Review Article - Interventional Cardiology (2014) Volume 6, Issue 5
A clinical update on the use of Resolute stents with dual anti-platelet therapy interruption
- Corresponding Author:
- Anthony
Mathur
Barts & The London NIHR Cardiovascular Biomedical Research Unit
London Chest Hospital, Barts Health NHS Trust, London, E2 9JX, UK
E-mail: a.mathur@qmul.ac.uk
Abstract
Percutaneous coronary intervention is currently the most commonly used revascularization procedure. This success is a direct consequence of the improved medium- and long-term target vessel patency achieved with drug-eluting stents (DES). However, DES are associated with stent thrombosis (ST), a rare but potentially catastrophic complication. Early interruption of dual anti-platelet therapy (DAPT) is a major risk factor for ST and guidelines recommend a minimum duration of DAPT of 6 to 12 months post implantation. Recently published observational data suggest that premature DAPT interruption is not associated with ST in patients treated with the second-generation Resolute zotarolimus-eluting stent. This article reviews the background to the development of Resolute DES and reviews recently published data on ST and DAPT interruption.
Introduction
Percutaneous coronary intervention (PCI) is currently the most common mode of coronary revascularization [1]. The use of drugeluting stents (DES) has improved clinical outcomes [2] but safety concerns over stent thrombosis (ST) have been raised [3–5]. Dual anti-platelet therapy (DAPT) is the mainstay of pharmacological treatment for the prevention of ST and contemporary clinical guidelines recommend a minimum duration of treatment ranging from 6 to 12 months post DES implantation [6–11].
Resolute (Medtronic CardioVascular, Santa Rosa, CA) and Resolute Integrity (Medtronic CardioVascular, Santa Rosa, CA) are second-generation DES with a biocompatible polymer providing prolonged zotarolimus elution [12]. Recently published data show that there is no increased risk of ST with DAPT interruption between 1 and 12 months post Resolute DES implantation [13].
This article provides the background to the development of Resolute and Resolute Integrity DES and reviews the recently published data on ST and DAPT interruption.
Background
the inception of percutaneous transluminal coronary balloon angioplasty and the pioneering work of Andreas Gruentzig and his co-workers in the 1970s [14], PCI has evolved to became a routine therapeutic modality [8,11]. In its earliest form, revascularization was achieved with plain balloon angioplasty and was limited by acute vessel closure and a high risk of target vessel revascularization (TVR) from late lumen loss secondary to negative remodelling [15–18]. The introduction of bare metal stents (BMS) in the 1980s [19] improved both acute- and medium-term outcomes and BMS soon became the new standard of care. [20] However, in-stent restenosis (ISR) occurs in approximately 30% of lesions treated with BMS [16,21] and is associated with not only with the reoccurrence of angina but also with myocardial infarction in a small but significant subgroup of patients [22]. The aetiology of ISR is incompletely understood and currently thought to be caused by an exuberant healing response to vascular injury [23,24]. ISR with BMS remained the Achilles’ heel of PCI until the development of DES in the late 1990s [25].
DES share the mechanical scaffolding properties of BMS and additionally function as delivery platforms for drugs which modulate the healing response to vascular injury to prevent ISR. Two drugs became the protagonists: paclitaxel (a chemotherapeutic agent) and sirolimus (rapamycin, a macrolide antibiotic) [26–28]. The first generation DES consisted of a BMS platform coated with a durable (nonbioerodable) polymer that eluted either sirolimus (Cypher) or paclitaxel (Taxus Express). Randomized controlled trials showed that first-generation DES reduced ISR to less than 10% [29,30], and subsequent meta-analyses suggested that sirolimus is superior to paclitaxel in reducing TVR and ISR [31,32].
Despite substantial progress, ISR remains a problem in a small subgroup of patients treated with DES. The pathophysiology of ISR in DES has recently been reviewed by Dangas et al. [33]; briefly, possible mechanisms include:
• Biological factors: genetic variants conferring resistance to anti-proliferative drugs [34] and hypersensitivity reactions to components of DES [35];
• Mechanical factors: stent fractures [36], polymer peeling [37] and non-uniform drug deposition [38];
• Technical factors: stent under-expansion [39].
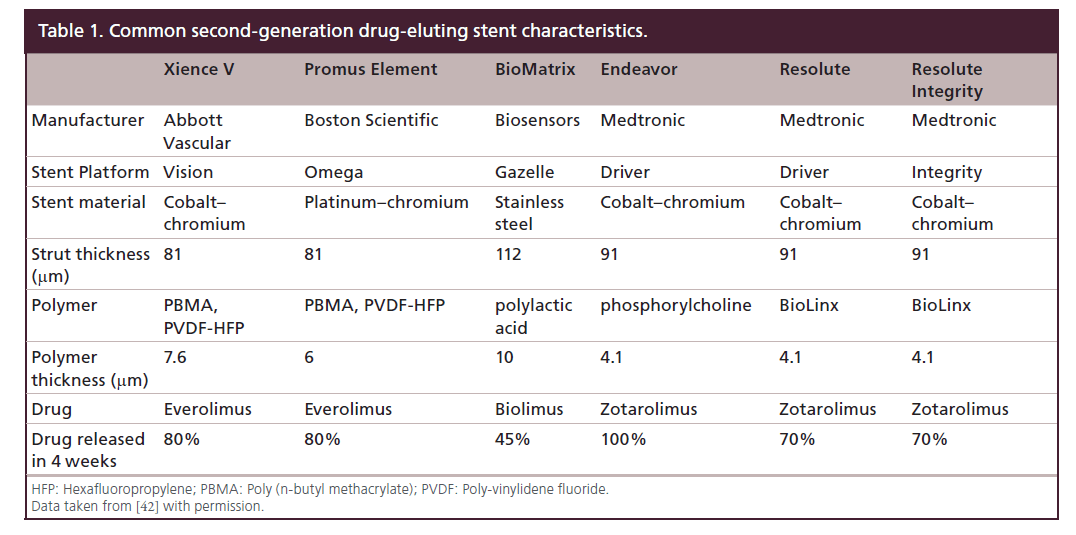
To address some of the factors predisposing to ISR, DES design progressed with the development of second-generation DES which are characterized by thinner struts and enhanced polymer biocompatibility and drug delivery [40,41]. The characteristics of commonly used second-generation DES are shown in Table 1 [42]. The rate of TVR at 1 year with second-generation DES is ≤7.5% [43] but some elements of DES design may predispose to stent thrombosis.
