Research Article - Clinical Investigation (2017) Volume 7, Issue 3
A randomized, double-blind, controlled study to evaluate clinical efficacy and safety of novel filmogen osmotic treatment for pharyngitis
- Corresponding Author:
- Monika Rousse
R&D Department, Vitro-Bio Research Institute, Issoire, France
E-mail: monika.rousse@vitrobio.com
Submitted: 27 June 2017; Accepted: 12 July 2017; Published online: 18 July 2017
Abstract
Background: Pharyngitis is caused principally by primary viral infection: virus growth causes lysis of throat mucosa cells, leading to inflammation, secondary bacterial infection, accumulation of contaminants on the throat, and clinical signs of sore throat infection. Currently available drugs only provide partial therapeutic action or relief. Since pharyngitis is a multifactorial condition, a multi target treatment should be more effective. We tested a novel hypertonic, highly osmotic, filmogen liquid bandage which can mechanically clean the throat surface to quickly alleviate the symptoms while also eliminating their causing pathogens. A clinical trial was conducted to evaluate its efficacy and safety. Methods and findings: A 14-day, randomized, placebo controlled, double blind, efficacy and safety study was conducted in 36 treated with Test Product (TP) v/s 18 treated with Comparator Product (CP, containing saline solution) patients suffering from sore throat infection. Products were applied as spray, every 20 to 30 min. during the first 2-3 hours then 3-4 times daily, for a maximum 14 days, and primary and secondary sore throat infection-related parameters were evaluated on days 1, 2, 3, 4, 7, and 14. Saline solution showed some beneficial effects on sore throat, but the TP proved significantly more effective, producing not only very rapid but also durable effects on all clinical signs of pharyngitis. The statistically significant effectiveness and rapidity of results obtained with the test product, led to faster recovery and reduced need for antibiotics compared to the CP group. No treatmentrelated undesirable or adverse effect was observed. Conclusions: This novel hypertonic, highly osmotic, filmogen liquid bandage is a safe and effective treatment for pharyngitis.
Keywords
Pharyngitis,Filmogen,Hyperosmotic,Antimicrobial and Broad spectrum
Protocol N°: NEX/HER-PAI/OBS/2016
Study Report N°: Clinical study NEX/HER-PAI/OBS/2016 report Version_1.0
Study title: “A comparative, randomized, double-blind, parallel group, observational clinical trial study to evaluate efficacy and safety of throat spray containing VB-Gy (filmogen glycerol) and Septicyanidin premix versus Saline spray as Comparator in the treatment of patients with sore throat.”
Introduction
Sore throat or pharyngitis is very common, largely seasonal conditions that are primarily caused by a viral infection of the upper respiratory tract (URT) [1-3]. While rhinoviruses, responsible for the common cough and cold, do not afflict significant damage to the respiratory mucosa, Influenza A infection can be much more serious [4]. As the virus weakens local defenses, secondary bacterial proliferation and surinfection often ensue [5]. Following initial infection, the virus enters the host cells for multiplication, but most virulent virus progeny is shed onto the throat surface [6-8]. As the virions again infect new healthy cells on the throat surface, cellular lysis ends up causing local inflammation and damage to the outer lining of the pharynx, which then presents a favorable ground for bacterial proliferation [9,10]. This secondary bacterial infection is the actual cause of most sore throat or strep throat infection symptoms [11,12].
Numerous drugs as well as traditional remedies are offered to the patient suffering from pharyngitis, but all deliver little more than symptomatic relief [13-16]. Antibiotics are prescribed to limit secondary bacterial infections [17,18], and last resort measures for severe infections include intracellular virus inhibitors, but those are not very effective to regularly treat throat infections.
A fast and effective treatment should therefore act on different pathogens at the same time and take into account an intricacy of factors, since viruses are complex and highly prone to mutation, act more often in symbiosis than in competition with bacteria, and their infectivity is aided by locally present proteases. To stop the pathogenic attacks on the URT mucosa epithelium cells, and therefore to curb the infection early in its tracks, a topical treatment, applied on the surface of the throat, would also seem a more strategically meaningful option.
