Case Report - Interventional Cardiology (2024) Volume 16, Issue 5
A case of ischemia with Non-Obstructive Coronary Arteries (INOCA) in a non-smoker 50-year-old female: Diagnostic challenges and management strategies
- Corresponding Author:
- Antonio Georgiev
Department of Cardiology, Ss. Cyril and Methodius University, Skopje, North Macedonia,
E-mail: antoniogeorgiev@yahoo.com
Received date: 23-Oct-2024, Manuscript No. FMIC-24-150748; Editor assigned: 25-Oct-2024, PreQC No. FMIC-24-150748 (PQ); Reviewed date: 08-Nov-2024, QC No. FMIC-24-150748; Revised date: 15-Nov-2024, Manuscript No. FMIC-24-150748 (R); Published date: 22-Nov-2024, ![]()
Abstract
Myocardial Infarction/Ischemia with Non-Obstructive Coronary Arteries (MINOCA/ INOCA) is a form of acute Myocardial Infarction (MI) without significant coronary artery blockage, making it diagnostically challenging. Accounting in up to 15% of all MI cases, INOCA disproportionately affects women and thus requires prompt recognition and targeted management to improve outcomes.
Our case has to do with a 50-year-old female patient, a non-smoker, who presented with chest pain characterized by tightening and pressure lasting for 30 min. Initial Electrocardiogram (ECG) revealed ST-segment elevations in leads V2-V5 and elevated initially troponin levels from 5,400 to 14,375 ng/L upon admission to our clinic, further confirming myocardial injury. Cardiac imaging revealed dyskinesia at the apex of the left ventricle with ischemic lesions but excluded myocarditis and Takotsubo syndrome, leading to the diagnosis of INOCA. A cardiac MRI showed transmural necrotic ischemic zones and fibrotic changes in the left ventricular apex. Thus we proceeded with a coronary angiography, revealing no significant stenosis, without using advanced imaging techniques such as Optical Coherence Tomography (OCT), Intravascular Ultrasound (IVUS) and Fractional Flow Reserve (FFR).
Treatment for INOCA must be individualized and should be tailored to the underlying cause, with different pharmacological strategies suggested based on factors such as plaque disruption, coronary spasm, or microvascular dysfunction, with a focus on addressing the ischemic origin. Accurate diagnosis requires careful evaluation through advanced imaging techniques such as OCT, IVUS and FFR. Early and precise intervention, combined with long-term monitoring, can improve outcomes and reduce the risk of future cardiac events.
Keywords
INOCA • Plaque disruption • Precise intervention • Cardiac events • Coronary angiography
Abbreviations
ECG: Electrocardiogram; OCT: Optical Coherence Tomography; IVUS: Intravascular Ultrasound FFR: Fractional Flow Reserve; CAD: Coronary Artery Disease; ESC: European Society of Cardiology; AMI: Acute Myocardial Infarction
Introduction
For many years, MI has been observed in patients without significant obstructive CAD or an identifiable culprit artery, until 2013 when this condition was formally termed “myocardial infarction/ischemia with non-obstructive coronary arteries-MINOCA/ INOCA” [1,2], followed by the paper of ESC in 2017 when was published a paper on MINOCA introducing diagnostic criteria based on the third universal definition of MI as follows:
• The presence of positive cardiac biomarker with clinical evidence of infarction
• Absence of stenosis (≥ 50%) in any epicardial coronary arteries on coronary angiography and
• Lack of any alternative diagnosis for the index presentation [3]
However, in recent times there has been a tendency to use the term INOCA, which means ischemia but without the presence of myocardial infarction, therefore in the following text, we will stick to this term even though the scientific papers that will be cited have the same MINOCA etiology and pathology.