Resolute & Resolute integrity drug-eluting stents
The Resolute Integrity DES was approved by the US FDA in 2012. Resolute Integrity consists of: cobaltbased alloy (MP35N) Integrity stent platform, a coat consisting of Parylene C primer and BioLinx polymer and zotarolimus. Resolute Integrity is the successor of the Resolute DES which was based on a Driver modular stent platform whilst Integrity is a continuous piece of wire with a sinusoidal shape wrapped into a cylinder. With the exception of the stent platform, the two stents have the same basic technical characteristics. BioLinx is a mixture of three polymers, each with a different function: the hydrophobic C10 controls drug release and provides rigidity to the mixture; the hydrophilic C19 polymer improves biocompatibility and polyvinyl pyrrolidone provides short peak in drug release [12,44]. The active pharmacological ingredient is zotarolimus, a sirolimus analogue which shares the same mode of action as the parent compound, but with a shorter half-life [45]. Resolute and Resolute Integrity stents elute 85% of zotarolimus within 60 days and drug elution is complete by 180 days [12]. Prolonged drug elution ensures that zotarolimus provides antiproliferative cover in parallel with delayed vascular healing [12].
The efficacy and safety of Resolute DES has been examined in a number of studies which were part of the Medtronic RESOLUTE Global Clinical Program:
• RESOLUTE First In Man was a prospective, non-randomized, multicenter study of 139 patients with de novo coronary lesions and showed a 0.22 ± 0.27 mm in-stent lumen loss at 9 months [46]. At 2 years, there was one case of TVR, one non-cardiac death and one possible ST [47];
• RESOLUTE US was a prospective, observational study in the USA designed to evaluate the clinical effectiveness of the Resolute DES in 1402 patients [48]. Target lesion failure (cardiac death, myocardial infarction or clinically-driven target lesion revascularization) was 3.7% at 12 months with Resolute DES and 6.5% using Endeavour DES historical data, meeting the non-inferiority criterion [48];
• RESOLUTE All Comers was a multi-center, single- blind, randomized trial comparing Resolute DES to Xience V everolimus-eluting stent in 2292 patients. Resolute had a similar safety and efficacy profile to Xience V at 2 years [49,50];
• RESOLUTE International Registry used data from 2349 patients and reported a 1-year incidence of cardiac death and target vessel myocardial infarction of 4.3% (95% CI: 3.5–5.2%). Definite and probable ST was observed in 0.9% [51];
• RESOLUTE Japan (ClinicalTrials.gov Identifier: NCT00927940) was designed to examine instent late lumen loss at 8 months in 100 participants. The results have not been published in peer reviewed journal (PubMed search: February 2014).
Pooled patient-level data from 5130 patients participating in the Medtronic RESOLUTE Global Clinical Program were used to examine Resolute DES in specific settings:
• Diabetes: the 12-month rate of target lesion failure was 7.8% in the pre-specified diabetic cohort and was significantly lower than the performance goal of 14.5% set by the FDA (p < 0.001) [52];
• Overlapping stents: the study reported comparable clinical outcomes in patients with overlapping and non-overlapping DES [53].
Finally, the TWENTE trial, an investigator-initiated study supported by Abbott Vascular and Medtronic, demonstrated the non-inferiority of Resolute DES compared with Xience V everolimus DES in a patient-blinded, randomized study which included 1391 patients (the findings were similar to RESOLUTE All Comers) [54].
ST & dual anti-platelet therapy
ST is a serious complication of PCI associated with acute myocardial infarction and high mortality [55–59]. Immediately after stent implantation, thrombogenic stent components, coronary dissection, in situ thrombus, stasis, and stent under-expansion may all contribute to the development of early ST (<30 days) [60]. Early ST can be effectively prevented by DAPT; aspirin and ticlopidine administration reduced early ST to <1% [61,62]. With the introduction of clopidogrel, ticolpidine became obsolete and the most commonly prescribed DAPT regimen is aspirin with clopidogrel [63]. Prasugrel and ticagrelor are alternatives to clopidogrel and may be better suited for patients with acute coronary syndromes undergoing stent implantation [64,65].