A new throat spray, containing a film-forming, osmotically active solution (F-VB-Gy, patented) for topical application, was recently designed, implementing a novel therapeutic approach consisting in cleaning the throat surface of any contaminants nondiscriminately (e.g. viruses, bacteria, dead cells, cell debris, impurities, foreign particles, pus) that are either directly causing the infection or contributing to the local damage and impeding local defenses and cellular regeneration. When sprayed on the throat surface, the solution forms an osmotically active film over the pharyngeal mucosa, whose action is to physically attract hypotonic fluid from the deeper parts of the throat, the exudation resulting in mechanical dislodging, drainage and removal of all contaminants present on the infected throat surface, including bacteria, free virus particles, dust particles, dead cells and cell debris. The product thus acts as a mechanical antiseptic and cleaning solution. The mechanical microbial removal would stop viruses topically present from infecting new mucosal cells and eliminate opportunistic bacteria. Due to its non-specific mechanical cleaning properties, this product can act as a multifactorial, non-chemical and cell-friendly device for the treatment of topical infections. The protective and hypertonic film also allows to moisturize the throat mucosa, thus alleviating pain and irritation, as well as stopping the inflammatory cascade, thus providing nearly instant symptomatic relief.
For durable film retention, thus longer-lasting activity, the solution’s filmogenic (i.e. film-forming and film retention) capacity was enhanced through the addition of selected plant-derived polymeric substances (e.g. Septicyanidins), and its sensory profile was ameliorated with aromatic essential oils which, being hydrophobic may thus also further help reduce dilution of the filmogen test product.
A randomized, double blind clinical trial was conducted using 0.9% NaCl as a control comparator, to verify the efficacy and safety of this new class of medical device.
Methods
The clinical part of this research was conducted at Nexus Clinical Research Center in India, affiliated to Nexus Clinical Research LLC, USA. The protocol and the study design were approved by the Institutional Ethical committee of India – Rajiv Gandhi Institute of medical sciences (EC Registration N° ECR/492/ Inst/2013 of 05-12-2013) and the trial was performed following the ICH-GCP guidelines as per the declaration of Helsinki to conduct ethical research on human subjects.
Study design and rationales
The study was designed as a multi-centric, randomized, comparator controlled, double-blind, clinical trial. The aim of the study was to compare the efficacy and safety of F-VB-Gy hyperosmotic filmogen spray (TP) versus isotonic sodium chloride solution as comparator product (CP), in patients suffering from pharyngitis. Isotonic sodium chloride solution was chosen as comparator product as this is as close to a placebo throat spray as possible, but it is seen as a comparator product as it is expected to have beneficial effects on its own. The TP was assessed for risk analyses and essential requirement conformity as per EU directive 93/43/EEC. Based on a previous pilot study [19] and assuming a power of 80%, the aim was to recruit at least 63 patients, randomized in a ratio of 1:2 (21 patients in Comparator group, 42 patients in Test group) so as to obtain final results (taking into account eventual dropouts or withdrawals) for at least 54 completed data sets for significant statistical analyses: 18 patients in the CP and 36 in the TP group completed the study. The doses were also selected based on the previous pilot dose range finding observational study in patients suffering from sore throat infection, where CP or TP were administered as topical applications of 4-5 sprays over the throat surface, every 30 minutes in the beginning of the treatment during the first 2-3 h, and 3-4 times per day afterwards. Products were applied for a maximum period of 14 days or up to complete healing. The key parameters were throat pain intensity and severity, recovery time, need for antibiotics as per physician’s decision, and total time to recovery.
Inclusion and exclusion criteria
At the time of recruitment in different study centers, patients were examined physically and patient’s medical, surgical, and allergic history was checked. Vital signs (e.g. blood biochemical profile for liver and kidney parameters, blood pressure, pulse rate, and respiratory rate) were recorded. Patients not suffering from any serious pathology were then examined for study inclusion and exclusion criteria. The main inclusion criteria included: Participants having clinical signs and symptoms of recent (less than 72 h) sore throat pharyngitis such as swollen tender anterior cervical lymph nodes, temperature >100.4°F (38°C), absence of cough, exudates or swelling of tonsils with >50% probability of positive culture for Streptococcus infection that is Cumulative symptom score of ≥4 at screening according to the Centor criteria. Patients with pain/sore throat score ≥6 on the Throat Pain Scale at baseline, male and female in the 18-65 years’ age group, no history of adverse effect or allergies to any ingredient used in the product composition, not under any antibacterial or antiviral treatment before recruitment and ready to give written consent for study participation and willing to follow the protocol as recommended.