MINOCA is recognized as a heterogeneous working diagnosis, with an estimated prevalence ranging from 3% to 15% (6% to 8% according to ESC) among all AMI patients [4,5]. This variability is partly due to differences in the conditions classified as MINOCA and the definitions applied. A pooled analysis of 23 studies found that the prevalence of MINOCA was 8.1% among 806,851 consecutive AMI patients [6]. National registries from countries such as the US, Japan, Poland and Sweden report MINOCA incidences between 2.9% and 10.2% [7-9]. Compared to myocardial infarction with obstructive CAD, MINOCA patients tend to be younger, with a median age of around 61 years and are more frequently identified in the Black and Hispanic populations [10,11]. Additionally, MINOCA patients are less likely to exhibit traditional cardiovascular risk factors, including hypertension, dyslipidemia, diabetes and current smoking history.
MINOCA disproportionately affects women, with recent metaanalyses indicating that females account for up to 50% of MINOCA patients [12]. In the VIRGO study (Variation in recovery: role of gender on outcomes of young AMI patients), young women with AMI had approximately five times the odds of experiencing MINOCA compared to men (14.9% vs. 3.5%, OR: 4.84, 95% CI: 3.29-7.13). Among the 269 women with MINOCA in the study, the majority (75%) had an undefined cause, while 4% were attributed to spasm, 21% to dissection and 1% to embolization. An analysis of MINOCA patients from the ACTION Registry- GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry-Get with the Guidelines) revealed a higher incidence of in-hospital Major Adverse Cardiovascular Events (MACE)-a composite of death, reinfarction, cardiogenic shock, or heart failure in females compared to males (5.4% vs. 4.1%; p<0.0001) [13].
At the time of angiography, INOCA is regarded as a provisional diagnosis, pending the exclusion of other causes of myocardial injury and identification of the underlying ischemic mechanisms [14].
The initial diagnostic investigations for identifying the causes of INOCA include clinical history, ECG, cardiac enzymes, echocardiography, coronary angiography and Left Ventricular (LV) angiography. Notably, regional wall motion abnormalities observed during LV angiography can indicate an “epicardial pattern” when limited to a single epicardial coronary artery territory which includes: Plaque rupture or erosion, coronary dissection and coronary artery spasm; or a “microvascular pattern” when they extend beyond this territory including Takotsubo cardiomyopathy, myocarditis, coronary thromboembolism, other forms of type 2 MI and INOCA of uncertain etiology [15].