In 2006 the success of first-generation DES in reducing ISR and target vessel revascularization was clouded by safety concerns over late (between 30 days and 1 year) and very late (beyond 1 year) ST [3–5]. Early investigators used variable definitions of ST which hampered the generalization and comparability of the research findings. Progress was made in 2007 when the Academic Research Consortium (ARC) proposed standardized definitions for ST [66]:
• Definite ST: angiographic or pathologic evidence of thrombus within a stent;
• Probable ST: any unexplained death within the first 30 days post stenting, or myocardial infarction that is related to acute ischemia in the territory of the implanted stent in the absence of any other obvious cause;
• Possible ST: any unexplained death from 30 days post stenting until end of trial follow-up.
ARC ST is also classified according to its temporal association with stenting: early (within 30 days), late (between 30 days and 1 year) and very late (beyond 1 year) [66].
A number of meta-analyses investigated ST using the ARC definitions and reported similarly low rates (<1%) of early and late ST for DES and BMS [67–71]. However, the meta-analyses demonstrated that firstgeneration DES were associated with a higher rate of very late ST (>1 year) than BMS [67–71]. Data from large registries indicate that the risk of very late ST persists at least 5 years post DES implantation, with an annual ST rate of approximately 0.5% [72,73,58]. Two larger network meta-analyses which included studies comparing second-generation DES with each other or with BMS, demonstrated that very late ARC definite ST was lower with everolimus DES than BMS [2,74]. Even though Resolute DES studies were included in the network meta-analyses [2,74], the relatively small number of patients involved make it difficult for solid conclusions to be drawn with regards to Resolute DES.
Overall, the available data on ST are reassuring but extensive DES utilization means that the population at risk is large and every possible step should be taken to prevent this complication. Risk factors of ST have recently been reviewed by Holmes et al [75] and Palmerini et al [76] and include:
• Patient factors: stenting in acute coronary syndromes, diabetes mellitus, premature DAPT discontinuation, DAPT non-responsiveness, prior brachytherapy, pro-thrombotic state;
• Lesion factors: lesion/stent length, vessel/stent diameter, complexity of lesion, saphenous vein graft intervention;
• Procedural factors: stent under-expansion, malapposition, edge dissection;
• Stent factors: hypersensitivity to drug coating or polymer, incomplete endothelialization, stent design, covered stents.
Premature DAPT interruption was identified as a strong predictor of ST following treatment with first-generation DES [56,77,78]. In the absence of data from randomized controlled trials examining the impact of DAPT duration on ST, current international guidelines recommend DAPT for 6 to 12 months based on consensus and observational data [6–11]. Even though these recommendations are reasonable for first-generation DES, the association of ST with DAPT interruption is less convincing with second-generation DES and other factors may play a dominant role in determining outcomes. The PRODIGY study examined ST relative to DAPT duration in 2013 patients [79]. The patients were randomized to a second-generation DES, a paclitaxel-eluting stent or a BMS and at 30 days randomized either to 6 or 24 months of DAPT with clopidogrel therapy. Treatment for 24 months did not confer a mortality benefit and did not reduce ST in second-generation DES [79]. In another study, Park et al. randomized 2701 DES recipients who had been free of major cardiovascular events and major bleeding for at least 12 months to receive either clopidogrel plus aspirin or aspirin alone. DAPT for longer than 12 months did not reduce myocardial infarction, death from cardiac causes or ST [80]. Finally, the PARIS registry examined adverse cardiac events and their relation to DAPT cessation in 5018 patients treated with BMS (11%), first-generation DES (15%) and second- generation DES (74%) [81]. The PARIS registry reported that the majority (74%) of a composite of cardiac death, definite or probable ST, myocardial infarction or target-lesion revascularization (MACE) at 2 years occurred whilst on DAPT [81]. The underlying pathology leading to DAPT interruption may be the dominant player in determining cardiac outcomes; DAPT interruption (<14 days) for surgery was not associated with a higher risk of MACE but DAPT disruption for bleeding was linked to increased MACE (HR: 1·41; 95% CI: 0·94–2·12; p = 0·10 and HR: 1·50; 95% CI: 1·14–1.97; p = 0·004 respectively when compared with patients on DAPT). Similar pattern of risk was observed for definite or probable ST [81].
DAPT interruption & ST with Resolute drug- eluting stents
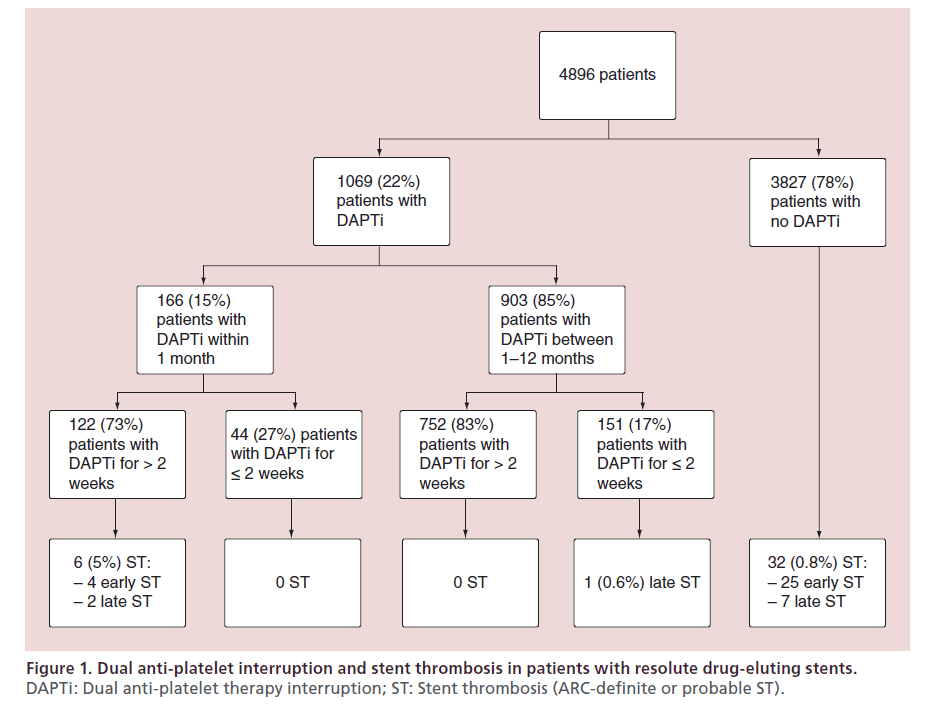
Silber et al. recently published a report on the occurrence of ST in patients treated with Resolute DES who subsequently interrupted DAPT [13]. For this analysis data were pooled from RESOLUTE-All Comers, RESOLUTE-International, RESOLUTE-Japan and RESOLUTE-US. Of the 4991 subjects who participated in the aforementioned trials, 95 (2%) patients were excluded primarily because of missing data on DAPT; the final cohort for this study consisted of 4896 patients. The majority of patients were in their seventh decade of life, 40% had an acute coronary syndrome and the prevalence of diabetes was approximately 30%. Most treated lesions were type B2/C.