The main exclusion criteria included presence of any serious respiratory disease (especially of the Lower Respiratory Tract), being under some medications, having a known allergy to Test Product (TP) components, and lack of willingness to participate in the study.
Randomization
After screening, patients satisfying all the inclusion criteria were enrolled and randomly allocated in 2:1 ratio as per randomization schedule to receive TP or CP (0.9% NaCl solution as comparator product). Treatments were allocated to patients by carrying out randomization using SAS Version 9.1.3 following a randomization schedule. Block Randomization methodology was employed for generating the list (within the block, treatments were distributed in the ratio of 2:1, as stated above).
Product presentation and administration
TP and CPs were supplied by Vitrobio, France (Issoire) and were presented identically (30 ml aluminum spray containing slightly viscous transparent liquid) except for the product code and the batch number. The TP contained an aqueous F-VB-Gy solution containing a specific association of Septicyanidins while CP contained 0.9% NaCl.
Rationale for accepting concomitant antibiotherapy
The use of antibiotics to treat any bacterial infection and prevent complication of viral infections, is very common in Europe but also in developing countries like India, creating a strong concern over antibiotic resistance, it was highly important to evaluate the effects of a bacterial-resistant free medical device on the need for antibiotics to treat relatively benign infections.
Parameters studied
The primary endpoints were. Change in score of Throat Pain Intensity, Difficulty Swallowing, and Swollen Throat, after 5 minutes and 2 h after 1st application on Day 1, and on Day 2, Day 3, Day 4, Day 7, and Day 14-15 or up to complete recovery (whichever was earlier) from baseline. Proportion of patients requiring antibiotics and the presence or absence of bacteria over the throat surface in Test compared to Comparator group (Time to complete recovery).
Change in score of local throat redness, throat irritation, fever, bacterial whitish deposits on throat from baseline were evaluated as secondary endpoints.
Changes were evaluated on a rating scale of 0 to 10 (0 being absence of symptoms) just before 1st treatment, after 5min and 2h and on Days 2, 3, 4, 7 and 14 by the investigator (Days 1 and 14) or by the patients.
Screening values were considered as baseline values and for ethical reasons; treatment was started just after patient’s enrollment in the study (Day 1). Each patient received a unique screening identification number, randomization code, and enrollment identification number.
Safety assessment: At the end of the study, subjects and investigators assessed any treatment-emergent adverse effect observed, and rated the tolerability and acceptability of TP and CP.
Statistics
The analyses were conducted with Microsoft Excel and XLStat using the available data.
Significant effects were those with a probability lower than α=0.05. For each score, repeated measures analysis of variance (RMANOVA) determined differences in symptoms scores across study visits. Nemenyi posthoc test provided pairwise comparisons in a group between baseline and the end of the study. Results were compared with baseline values (scores obtained just before 1st treatment on Day 1) in the same group and with the CP, at each time point.
Results
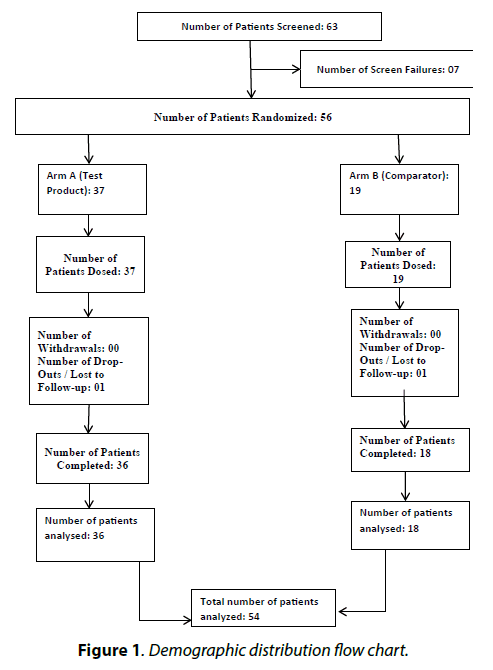
Demographic distribution flow chart shows that the population enrolled was corresponding to the study protocol (Figure 1).
Among 63 patients enrolled in the study, 36 in the TP group and 18 in the CP group completed the study. No patient was withdrawn due to any undesired effects but 1 patient in CP and 1 in TP were lost to follow up during the study. The demography of patients included in the study was considered fairly homogenous between the groups.