In 2018, the universal definition of MI was revised to encompass only ischemic mechanisms linked to myocardial injury, thereby excluding non-ischemic conditions such as Takotsubo cardiomyopathy, myocarditis and non-ischemic cardiomyopathy. Consequently, the American Heart Association (AHA) scientific statement in 2019 refined the definition of MINOCA by excluding these non-ischemic mechanisms, categorizing them as MINOCA mimickers [16].
INOCA patients present a clinical challenge due to the wide range of possible etiologies and pathogenic mechanisms linked to the condition. Therefore, selecting the appropriate treatment often necessitates further diagnostic evaluation.
Case Presentation
Case history and examination
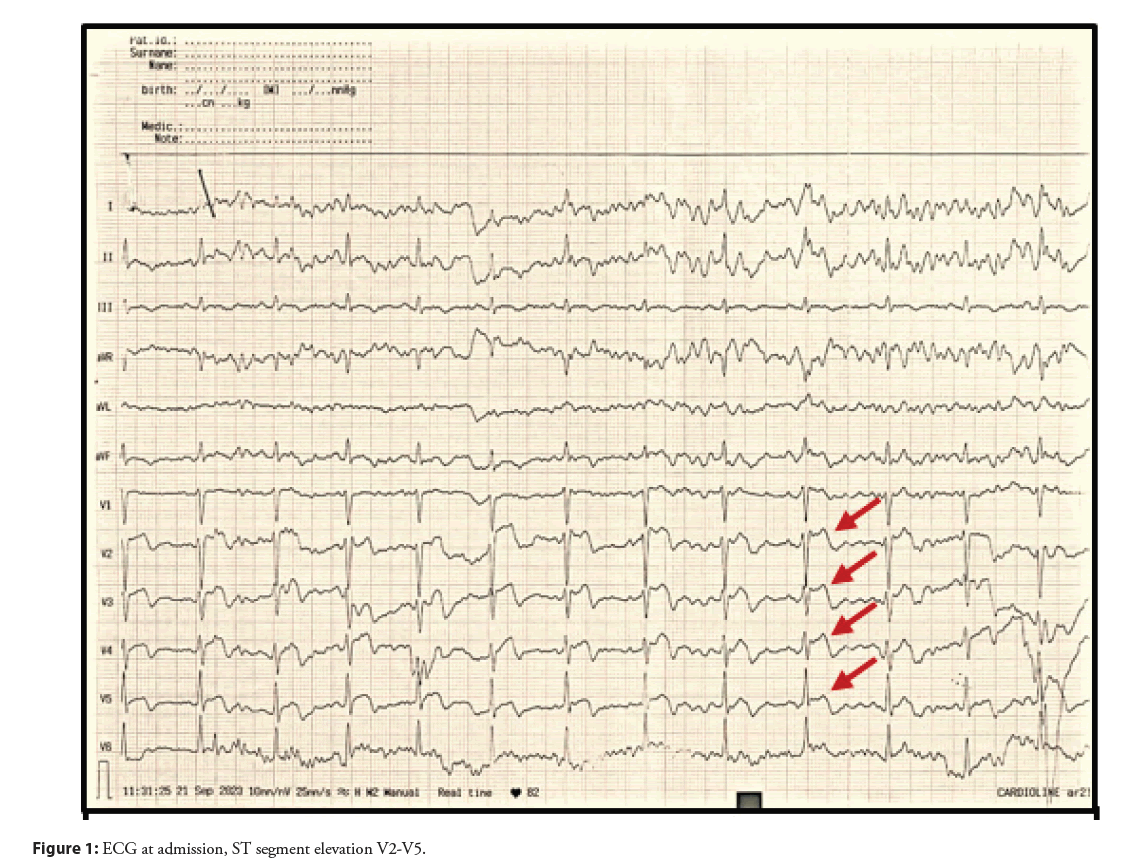
A 50-year-old female patient, a non-smoker, presented to the primary care health institution with chest pain described as a tightening sensation and pressure lasting 30 min. The ECG revealed ST segment elevations in leads V2-V5 (Figure 1) and her troponin level was significantly elevated at 5400 ng/L initially. Patient received treatment in accordance with myocardial infarction protocols and was subsequently referred to the cardiology clinic for further investigation and treatment. Her past medical history includes hypertension, diagnosed 2 years prior and a right leg fracture 2 months ago (July, 2023). Family history is non-contributory and patient is currently taking Enalapril 5 mg twice daily, with no regular measurements and follow-up by the family doctor.
Diagnosis, investigation and treatment
From the laboratory findings: Troponin: 14375 ng/L, C-Reactive Protein (CRP): 2.8 mg/L, Na+: 137 mmol/L, K+: 4.4 mmol/L, Urea: 5.9 mmol/L, Creatinine: 65 μmol/L, Fasting Plasma Glucose (FPG): 5.6 mmol/L, Hgb: 131 g/L, RBC: 4.2 × 10^12 cells/L, WBC: 10.5 × 10^9 cells/L, PLT: 223 × 10^9 cells/l, D-dimer: 326 ng/ml. These results indicate significant elevation in troponin levels, consistent with myocardial injury, with a normal range of the blood count, electrolyte status, degradation products, inflammation marker, fasting plasma glucose and D-dimer, which in this case could indicate one of the theories of the pathophysiological causes of INOCA in the scope of microvascular pattern, respectively coronary thromboembolism, which in this case is also reasonable to suspect due to the fracture that the patient had two months before this event.