All patients were recommended long-term treatment with aspirin with co-administration of a thienopyridine (clopidogrel or ticlopidine) for a minimum of 6 months and ideally for 12 months post stent implantation. DAPT interruption was defined as treatment discontinuation for more than 1 day. The outcome of the study was definite ST or probable ST as defined by the ARC. ST was examined in three groups: patients with continuous DAPT, DAPT interruption in the first month and DAPT interruption after 1 to 12 months following stent placement.
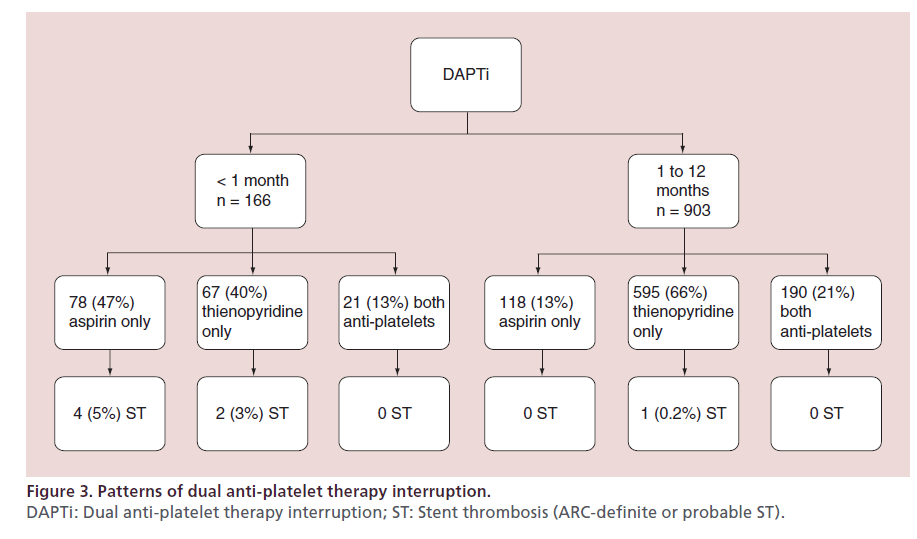
In all, DAPT interruption was reported in 1069 patients (22%): thienopyridine alone in 62%, aspirin alone in 18% and both anti-platelets in 20%. Medical, dental and surgical procedures were the most common causes of temporary DAPT interruption. Interestingly, bleeding was not a cause of DAPT interruption within the first month. Permanent DAPT interruption occurred mostly because of physician-directed with-drawal after a period of 6 months. The reported prevalence and patterns of DAPT interruption were broadly similar to the PARIS observational study [81].