At the time of their recruitment, all enrolled subjects were diagnosed with moderate to severe sore throat, and were positive for throat swab bacterial cultures with Streptococcus as main causative organism. Only those patients suffering from sore throat for less than 72 h, with related symptoms including throat pain, swollen throat, difficulty swallowing, throat redness, throat irritation, whitish deposits on the throat surface, and possibly fever, were included in the study (Table 1). Patients diagnosed with diseases of the lower respiratory tract: Inflammations of the larynx, trachea, bronchi, pneumonia, asthma, sinusitis, allergic rhinitis, as well as heart disease, were not enrolled in the study. Participants were asked to refrain from smoking during the treatment period but this parameter was difficult to monitor. The baseline mean symptom scores were fairly homogenous between the groups, with only minor, non-significant (p>0.05, close to 1) differences, as shown in the figures.
| Group | Time-points | Day 1 - T0 Baseline (Visit 1) | Day 1 + 5mn (Visit 1) |
Day 1 + 2h (Visit 1) |
Day 2 (Visit 2) |
Day 3 (Visit 3) |
Day 4 (Visit 4) |
Day 7 (Visit 5) |
Day 14 (Visit 6) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| THROAT PAIN INTENSITY | CP | Means | 7,389 | 7,278 | 7,000 | 6,444 | 5,889 | 5,667 | 5,278 | 3,333 |
| SD ± | 1,092 | 0,958 | 1,188 | 1,097 | 1,231 | 1,029 | 1,018 | 2,301 | ||
| % Change vs T0 | - | -1,50% | -5,26% | -12,78% | -20,30% | -23,31% | -28,57% | -54,89% | ||
| p value | - | 1.000 | 0.980 | 0.142 | 0.001 | 0.00016 | < 0.0001 | < 0.0001 | ||
| TP | Means | 7,361 | 6,667 | 5,972 | 5,111 | 4,111 | 2,889 | 1,861 | 0,222 | |
| SD ± | 1,150 | 1,042 | 0,878 | 1,008 | 1,237 | 1,036 | 1,334 | 0,485 | ||
| % Change vs T0 | - | -9,43% | -18,87% | -30,57% | -44,15% | -60,75% | -74,72% | -96,98% | ||
| p value | - | 0.811 | 0.050 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| % SeverityDifference TP vs CP | -0,38% | -8,40% | -14,68% | -20,69% | -30,19% | -49,02% | -64,74% | -93,33% | ||
| p value | 0.933 | 0.042 | 0.001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| DIFFICULTY SWALLOWING | CP | Means | 6,944 | 6,944 | 6,611 | 6,222 | 5,722 | 5,389 | 4,889 | 2,500 |
| SD ± | 0,998 | 0,998 | 1,290 | 1,309 | 1,638 | 1,501 | 1,491 | 2,455 | ||
| % Change vs T0 | - | 0,00% | -4,80% | -10,40% | -17,60% | -22,40% | -29,60% | -64,00% | ||
| p value | - | 1.000 | 0.980 | 0.479 | 0.018 | 0.001 | < 0.0001 | < 0.0001 | ||
| TP | Means | 6,972 | 6,722 | 5,917 | 4,722 | 3,611 | 2,417 | 1,333 | 0,167 | |
| SD ± | 1,028 | 1,031 | 0,937 | 0,914 | 0,903 | 0,770 | 1,014 | 0,447 | ||
| % Change vs T0 | -3,59% | -15,14% | -32,27% | -48,21% | -65,34% | -80,88% | -97,61% | |||
| p value | - | 1.000 | 0.392 | 0.001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| % SeverityDifference TP vs CP | +0,40% | -3,20% | -10,50% | -24,11% | -36,89% | -55,15% | -72,73% | -93,33% | ||