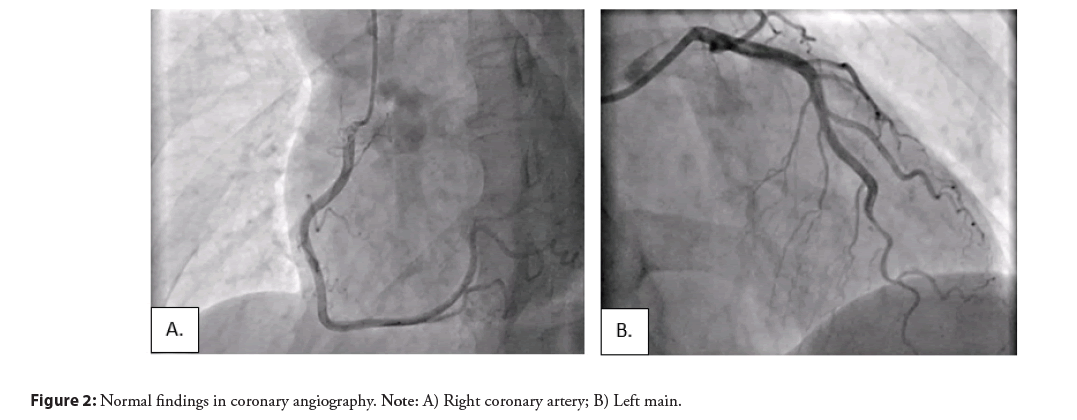
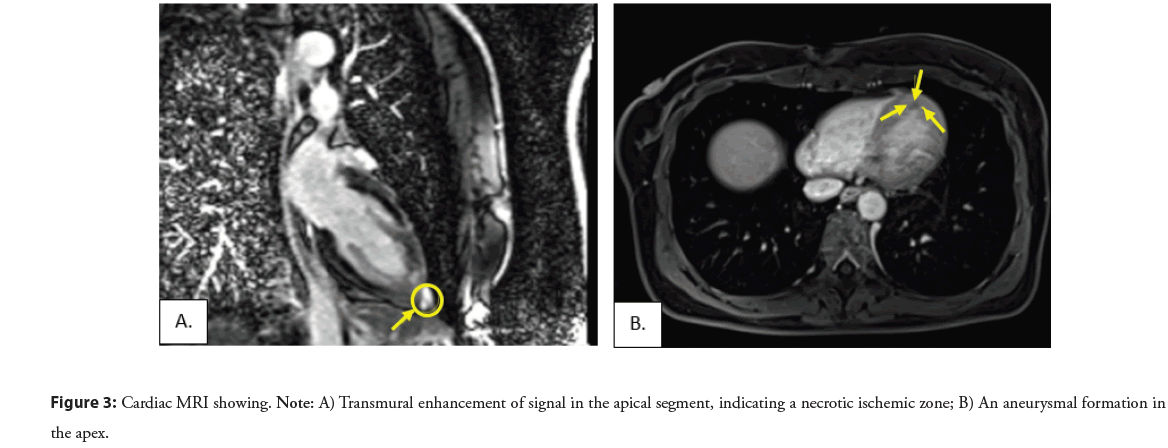
Because of the complexity of the patient and situation, in the beginning, a coronary angiography was performed, revealing no significant stenosis and normal findings were reported (Figure 2), and after that the next day an echocardiogram was indicated showing an impaired global longitudinal deformation with a Global Longitudinal Strain (GLS) 15% (ref values >-20%) of the left ventricle involving the entire myocardium, except the basal segments of the anterior and inferior walls of the left ventricle with an impression of dyskinetic motion at the apex of the left ventricle, necessitating further investigation, with a normal systolic function (EF 63%). According to ESC guidelines for the diagnosis of INOCA, a cardiac MRI was performed with early post-contrast series showing transmural enhancement of signal in the apical segment, indicating a necrotic ischemic zone. A delayed post-contrast series reveals clear enhancement along the apical inferior wall and towards the mid-septal anteroseptal and inferoseptal walls, as well as significant enhancement of the aneurysmal dilated apex, predominantly due to fibrotic changes, changes which correspond to ischemic lesions, excluding myocarditis and Takotsubo syndrome and confirming the INOCA (Figure 3).