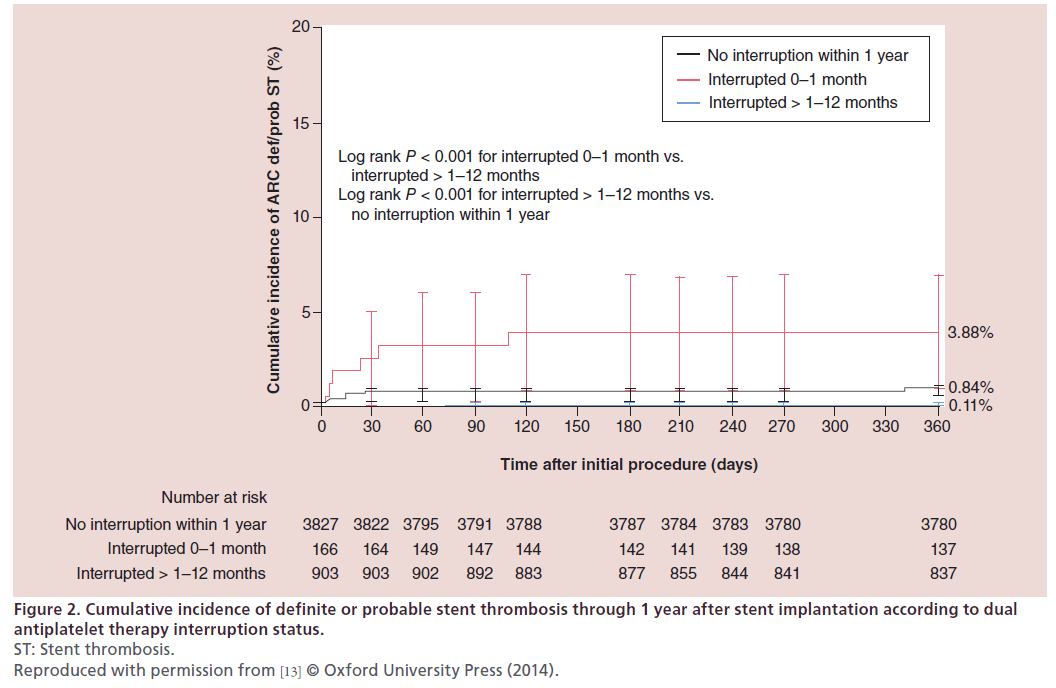
Within 1 year of treatment with Resolute DES, a total of 39 (0.8%) ST were observed in 4896 patients. The occurrence of ST stratified according to DAPT adherence is summarized in Figure 1. ST was most common after DAPT interruption within the first month post stenting, with a 3.9% cumulative incidence of ST at 1 year. There was a single case of definite late ST out of 903 patients who interrupted DAPT between 1 and 12 months (1-year cumulative incidence of 0.11%). This patient had a prior history of ST and stopped clopidogrel 69 days post intervention with ST developing 3 days later. There were 32 ST in patients with continuous DAPT, with a 0.84% cumulative incidence of ST at 1 year. Very late ST was not observed during the duration of the study. The Kaplan–Meier curves for each prespecified group is shown in Figure 2. DAPT interruption between 1 and 12 months was not associated with an increased risk of ST when compared with patients on continuous DAPT. The patterns of DAPT interruption and their relation to ST are shown in Figure 3. Notably, ST was not reported with the simultaneous interruption of aspirin and thienopyridne. The six ST within the first month of stenting occurred in patients with DAPT interruption of >14 days. No ST were reported in patients with prolonged DAPT interruption between 1 to 12 months.
Figure 2: Cumulative incidence of definite or probable stent thrombosis through 1 year after stent implantation according to dual
antiplatelet therapy interruption status.
ST: Stent thrombosis.
Reproduced with permission from [13] © Oxford University Press (2014).
The reported rates of ST by Silber et al. [13] are in keeping with the TWENTE trial which tested the non-inferiority of Resolute against Xience V [54]. In the TWENTE trial five definite or probable late ST (0.5%) were observed in patients treated with Resolute DES with one ST-related to DAPT interruption [54].
The exact mechanism responsible for the low incidence of ST with DAPT interruption in Resolute DES is not clear. The authors postulate that their findings may be explained by the highly biocompatible nature of the BioLinx polymer which provides a minimally pro-inflammatory hydrophilic surface as shown in a porcine coronary model [13,44,82]. In humans, optical coherence tomographic studies of Resolute DES have shown improved early neointimal coverage compared with first-generation DES [83,84].
DAPT interruption with other second-generation drug-eluting stents has also been associated with a low incidence of ST. Results from the 8061-patient XIENCE V US study showed that the incidence of definite/probable ST was 0.8% at 1 year and DAPT interruption between 1 to 12 months was not associated with an increased risk [85]. A polled analysis of SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials also showed that DAPT interruption after 6 months was not associated with ST in patients treated with Xience V stents [86].
Clinical implications
DAPT interruption within 1 year of DES implantation is unavoidable in approximately 15% of patients [81]. Cardiologists and patients commonly face difficult decisions because of unexpected bleeding and essential surgical/dental procedures unforeseen at the time of coronary intervention. To minimize the risk of ST with essential surgical procedures, guidelines recommend continuing aspirin whenever possible but difficult situations arise when both aspirin and thienopyridine have to be discontinued [87].
Data from Silber et al. [13] offer some reassurance that the incidence of ST is low following DAPT interruption (including both aspirin and thienopyridine) between 1 to 12 months post Resolute DES treatment. This observation should be interpreted cautiously as it is based on observational data rather than a randomized controlled trial examining the optimal duration of DAPT and should not change the current practice of DAPT for 6 to 12 months post DES [6–11]. The results also highlight that procedural and lesion factors are more important in determining ST than DAPT duration in the majority of patients as most ST occurred whilst on DAPT [13], in keeping with the PARIS registry data [81]. An unanswered question is whether these data from Resolute DES can be applied to patients treated with the newer Resolute Integrity stent which has a different stent platform but the same polymer and drug (zotarolimus).
Silber et al. also showed that DAPT interruption within the first month of Resolute DES was associated with a higher risk of ST [13] and should thus be avoided whenever possible. If absolutely essential, DAPT interruption should be limited to less than 2 weeks.
Future perspective
With the development of second-generation DES, the risk of ST thrombosis appears to have declined and randomized controlled trials have shown that DAPT for 24 months is not beneficial [79,80]. The optimal DAPT duration with second-generation DES needs to be determined in an adequately powered prospective clinical trial. However, the low incidence of ST means makes this challenging as a very large number of patients will need to be enrolled.
Executive summary
Background
• Stent thrombosis (ST) is an uncommon but serious complication of coronary stenting.
Stent thrombosis & dual anti-platelet therapy
• Dual anti-platelet therapy (DAPT) interruption within the first month of stenting is unequivocally associated with a very high risk of ST and should be avoided at all costs.