| p value | 0.925 | 0.454 | 0.028 | <0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| SWOLLEN THROAT | CP | Means | 6,278 | 6,278 | 6,278 | 5,889 | 5,611 | 5,333 | 5,056 | 2,722 |
| SD ± | 1,074 | 1,074 | 1,074 | 1,367 | 1,501 | 1,534 | 1,862 | 2,675 | ||
| % Change vs T0 | - | 0,00% | 0,00% | -6,19% | -10,62% | -15,04% | -19,47% | -56,64% | ||
| p value | - | 1.000 | 1.000 | 0.888 | 0.479 | 0.066 | 0.011 | < 0.0001 | ||
| TP | Means | 6,250 | 6,250 | 5,917 | 4,778 | 3,639 | 2,444 | 1,500 | 0,222 | |
| SD ± | 0,937 | 0,937 | 0,996 | 1,017 | 0,931 | 0,939 | 0,775 | 0,422 | ||
| % Change vs T0 | - | 0,00% | -5,33% | -23,56% | -41,78% | -60,89% | -76,00% | -96,44% | ||
| p value | - | 1.000 | 0.989 | 0.005 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| % SeverityDifference TP vs CP | -0,44% | -0,44% | -5,75% | -18,87% | -35,15% | -54,17% | -70,33% | -91,84% | ||
| p value | 0.922 | 0.922 | 0.227 | 0.001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| THROAT IRRITATION | CP | Means | 6,222 | 6,167 | 5,944 | 5,222 | 4,778 | 4,611 | 4,278 | 2,889 |
| SD ± | 1,060 | 1,150 | 1,259 | 1,437 | 1,478 | 1,335 | 1,565 | 2,055 | ||
| % Change vs T0 | - | -0,89% | -4,46% | -16,07% | -23,21% | -25,89% | -31,25% | -53,57% | ||
| p value | - | 1.000 | 0.997 | 0.121 | 0.006 | 0.00045 | < 0.0001 | < 0.0001 | ||
| TP | Means | 6,222 | 5,694 | 5,306 | 4,389 | 3,306 | 2,083 | 0,917 | 0,167 | |
| SD ± | 1,149 | 1,009 | 1,064 | 1,128 | 1,117 | 1,105 | 1,131 | 0,737 | ||
| % Change vs T0 | - | -8,48% | -14,73% | -29,46% | -46,88% | -66,52% | -85,27% | -97,32% | ||
| p value | - | 0.889 | 0.320 | 0.0004 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| % SeverityDifference TP vs CP | 0,00% | -7,66% | -10,75% | -15,96% | -30,81% | -54,82% | -78,57% | -94,23% | ||
| p value | 1.000 | 0.128 | 0.056 | 0.024 | 0.00015 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| THROAT REDNESS | CP | Means | 5,333 | 5,333 | 5,278 | 5,000 | 4,833 | 4,556 | 4,444 | 2,444 |
| SD ± | 1,029 | 1,029 | 1,127 | 1,138 | 1,249 | 1,199 | 1,247 | 2,255 | ||
| % Change vs T0 | 0,00% | -1,04% | -6,25% | -9,38% | -14,58% | -16,67% | -54,17% | |||
| p value | - | 1.000 | 1.000 | 0.934 | 0.502 | 0.072 | 0.060 | < 0.0001 | ||
| TP | Means | 5,361 | 5,361 | 5,000 | 3,917 | 2,861 | 1,833 | 0,861 | 0,222 | |
| SD ± | 0,867 | 0,867 | 0,894 | 1,052 | 1,268 | 1,384 | 1,046 | 0,637 | ||
| % Change vs T0 | 0,00% | -6,74% | -26,94% | -46,63% | -65,80% | -83,94% | -95,85% | |||
| p value | - | 1.000 | 0.955 | 0.010 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| % SeverityDifference TP vs CP | +0,52% | +0,52% | -5,26% | -21,67% | -40,80% | -59,76% | -80,63% | -90,91% | ||
| p value | 0.917 | 0.917 | 0.329 | 0.001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||
| BACTERIAL WHITISH DEPOSITS | CP | Means | 4,611 | 4,611 | 4,611 | 4,167 | 3,944 | 3,222 | 3,056 | 1,444 |
| SD ± | 1,720 | 1,720 | 1,852 | 1,886 | 1,765 | 1,629 | 1,662 | 2,255 | ||
| % Change vs T0 | - | 0,00% | 0,00% | -9,64% | -14,46% | -30,12% | -33,73% | -68,67% | ||