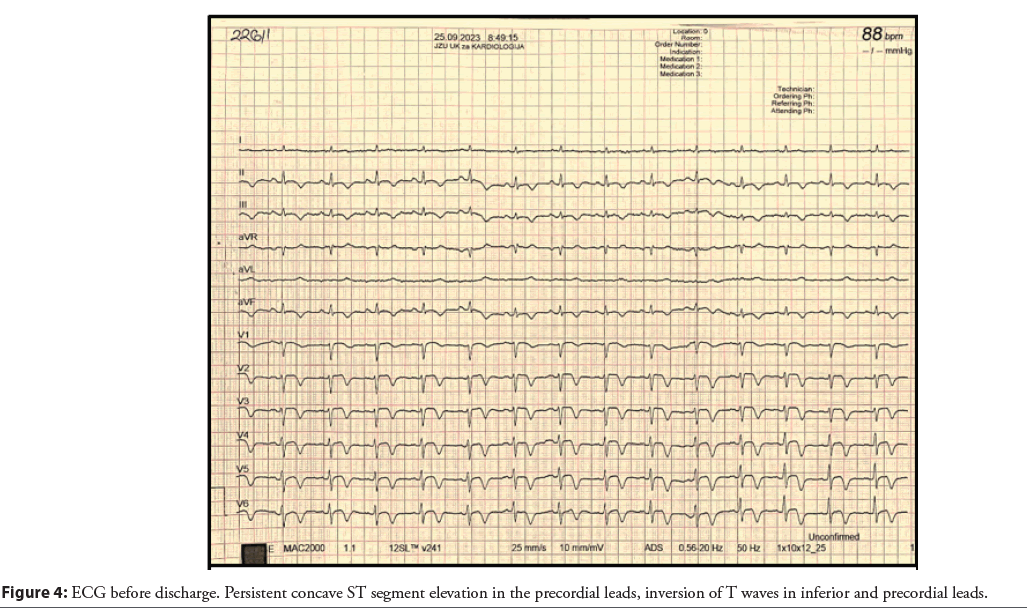
In cases of diagnostic uncertainty like in our case, advanced intracoronary imaging modalities such as OCT, Intravascular Ultrasound (IVUS) and FFR may be considered but these techniques were not available at the time to perform. However, these should be used cautiously due to the potential risk of propagating coronary dissection. Regarding our case, it was decided to discharge the patient with the following therapy due to the extensive areas of ischemia guided by the findings in MRI and ECG changes before admission and discharge (Figure 4): Tbl. Acetyl-salicylic acid a 100 mg 1 × 1, Tbl. Clopidogrel a 75 mg 1 × 1, Tbl. Rosuvastatin a 40 mg 1 × 1, Tbl. Zofenopril 75 mg 2 × 1, Tbl. Pantoprazole a 40 mg 1 × 1.
Outcome and follow-up
Following the administration of the therapy recommended by the expert opinion and studies conducted in this domain, the patient showed significant improvement clinically, but not only, the highly sensitive troponin which was 14375 ng/L in the admission, four days after we noticed a significant decrease before discharge from the hospital, 2720.7 ng/L and all other laboratory findings were in the normal range. The patient was discharged hemodynamically and rhythmically stable with a recommendation of a hygienedietetic regimen and follow-up in our clinic after one month. In the first consultation after a month the patient showed no complications or subjective complaints. After six months tbl. Clopidogrel was discontinued and the patient had an appointment on November 2024, for a control echocardiography and magnetic resonance of the heart.
Results and Discussion
Patients with an identified underlying cause of INOCA benefit from targeted, cause-specific treatment. Additionally, secondary atherothrombotic prevention measures should be considered based on the specific etiology. No randomized clinical trials have been published for the treatment of INOCA, so management should be individualized and tailored to the underlying cause of each case, but there are few randomized controlled trials and observational studies have reported lower mortality in MINOCA patients treated with Angiotensin-Converting Enzyme (ACE) inhibitors/angiotensin II receptor blockers and statins. One such study, involving 9,138 patients from the SWEDHEART registry, demonstrated improved outcomes with these treatments, analyzes the positive effect of treatment with beta-blockers and the neutral effect of dual antiplatelet therapy [17,18].