DAPT interruption with Resolute stents
• Recently published data from the Medtronic RESOLUTE Global Clinical Program indicate that DAPT interruption between 1 to 12 months of Resolute DES implantation is not associated with a higher risk of ST.
Clinical implications
• Patients treated with Resolute DES should still be advised to continue DAPT for 6 to 12 months post stenting in accordance to international guidelines and can be reassured that DAPT interruption for concurrent medical/surgical illnesses or bleeding is not likely to lead to catastrophic ST.
Future perspective
• The optimal DAPT duration with second-generation DES needs to be determined in an adequately powered prospective clinical trial.
References
Papers of special note have been highlighted as:
•• of considerable interest.
- Lenzen MJ, Boersma E, Bertrand ME et al. Management and outcome of patients with established coronary artery disease: the Euro Heart Survey on coronary revascularization. Eur. Heart J. 26(12), 1169–1179 (2005).
- Bangalore S, Kumar S, Fusaro M et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation 125(23), 2873–2891 (2012).
- McFadden EP, Stabile E, Regar E et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 364(9444), 1519–1521 (2004).
- Ong AT, McFadden EP, Regar E, de Jaegere PP, van Domburg RT, Serruys PW. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J. Am. Coll. Cardiol. 45(12), 2088–2092 (2005).
- Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation 115(11), 1440–1455 (2007).
- Silber S, Albertsson P, Aviles FF et al. Guidelines for percutaneous coronary interventions. The task force for percutaneous coronary interventions of the european society of cardiology. Eur. Heart J. 26(8), 804–847 (2005).
- Wijns W, Kolh P, Danchin N et al. Guidelines on myocardial revascularization. Eur. Heart J. 31(20), 2501–2555 (2010).
- Levine GN, Bates ER, Blankenship JC et al. ACCF/AHA/ SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/ American Heart Association Task Force on practice guidelines and the society for cardiovascular angiography and interventions. J. Am. Coll. Cardiol. 58(24), e44–e122 (2011).
- Jneid H, Anderson JL, Wright RS et al. ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 126(7), 875–910 (2012).
- O’Gara PT, Kushner FG, Ascheim DD et al. 2013 ACCF/ AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 127(4), e362–e425 (2013).
- Montalescot G, Sechtem U, Achenbach S et al. ESC guidelines on the management of stable coronary artery disease: The Task Force On The Management of Stable Coronary Artery Disease of The European Society of Cardiology. Eur. Heart J. 34, 2949–3003 (2013).
- Udipi K, Melder RJ, Chen M et al. The next generation Endeavor Resolute Stent: role of the BioLinx Polymer System. EuroIntervention 3(1), 137–139 (2007).
- Silber S, Kirtane AJ, Belardi JA et al. Lack of association between dual antiplatelet therapy use and stent thrombosis between 1 and 12 months following resolute zotarolimuseluting stent implantation. Eur. Heart J. 35(29), 1949–1956 (2014).
- Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 301(2), 61–68 (1979).
- Detre KM, Holmes DR, Jr, Holubkov R et al. Incidence and consequences of periprocedural occlusion. The 1985–1986 National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation 82(3), 739–750 (1990).
- Serruys PW, de Jaegere P, Kiemeneij F et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N. Engl. J. Med. 331(8), 489–495 (1994).
- Mintz GS, Popma JJ, Pichard AD et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation 94(1), 35–43 (1996).
- Pasterkamp G, Peters RJ, Kok WE, van Leeuwen TG, Borst C. Arterial remodeling after balloon angioplasty of the coronary artery: an intravascular ultrasound study. PICTURE Investigators. PostTreatment IntraCoronary Transluminal Ultrasound Result Evaluation. Am. Heart J. 134(4), 680–684 (1997).
- Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 316(12), 701–706 (1987).
- van Hout BA, van der WT, de Jaegere PP et al. Cost effectiveness of stent implantation versus PTCA: the BENESTENT experience. Semin. Interv. Cardiol. 1(4), 263–268 (1996).
- Fischman DL, Leon MB, Baim DS et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 331(8), 496–501 (1994).
- Chen MS, John JM, Chew DP, Lee DS, Ellis SG, Bhatt DL. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 151(6), 1260–1264 (2006).
- Hoffmann R, Mintz GS, Dussaillant GR et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation 94(6), 1247–1254 (1996).
- Scott NA. Restenosis following implantation of bare metal coronary stents: pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 58(3), 358–376 (2006).
- Sousa JE, Costa MA, Abizaid A et al. Lack of neointimal proliferation after implantation of sirolimus-coated stents in human coronary arteries: a quantitative coronary angiography and three-dimensional intravascular ultrasound study. Circulation 103(2), 192–195 (2001).
- Axel DI, Kunert W, Goggelmann C et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 96(2), 636–645 (1997).
- Poon M, Marx SO, Gallo R, Badimon JJ, Taubman MB, Marks AR. Rapamycin inhibits vascular smooth muscle cell migration. J. Clin. Invest. 98(10), 2277–2283 (1996).
- Marx SO, Jayaraman T, Go LO, Marks AR. Rapamycin- FKBP inhibits cell cycle regulators of proliferation in vascular smooth muscle cells. Circ. Res. 76(3), 412–417 (1995).
- Moses JW, Leon MB, Popma JJ et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 349(14), 1315–1323 (2003).
- Stone GW, Ellis SG, Cox DA et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 350(3), 221–231 (2004).
- Kastrati A, Dibra A, Eberle S et al. Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA 294(7), 819–825 (2005).
