Case Report - Interventional Cardiology (2025) Volume 17, Issue 2
A Cardiac Thrombus: The Risk Of Anticoagulant Noncompliance In Antiphospholipid Syndrome
- Corresponding Author:
- Noor Abu Hantash
School of Medicine, University of Jordan, Amman, Jordan
E-mail: abuhantashnoor91@gmail.com
Received date: 01-Apr-2025, Manuscript No. FMIC-25-166109; Editor assigned: 03- Apr -2025, PreQC No. FMIC-25-166109 (PQ); Reviewed date: 17- Apr -2025, QC No. FMIC-25-166109; Revised date: 24- Apr -2025, Manuscript No. FMIC-25-166109 (R); Published date: 01-May-2025, DOI: 10.37532/1755-5310.2025.17(2):985
Abstract
Introduction: Primary Antiphospholipid Syndrome (PAPS) is an autoimmune disorder associated with a 40%-50% risk of thrombotic events and a 16% prevalence of intracardiac thrombosis. We report a treatment-refractory case of intracardiac thrombosis in a PAPS patient, the first documented in the Middle East and North Africa (MENA) region. This case highlights the critical role of cardiovascular risk stratification and management strategies in this population. Case Presentation: A 47-year-old woman with a history of PAPS presented with worsening shortness of breath and chest pain over five days. Her medical history was significant for multiple episodes of Pulmonary Embolism (PE) and Deep Vein T hrombosis (DVT). She had discontinued anticoagulation therapy three years earlier *due to socioeconomic challenges during the COVID-19 pandemic. Investigations revealed a chest X-ray demonstrating left upper zone ground-glass opacification and right middle zone collapse, consistent with multiple PEs. Cardiac Magnetic Resonance Imaging (MRI) identified *non-enhancing filling defects in the right atrium, protruding through the tricuspid valve during diastole, accompanied by right ventricular dilation and hypokinesia. Her ejection fraction was 55%, with no evidence of pericardial effusion. The patient was non-responsive to medical therapy and was referred for surgical thrombectomy. Conclusion: While thrombotic complications in PAPS are well-documented, surveillance primarily targets PE, DVT, and obstetric morbidity, often overlooking intracardiac thrombi. This case underscores the need for heightened clinical suspicion and routine cardiovascular assessment in PAPS patients, particularly those with a history of anticoagulation Non-adherence. Incorporating cardiac imaging into thrombotic risk evaluation may facilitate early detection and improve outcomes in this high-risk population.
Keywords
Intracardiac thrombosis • Autoimmune disorder • COVID-19 • Pandemic • Embolism
Introduction
Primary Antiphospholipid Syndrome (PAPS) is an autoimmune disorder characterized by thrombotic events and persistent antiphospholipid antibodies (aPL), including lupus anticoagulant, anticardiolipin antibodies, and anti-ß2-glycoprotein I antibodies [1]. The syndrome carries a 40%-50% lifetime risk of venous or arterial thrombosis, with intracardiac thrombosis occurring in approximately 16% of cases [2]. While Pulmonary Embolism (PE) and Deep Vein Thrombosis (DVT) are well-recognized complications, intracardiac thrombi remain underdiagnosed due to limited routine cardiac imaging in Antiphospholipid Syndrome (APS) management protocols [3].
Anticoagulation remains the cornerstone of therapy for thrombotic APS, with lifelong warfarin recommended for secondary prevention [4]. However, nonadherence to anticoagulation, driven by socioeconomic barriers, bleeding concerns, or inadequate follow-up, significantly elevates the risk of recurrent thrombosis [5]. The COVID-19 pandemic exacerbated healthcare disparities, particularly in low- and middle-income regions, where access to medications and monitoring became further constrained [6]. This report presents the first documented case of intracardiac thrombosis in a PAPS patient from the Middle East and North Africa (MENA) region, emphasizing the consequences of anticoagulant noncompliance and gaps in cardiovascular risk stratification.
Case Presentation
A 47-year-old woman with a confirmed diagnosis of PAPS (per the 2006 Sydney criteria: Recurrent DVTs, positive lupus anticoagulant, and anticardiolipin IgG) presented to the emergency department with progressive dyspnea and chest pain over five days [7]. Her medical history included three episodes of PE and two DVTs over the past decade, managed initially with warfarin (target INR 2-3). However, she discontinued anticoagulation three years prior due to financial constraints during the COVID-19 pandemic, citing inability to afford regular INR testing or medication refills.