| p value | - | 1.000 | 1.000 | 0.951 | 0.619 | 0.008 | 0.006 | < 0.0001 | ||
| TP | Means | 4,639 | 4,639 | 4,111 | 3,111 | 2,167 | 1,194 | 0,306 | 0,111 | |
| SD ± | 1,515 | 1,515 | 1,450 | 1,450 | 1,108 | 0,920 | 0,822 | 0,523 | ||
| % Change vs T0 | - | 0,00% | -11,38% | -32,93% | -53,29% | -74,25% | -93,41% | -97,60% | ||
| p value | - | 1.000 | 0.859 | 0.006 | < 0.0001 | < 0.0001 | < 0.0001 | 0.001 | ||
| % SeverityDifference TP vs CP | +0,60% | +0,60% | -10,84% | -25,33% | -45,07% | -62,93% | -90,00% | -92,31% | ||
| p value | 0.952 | 0.952 | 0.282 | 0.027 | < 0.0001 | < 0.0001 | < 0.0001 | 0.001 | ||
Table 1: Sore Throat Symptom Evolution: Mean Values and Comparisons at each time-point in both Groups (Comparator and Test)
Effect on throat pain
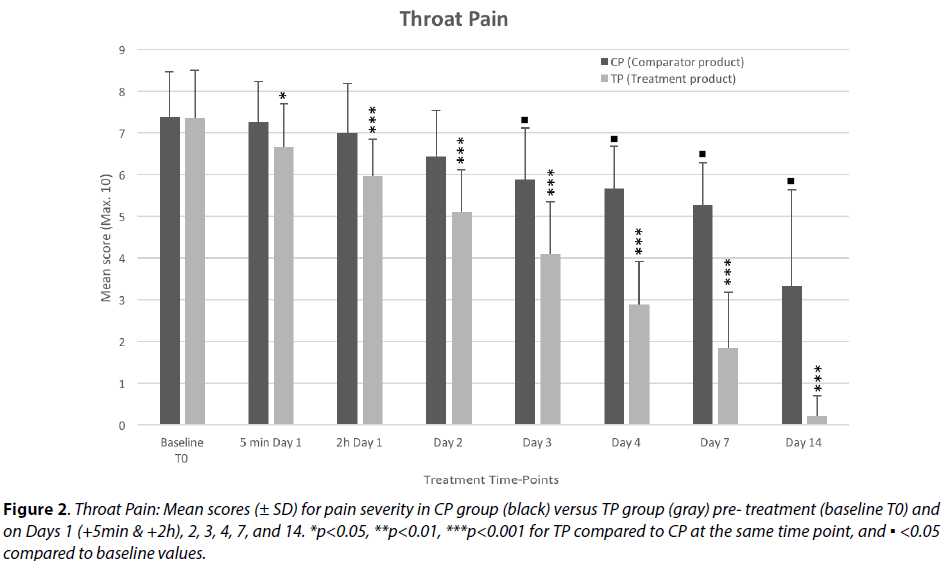
Five minutes after the 1st treatment application, the throat pain intensity (Figure 2) was nearly not affected in the Comparator Product (CP) group (-1.50%) but was diminished by 9.43% in the Test Product (TP) group.
Although the difference in change at 5 min versus baseline between the 2 groups is only 8.40 %, it is statistically significant (p≤0.05). Taking into consideration the time of observation (only 5 minutes after very first application), a high reduction in pain such as with topical chemical anesthetics cannot be expected with a treatment that does not include any analgesic, nevertheless the effect observed at that timepoint is pronounced and very encouraging.
Two hours after the 1st product application, the TP produced a reduction of 18.87% (p=0.05) in throat pain compared to baseline, showing a 14.68% severity difference compared to CP group, confirming the results observed within 5 minutes of first application, that the TP helps alleviate throat pain with statistically significant difference (p=0.001) compared to CP.
In the CP group, the pain score decreased slightly but progressively up to Day 7 (-28.6%) with a reduction of nearly 55% on Day 14. This shows statistically significant (p<0.0001) but only fairly moderate efficacy of the comparator product, as natural healing also helps reduce pain over time.
In the TP group, throat pain decreased progressively but rapidly from Day 2 (-44 %, p<0.0001) this strong and significant reduction further augmented by days 3 and 4 (-60% and -75 % respectively, p<0.0001). On day 7, the mean pain score was reduced by nearly 75% (p<0.0001) compared to pre-treatment, with a severity mean score 75% lower compared to the CP group (p< 0.0001). Throat pain had nearly totally subsided by Day 14 (-97%) with only a few patients still reporting some residual pain, showing a drastic 93% severity difference with the comparator group (p<0.0001).
Topical application of TP produces nearly instant reduction of pain within just 5 minutes of 1st application with a strong and progressive analgesic effect, achieving durable efficacy from the 1st week of treatment in most patients.