MINOCA-BAT is a study involving 3500 patients investigating the effects of beta-blockers and ACE/ARB compared to placebo. It is being conducted to assess mortality, recurrent myocardial infarction, stroke, or heart failure, with the study expected to conclude in 2025 [19]. The PROMISE trial, will recruit 180 patients diagnosed with MINOCA and randomly assign them into two groups. One group will undergo a precision medicine approach that includes coronary OCT, Cardiac Magnetic Resonance Imaging (CMRI) and coronary spasm testing to guide tailored medical therapy. The other group will follow the standard approach to managing acute coronary syndrome. This study aims to evaluate the effectiveness of personalized diagnostics and treatment strategies compared to conventional care in INOCA patients [20].
It is important to highlight that current expert recommendations for INOCA management are not fully aligned, with conflicting opinions and differing interpretations. For example, the European Society of Cardiology (ESC) guidelines for managing non-ST segment elevation acute coronary syndromes recommend using conventional secondary prevention medications for INOCA, similar to Myocardial Infarction with Obstructive Coronary Artery Disease (MI-CAD), when the underlying cause is unclear [21].
The ESC advises routine use of aspirin, statins and Calcium Channel Blockers (CCBs) for vasospasm. In contrast, the American Heart Association (AHA) suggests that statins and antiplatelet therapy should only be used in INOCA cases involving plaque disruption and avoided in type 2 MI, where they may be contraindicated [22].
Currently, targeted therapies for INOCA based on specific pathophysiological mechanisms have not been sufficiently studied and in the Table 1, we have listed with minimal corrections the optimal therapeutic treatment based on the etiology of INOCA. It remains uncertain whether conventional secondary preventive therapies are effective in reducing post-infarct angina in INOCA patients. An individualized approach should be adopted based on the underlying cause (etiology) of its development.
| MINOCA/INOCA | Etiology | Treatment |
| Plaque disruption | ASA, Clopidogrel or Ticagrelor, Statins, RAASi, β-blockers | |
| Epicardial coronary vasospasm | Isosorbid dinitrate, CCBs (low-dose ASA, statins, β-blockers) | |
| Coronary microvascular dysfunction | β-blockers, CCBs, (RAASi, statins, nitrates) | |
| Spontaneous coronary artery dissection | Conservative therapy (β-blockers, statins, RAASi, ASA, Clopidogrel or Ticagrelor) | |
| Coronary artery embolism | PFO closure, OAC |
Abbreviations: ASA: Acetylsalicylic Acid; RAASi: Renin Angiotensin Adrenergic Antagonists; CCBs: Calcium Channel Blockers; OAC: Oral Anticoagulation; PFO: Patent Foramen Ovale
Table 1: Therapeutic target based on the etiology of INOCA (adopted from Samaras et al. [22]
Conclusion
INOCA is a syndrome that predominantly affects women and presents as a heterogeneous working diagnosis, often understudied, underdiagnosed and undertreated. Despite the absence of significant coronary artery blockages, patients with INOCA experience significant cardiovascular events and adverse outcomes, highlight the need for thorough diagnostic evaluation, including advanced multimodality imaging, to identify underlying mechanisms and guide treatment. While randomized clinical trials on secondary prevention are ongoing, there is an urgent need for large, multicenter studies to better understand the underlying mechanism of INOCA and to define evidence-based therapeutic strategies, which could improve patient outcomes and quality of life.
Consent
Written informed consent was obtained from patient to publish this report in accordance with the journal’s patient consent policy.
References
- Yildiz M, Ashokprabhu N, Shewale A, et al. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA). Front Cardiovasc Med. 9:1032436 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Beltrame JF. Assessing patients with Myocardial Infarction and Nonobstructed Coronary Arteries (MINOCA). J Intern Med. 273(2) (2013).
