 (PTP4A3/PRL-3) is associated with tumor differentiation and a poor prognosis in human hepatocellular carcinoma. Ann Surg Oncol 20: 305-317 (2013).
- Ren T, Jiang B, Xing X, et al. Prognostic significance of phosphatase of regenerating liver-3 expression in ovarian cancer. Pathol Oncol Res 15: 555-560 (2009).
- Xing X, Peng L, Qu L, et al. Prognostic value of PRL-3 overexpression in early stages of colonic cancer. Histopathology 54: 309-318 (2009).
- Qu S, Bin Liu, Guo X, et al. Independent oncogenic and therapeutic significance of phosphatase PRL-3 in FLT3-ITD-negative acute myeloid leukemia. Cancer 120: 2130-2141 (2014).
- Fagerli UM, Holt RU, Holien T, et al. Overexpression and involvement in migration by the metastasis-associated phosphatase PRL-3 in human myeloma cells. Blood 111: 806-815 (2008).
- Hjort MA, Abdollahi P, Vandsemb EN, et al. Phosphatase of regenerating liver-3 is expressed in acute lymphoblastic leukemia and mediates leukemic cell adhesion, migration and drug resistance. Oncotarget 9: 3549-3561 (2018).
- Hjort MA, Hov H, Abdollahi P, et al. Phosphatase of regenerating liver-3 (PRL-3) is overexpressed in classical Hodgkin lymphoma and promotes survival and migration. Exp Hematol Oncol 7: 1-13 (2018).