Difficulty swallowing
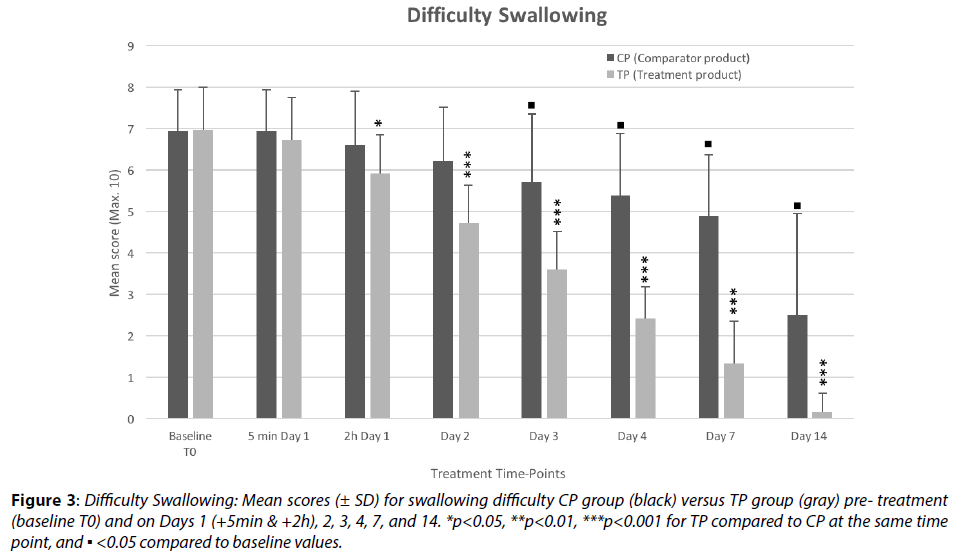
In the comparator group, the mean score of difficulty swallowing (Figure 3) started decreasing progressively and slowly from Day 1 up to Day 14 (-64%), with significant results only from Day 3.
Figure 3: Difficulty Swallowing: Mean scores (± SD) for swallowing difficulty CP group (black) versus TP group (gray) pre- treatment (baseline T0) and on Days 1 (+5min & +2h), 2, 3, 4, 7, and 14. *p<0.05, **p<0.01, ***p<0.001 for TP compared to CP at the same time point, and ▪ <0.05 compared to baseline values.
In the TP treated group, there was no significant effect on this parameter 5 minutes after the 1st treatment but the mean score started decreasing from Day 1 (5.91/10 just 2h after first application) with significant reduction within 24 h: 32% reduction on Day 2, p<0.0001. It took 3 days with TP to obtain the same reduction in severity it took CP 2 weeks to show (-65%), and this remarkable rapidity of results induced by the TP (mean score of 1.33/10 on Day 7 and 0.16/10 on Day 14, indicating 81% and 97% reduction) shows that the TP treatment starts acting significantly within 24h and reduces considerably the swallowing difficulty within 3-4 days. The significant difference of mean scores between the CP and TP groups (-10.50%, p<0.05 at 2h on Day 1; -72.7%, p<0.0001 on Day 7; -93%, p<0.0001 on Day 14) is indicative of the high efficacy of the TP on this parameter as the results were marked, homogenous, significant and sustained through time, and followed a common pattern with the observations for the other parameters evaluated in this study.
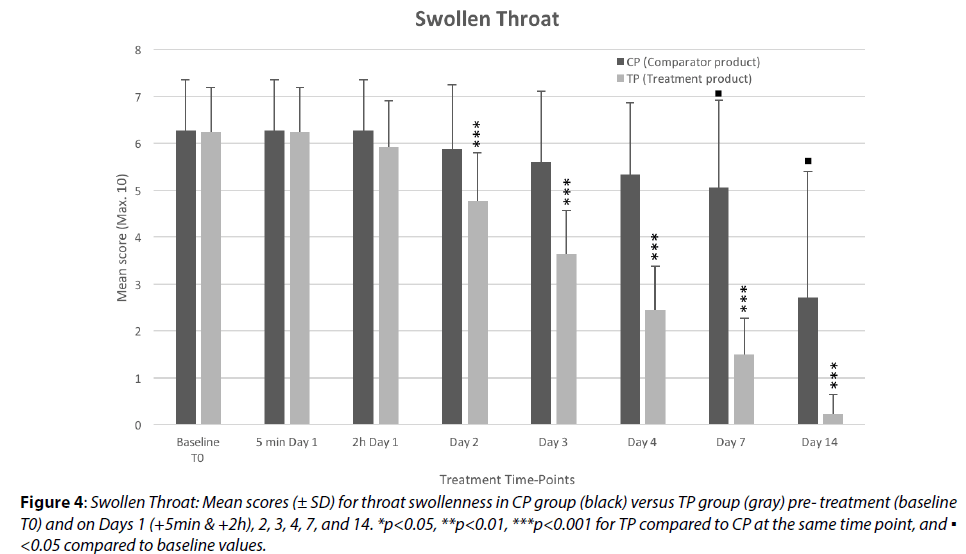
Effect on swollen throat
The mean scores for severity of throat swelling (Figure 4) in the comparator group show no change in this parameter 2h after 1st treatment, but a very slight reduction is reported during the 1st week of treatment. This evolution, however, is not statistically significant (-15%, p=0.66 on Day 4) until Day 7 (-19.5%, p=0.011). The swelling was then reduced by nearly 57% during the second week of treatment, however this is considered to be more probably related to the process of natural healing overtime and especially to the use of antibiotics in some CP group patients.