- Schomig A, Dibra A, Windecker S et al. A meta-analysis of 16 randomized trials of sirolimus-eluting stents versus paclitaxel-eluting stents in patients with coronary artery disease. J. Am. Coll. Cardiol. 50(14), 1373–1380 (2007).
- Dangas GD, Claessen BE, Caixeta A, Sanidas EA, Mintz GS, Mehran R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 56(23), 1897–1907 (2010).
- Huang S, Houghton PJ. Mechanisms of resistance to rapamycins. Drug Resist. Updat. 4(6), 378–391 (2001).
- Nebeker JR, Virmani R, Bennett CL et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J. Am. Coll. Cardiol. 47(1), 175–181 (2006).
- Doi H, Maehara A, Mintz GS et al. Classification and potential mechanisms of intravascular ultrasound patterns of stent fracture. Am. J. Cardiol. 103(6), 818–823 (2009).
- Levy Y, Mandler D, Weinberger J, Domb AJ. Evaluation of drug-eluting stents’ coating durability‐‐clinical and regulatory implications. J. Biomed. Mater. Res. B Appl. Biomater. 91(1), 441–451 (2009).
- Balakrishnan B, Tzafriri AR, Seifert P, Groothuis A, Rogers C, Edelman ER. Strut position, blood flow, and drug deposition: implications for single and overlapping drug-eluting stents. Circulation 111(22), 2958–2965 (2005).
- Mintz GS. Features and parameters of drug-eluting stent deployment discoverable by intravascular ultrasound. Am. J. Cardiol. 100(8B), 26M–35M (2007).
- Garg S, Serruys PW. Coronary stents: looking forward. J. Am. Coll. Cardiol. 56(Suppl. 10), S43–S78 (2010).
- Garg S, Serruys PW. Coronary stents: current status. J. Am. Coll. Cardiol. 56(Suppl. 10), S1–S42 (2010).
- Iqbal J, Gunn J, Serruys PW. Coronary stents: historical development, current status and future directions. Br. Med. Bull. 106, 193–211 (2013).
- Navarese EP, Tandjung K, Claessen B et al. Safety and efficacy outcomes of first and second generation durable polymer drug eluting stents and biodegradable polymer biolimus eluting stents in clinical practice: comprehensive network meta-analysis. BMJ 347, f6530 (2013).
- Hezi-Yamit A, Sullivan C, Wong J et al. Impact of polymer hydrophilicity on biocompatibility: implication for DES polymer design. J. Biomed. Mater. Res. A 90(1), 133–141 (2009).
- Chen YW, Smith ML, Sheets M et al. Zotarolimus, a novel sirolimus analogue with potent anti-proliferative activity on coronary smooth muscle cells and reduced potential for systemic immunosuppression. J. Cardiovasc. Pharmacol. 49(4), 228–235 (2007).
- Meredith IT, Worthley S, Whitbourn R et al. Clinical and angiographic results with the next-generation resolute stent system: a prospective, multicenter, first-in-human trial. JACC Cardiovasc. Interv. 2(10), 977–985 (2009).
- Meredith IT, Worthley SG, Whitbourn R et al. Longterm clinical outcomes with the next-generation Resolute Stent System: a report of the two-year follow-up from the RESOLUTE clinical trial. EuroIntervention 5(6), 692–697 (2010).
- Yeung AC, Leon MB, Jain A et al. Clinical evaluation of the Resolute zotarolimus-eluting coronary stent system in the treatment of de novo lesions in native coronary arteries: the RESOLUTE US clinical trial. J. Am. Coll. Cardiol. 57(17), 1778–1783 (2011).
- Serruys PW, Silber S, Garg S et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N. Engl. J. Med. 363(2), 136–146 (2010).
- Silber S, Windecker S, Vranckx P, Serruys PW. Unrestricted randomised use of two new generation drug-eluting coronary stents: 2-year patient-related versus stent-related outcomes from the RESOLUTE All Comers trial. Lancet 377(9773), 1241–1247 (2011).
- Neumann FJ, Widimsky P, Belardi JA. One-year outcomes of patients with the zotarolimus-eluting coronary stent: RESOLUTE International Registry. EuroIntervention 7(10), 1181–1188 (2012).
- Silber S, Serruys PW, Leon MB et al. Clinical outcome of patients with and without diabetes mellitus after percutaneous coronary intervention with the resolute zotarolimus-eluting stent: 2-year results from the prospectively pooled analysis of the international global RESOLUTE program. JACC Cardiovasc. Interv. 6(4), 357–368 (2013).
- Farooq V, Vranckx P, Mauri L et al. Impact of overlapping newer generation drug-eluting stents on clinical and angiographic outcomes: pooled analysis of five trials from the international Global RESOLUTE Program. Heart 99(9), 626–633 (2013).
- von Birgelen C, Basalus MW, Tandjung K et al. A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J. Am. Coll. Cardiol. 59(15), 1350–1361 (2012).
- Wenaweser P, Rey C, Eberli FR et al. Stent thrombosis following bare-metal stent implantation: success of emergency percutaneous coronary intervention and predictors of adverse outcome. Eur. Heart J. 26(12), 1180–1187 (2005).
- Iakovou I, Schmidt T, Bonizzoni E et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293(17), 2126–2130 (2005).
- Vaknin-Assa H, Assali A, Ukabi S, Lev EI, Kornowski R. Stent thrombosis following drug-eluting stent implantation. A single-center experience. Cardiovasc. Revasc. Med. 8(4), 243–247 (2007).