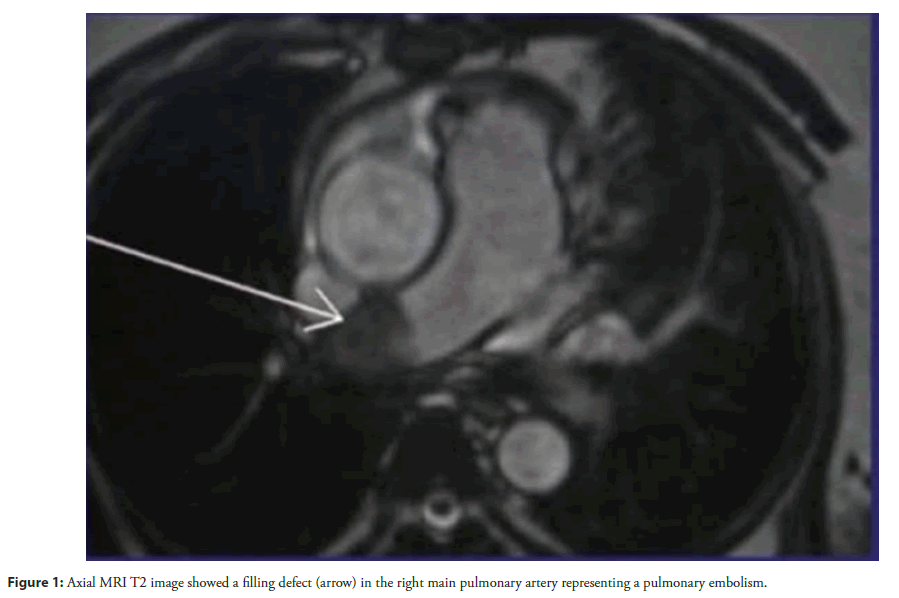
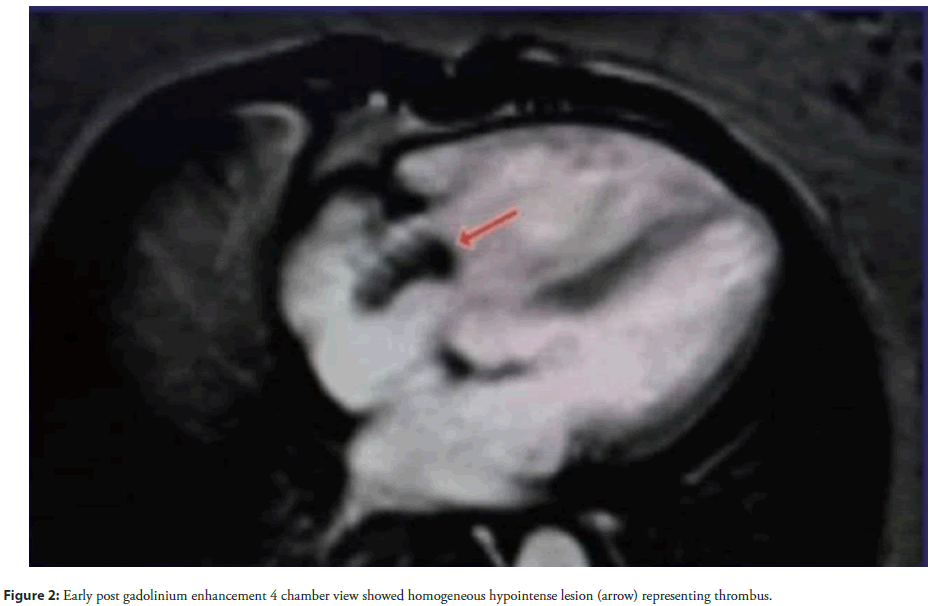
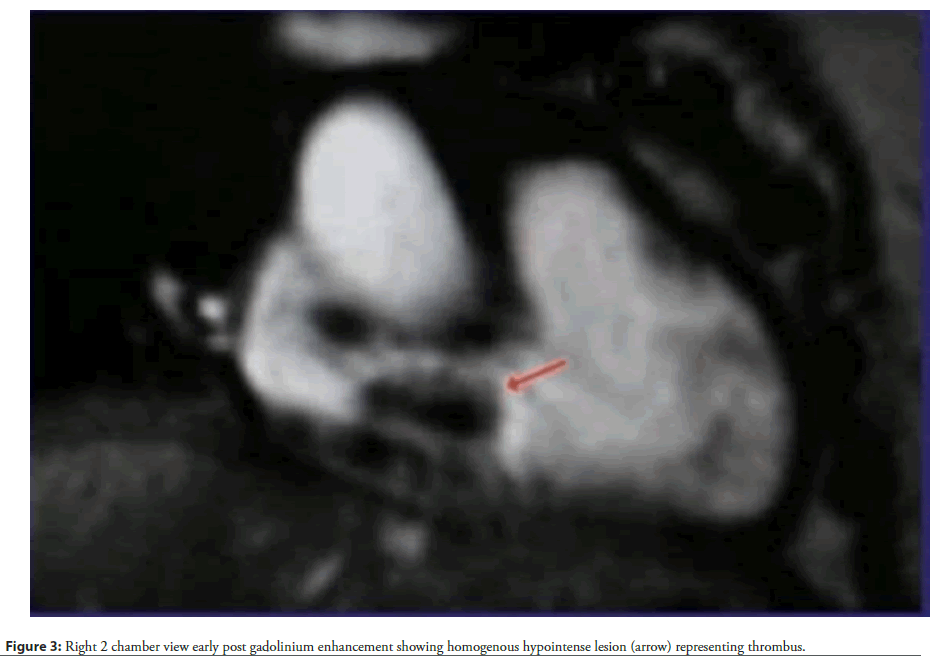
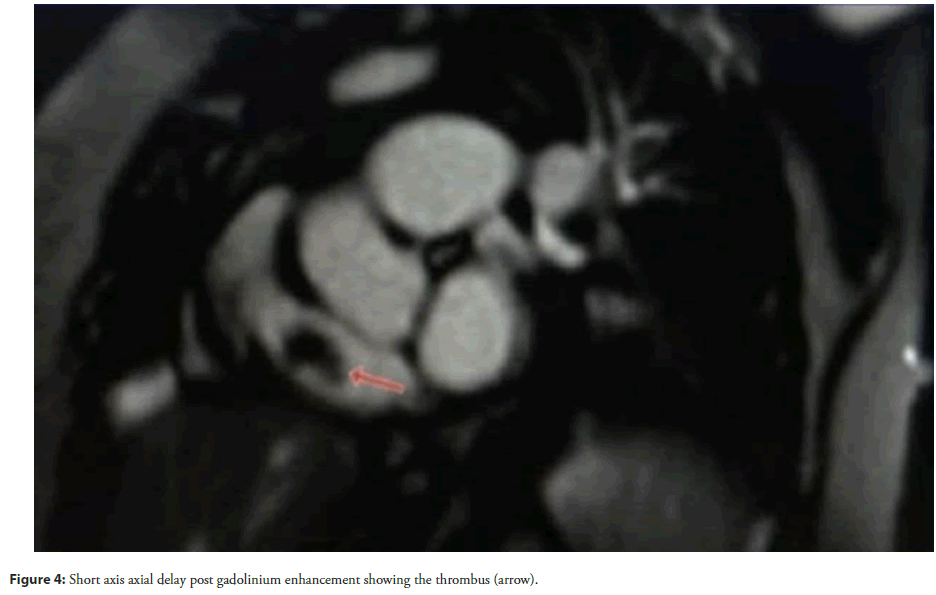
Physical examination revealed tachycardia (110 bpm), hypotension (90/60 mmHg), and hypoxia (SpO2 88% on room air). Cardiovascular findings included jugular venous distension, a right parasternal heave, and a holosystolic murmur at the left lower sternal border, suggestive of tricuspid regurgitation. Laboratory studies demonstrated elevated D-dimer (2.5 µg/mL) and persistently positive lupus anticoagulant and anticardiolipin IgG (65 GPL). Chest X-ray revealed left upper lobe ground-glass opacities and right middle lobe collapse, consistent with recurrent PE. Cardiac Magnetic Resonance Imaging (MRI) identified a large (2.5 × 3.8 cm), non-enhancing mass in the right atrium, prolapsing through the tricuspid valve during diastole (Figures 1-4).
Accompanying findings included right ventricular dilation (end-diastolic volume 150 mL/m²), hypokinesia, and preserved left ventricular ejection fraction (55%). Transthoracic echocardiography corroborated these findings, revealing a mobile thrombus and moderate tricuspid regurgitation. Despite immediate anticoagulation with intravenous heparin (target aPTT 60-80 seconds), the thrombus persisted, necessitating surgical thrombectomy. Intraoperative findings confirmed a friable, organized thrombus adherent to the right atrial wall.