[CrossRef] [Google Scholar] [PubMed]
- Agewall S, Beltrame JF, Reynolds HR, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. 38(3):143-153 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Choo EH, Chang K, Lee KY, et al. Prognosis and predictors of mortality in patients suffering myocardial infarction with nonâobstructive coronary arteries. J Am Heart Assoc. 8(14):e011990 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Dreyer RP, Tavella R, Curtis JP, et al. Myocardial infarction with non-obstructive coronary arteries as compared with myocardial infarction and obstructive coronary disease: Outcomes in a medicare population. Eur Heart J.41(7):870-878 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Pasupathy S, Lindahl B, Litwin P, et al. Survival in patients with suspected myocardial infarction with nonobstructive coronary arteries: A comprehensive systematic review and meta-analysis from the MINOCA global collaboration. Circ Cardiovasc Qual Outcomes. 14(11):e007880 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Gasior P, Desperak A, Gierlotka M, et al. Clinical characteristics, treatments, and outcomes of patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Results from a multicenter national registry. J Clin Med. 9(9):2779 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Lindahl B, Baron T, Erlinge D, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 135(16):1481-1489 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Ishii M, Kaikita K, Sakamoto K, et al. Characteristics and in-hospital mortality of patients with myocardial infarction in the absence of obstructive coronary artery disease in super-aging society. Int J Cardiol. 301:108-113 (2020).
[CrossRef] [Google Scholar] [PubMed]
- Larsen AI, Nilsen DW, Yu J, et al. Long-term prognosis of patients presenting with ST-segment elevation myocardial infarction with no significant coronary artery disease (from the HORIZONS-AMI trial). Am J Cardiol. 111(5):643-648 (2013).
[CrossRef] [Google Scholar] [PubMed]
- Safdar B, Spatz ES, Dreyer RP, et al. Presentation, clinical profile, and prognosis of young patients with Myocardial Infarction with Nonobstructive Coronary Arteries (MINOCA): Results from the VIRGO study. J Am Heart Assoc. 7(13):e009174 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Pacheco CC, Quesada O, Pepine CJ, et al. Why names matter for women: MINOCA/INOCA (myocardial infarction/ischemia and no obstructive coronary artery disease). Clin Cardiol. 41(2):185-193 (2018).
[CrossRef] [Google Scholar] [PubMed]
- Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry-GWTG (Acute coronary treatment and intervention outcomes network registry-get with the guidelines). Circ Cardiovasc Qual Outcomes. 10(12):e003443 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018). Circulation.138(20):e618-e651 (2018).
- Scalone G, Niccoli G, Crea F, et al. Editor’s choice-pathophysiology, diagnosis and management of MINOCA: An update. Eur Heart J Acute Cardiovasc Care. 8(1):54-62 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: A scientific statement from the American Heart Association. Circulation. 139(18):e891-e908 (2019).
[CrossRef] [Google Scholar] [PubMed]
- Alabas OA, Gale CP, Hall M, et al. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: National cohort study using the SWEDEHEART registry. J Am Heart Assoc. 6(12):e007123 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Wallert J, Held C, Madison G, et al. Temporal changes in myocardial infarction incidence rates are associated with periods of perceived psychosocial stress: A SWEDEHEART national registry study. Am Heart J. 191:12-20 (2017).
[CrossRef] [Google Scholar] [PubMed]
- Mooney J. Randomized evaluation of beta blocker and ACE-inhibitor/angiotensin receptor blocker treatment for post infarct angina in patients with myocardial infarction with non-obstructive coronary arteries: A MINOCA-BAT sub study rationale and design. Front Cardiovasc Med. 8:8:717526 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Montone RA, Cosentino N, Graziani F, et al. Precision medicine versus standard of care for patients with Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA): Rationale and design of the multicentre, randomised PROMISE trial. EuroIntervention.18(11):e933 (2022).
[CrossRef] [Google Scholar] [PubMed]
- Collet JP, Thiele H, Barbato E et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Rev Esp Cardiol (Engl Ed).42(14):1289-1367 (2021).
[CrossRef] [Google Scholar] [PubMed]
- Samaras A, Moysidis DV, Papazoglou AS, et al. Diagnostic puzzles and cause-targeted treatment strategies in myocardial infarction with non-obstructive coronary arteries: An updated review. J Clin Med. 12(19):6198 (2023).
[CrossRef] [Google Scholar] [PubMed]