- Pinto Slottow TL, Steinberg DH, Roy PK et al. Observations and outcomes of definite and probable drug-eluting stent thrombosis seen at a single hospital in a four-year period. Am. J. Cardiol. 102(3), 298–303 (2008).
- Almalla M, Schroder J, Hennings V, Marx N, Hoffmann R. Long-term outcome after angiographically proven coronary stent thrombosis. Am. J. Cardiol. 111(9), 1289–1294 (2013).
- Fujii K, Carlier SG, Mintz GS et al. Stent underexpansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: an intravascular ultrasound study. J. Am. Coll. Cardiol. 45(7), 995–998 (2005).
- Leon MB, Baim DS, Popma JJ et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N. Engl. J. Med. 339(23), 1665–1671 (1998).
- Cutlip DE, Baim DS, Ho KK et al. Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103(15), 1967–1971 (2001).
- Mehta SR, Yusuf S, Peters RJ et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 358(9281), 527–533 (2001).
- Wiviott SD, Braunwald E, McCabe CH et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357(20), 2001–2015 (2007).
- Cannon CP, Harrington RA, James S et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 375(9711), 283–293 (2010).
- Cutlip DE, Windecker S, Mehran R et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 115(17), 2344–2351 (2007).
- Spaulding C, Daemen J, Boersma E, Cutlip DE, Serruys PW. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 989–997 (2007).
- Stone GW, Moses JW, Ellis SG et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N. Engl. J. Med. 356(10), 998–1008 (2007).
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N. Engl. J. Med. 356(10), 1020–1029 (2007).
- Roukoz H, Bavry AA, Sarkees ML et al. Comprehensive metaanalysis on drug-eluting stents versus bare-metal stents during extended follow-up. Am. J. Med. 122(6), 581–510 (2009).
- Kastrati A, Mehilli J, Pache J et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 356(10), 1030–1039 (2007).
- Daemen J, Wenaweser P, Tsuchida K et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxeleluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369(9562), 667–678 (2007).
- Wenaweser P, Daemen J, Zwahlen M et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J. Am. Coll. Cardiol. 52(14), 1134–1140 (2008).
- Palmerini T, Biondi-Zoccai G, Della RD et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet 379(9824), 1393–1402 (2012).
- Holmes DR, Jr., Kereiakes DJ, Garg S et al. Stent thrombosis. J. Am. Coll. Cardiol. 56(17), 1357–1365 (2010).
- Palmerini T, Biondi-Zoccai G, Della RD et al. Stent thrombosis with drug-eluting stents: is the paradigm shifting? J. Am. Coll. Cardiol. 62(21), 1915–1921 (2013).
- Pfisterer M, Brunner-La Rocca HP, Buser PT et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drugeluting versus bare-metal stents. J. Am. Coll. Cardiol. 48(12), 2584–2591 (2006).
- 78 Eisenstein EL, Anstrom KJ, Kong DF et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA 297(2), 159–168 (2007).
- Valgimigli M, Borghesi M, Tebaldi M, Vranckx P, Parrinello G, Ferrari R. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur. Heart J. 34(12), 909–919 (2013).
- Park SJ, Park DW, Kim YH et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N. Engl. J. Med. 362(15), 1374–1382 (2010).
- Mehran R, Baber U, Steg PG et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 382(9906), 1714–1722 (2013).
- Udipi K, Chen M, Cheng P et al. Development of a novel biocompatible polymer system for extended drug release in a next-generation drug-eluting stent. J. Biomed. Mater. Res. A 85(4), 1064–1071 (2008).
- Kim JS, Kim JS, Shin DH et al. Optical coherence tomographic comparison of neointimal coverage between sirolimus- and resolute zotarolimus-eluting stents at 9 months after stent implantation. Int. J. Cardiovasc. Imaging 28(6), 1281–1287 (2012).
- Kim JS, Kim BK, Jang IK et al. ComparisOn of neointimal coVerage betwEen zotaRolimus-eluting stent and everolimuseluting stent using Optical Coherence Tomography (COVER OCT). Am. Heart J. 163(4), 601–607 (2012).
- Naidu SS, Krucoff MW, Rutledge DR et al. Contemporary incidence and predictors of stent thrombosis and other major adverse cardiac events in the year after XIENCE V implantation: results from the 8,061-patient XIENCE V United States study. JACC Cardiovasc. Interv. 5(6), 626–635 (2012).
- Kedhi E, Stone GW, Kereiakes DJ et al. Stent thrombosis: insights on outcomes, predictors and impact of dual antiplatelet therapy interruption from the SPIRIT II, SPIRIT III, SPIRIT IV and COMPARE trials. EuroIntervention 8(5), 599–606 (2012).
- Darvish-Kazem S, Gandhi M, Marcucci M, Douketis JD. Perioperative management of antiplatelet therapy in patients with a coronary stent who need noncardiac surgery: a systematic review of clinical practice guidelines. Chest 144(6), 1848–1856 (2013).
•• Major meta-analysis comparing stent thrombosis in secondgeneration drug-eluting stents and bare metal stents.
•• Detailed description of the relation of dual anti-platelet therapy interruption and stent thrombosis with Resolute drug-eluting stents.
•• Major meta-analysis comparing stent thrombosis in secondgeneration drug-eluting stents and bare metal stents.
•• Up-to-date review of stent thrombosis.
•• A large registry study examining the patterns of nonadherence to anti-platelet regimens post percutaneous coronary intervention.