Postoperatively, the patient resumed warfarin with bridging enoxaparin, achieving a therapeutic INR (2.5) within one week. At six-month follow-up, repeat cardiac MRI demonstrated no thrombus recurrence, and her functional status improved to NYHA Class I.
Discussion
This case underscores the life-threatening consequences of anticoagulant noncompliance in PAPS, particularly in resource-limited settings. Intracardiac thrombi, though rare, are associated with a mortality rate of up to 30% due to embolic complications or obstructive shock [8]. The right atrial location observed here is atypical, as most APS-associated intracardiac thrombi occur in the left ventricle or atrium [9]. This deviation may reflect prolonged stasis secondary to recurrent PE-induced right ventricular dysfunction, exacerbated by hypercoagulability from untreated APS [10].
Anticoagulant nonadherence remains a critical challenge in APS management. Studies indicate that up to 40% of APS patients discontinue anticoagulation within five years, often due to financial barriers or inadequate education on thrombotic risks [11]. The COVID-19 pandemic amplified these disparities, with global reports of disrupted anticoagulant access and reduced clinic follow-ups [12]. In the MENA region, where this case originated, fragmented healthcare systems and out-of-pocket payment models further exacerbate nonadherence [13].
Current APS guidelines prioritize obstetric and peripheral thrombotic surveillance, with limited emphasis on cardiac evaluation [14]. However, emerging evidence suggests that silent intracardiac thrombi may precede catastrophic events, warranting routine echocardiography in high-risk patients [15]. Cardiac MRI, as utilized here, offers superior sensitivity for detecting thrombi and assessing ventricular function, though its cost limits accessibility in low-resource settings [16].
Surgical intervention, while lifesaving in refractory cases, carries inherent risks, including perioperative bleeding and thrombus fragmentation. Multidisciplinary care incorporating hematology, cardiology, and social work is essential to address both medical and socioeconomic barriers to adherence.
Conclusion
This case highlights intracardiac thrombosis as a rare but devastating complication of APS, particularly in anticoagulant-noncompliant patients. It underscores the need for enhanced cardiovascular risk stratification, including routine cardiac imaging, and public health initiatives to improve medication access in underserved regions. Future research should explore cost-effective surveillance strategies and patient-centered interventions to mitigate nonadherence in APS populations.
References
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite Antiphospholipid Syndrome (APS). J Thromb Haemost. 4(2):295-306 (2006).
- Cervera R, Piette JC, Font J, et al. Antiphospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 46(4):1019-1027 (2002).
- Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med. 378(21):2010-2021 (2018).
- Ruiz-Irastorza G, Cuadrado MJ, Ruiz-Arruza I, et al. Evidence-based recommendations for the prevention and long-term management of thrombosis in antiphospholipid antibody-positive patients: Report of a task force at the 13th International Congress on antiphospholipid antibodies. Lupus. 20(2):206-218 (2011).
- Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis. 78(10):1296-1304 (2019).
- Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 26(7):1017-1032 (2020).
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. J Thromb Haemost. 7(10):1737-1740 (2009).
- Ata AA, Hantash NA, Al-Abed Z, et al. A cardiac thrombus: The risk of anticoagulant noncompliance in antiphospholipid syndrome.
- Roldan CA, Gelgand EA, Qualls CR, et al. Valvular heart disease as a cause of cerebrovascular disease in patients with systemic lupus erythematosus. Am J Cardiol. 95(12):1441-1447 (2005).
- Zuily S, Regnault V, Selton-Suty C, et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: Meta-analysis of echocardiographic studies. Circulation. 124(2):215-224 (2011).
- Dar S, Koirala S, Khan A, et al. A comprehensive literature review on managing systemic lupus erythematosus: Addressing cardiovascular disease risk in females and its autoimmune disease associations. Cureus. 15(8) (2023).
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 75(23):2950-2973 (2020).
- Al-Hussein FA, Alshammari SA, Alharbi AM, et al. Healthcare challenges in the Middle East: Access to anticoagulation in Saudi Arabia. Saudi Med J. 42(5):553-559 (2021).
- Tektonidou MG. Cardiovascular disease risk in antiphospholipid syndrome: Thrombo-inflammation and atherothrombosis. J Autoimmun. 128:102813 (2022).
- Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis. Revista Española de Cardiología (English Edition). 69(1) (2016).
- Kramer CM, Barkhausen J, Bucciarelli-Ducci C, et al. Standardized Cardiovascular Magnetic Resonance Imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 22(1):17 (2020).