Figure 4: Swollen Throat: Mean scores (± SD) for throat swollenness in CP group (black) versus TP group (gray) pre- treatment (baseline T0) and on Days 1 (+5min & +2h), 2, 3, 4, 7, and 14. *p<0.05, **p<0.01, ***p<0.001 for TP compared to CP at the same time point, and ▪ <0.05 compared to baseline values.
In the TP treated group, the throat swelling was significantly reduced during the 1st 24 h (-23.6%, p=0.005), but the reduction in swelling was then sharply more marked and faster with a 76% reduction (p<0.0001) on Day 7, with a mean severity score about 70% lower (p<0.0001) compared to the CP group.
Strong effects of the TP on throat swelling were very quickly observed, as early as within the 1st 2-3 days of product application.
These results are coherent with the beneficial effects observed on other sore throat parameters evaluated in this study and the mode of action of the product. TP is purported to attract hypotonic liquid from the inner parts of the throat tissues, thereby reducing throat swelling and hydrating the throat surface at the same time.
Effect on throat irritation/itching
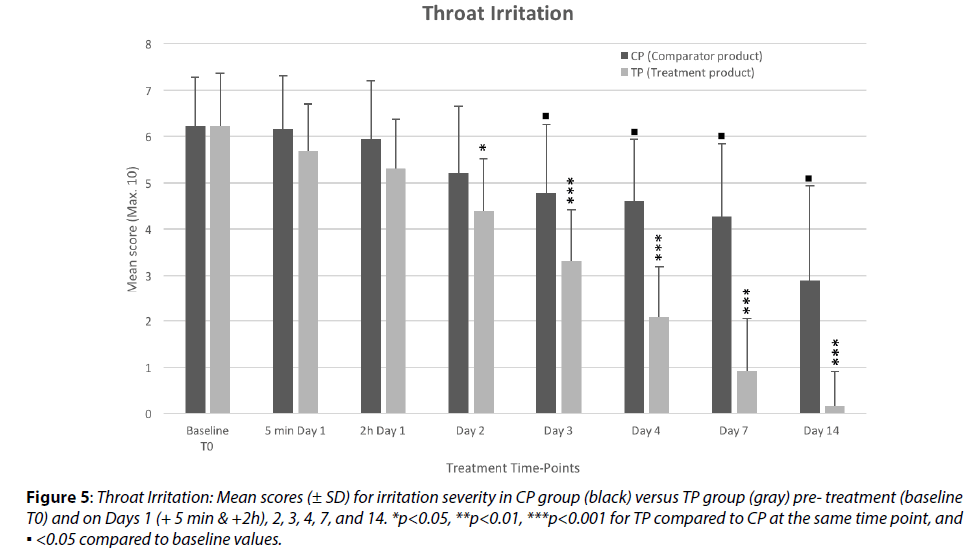
The mean values show that the throat irritation (Figure 5) decreased very progressively in the CP group throughout the study period with about 31% reduction on Day 7 and 53.6% on Day 14 compared to the baseline value (each p<0.0001). In comparison, during the same period, the mean values in the TP group was reduced by 30% compared to baseline much sooner: on Day 2 (p<0.001) and showed further significant reduction of 78% on Day 7 and 94% on Day 14, indicating a very strong soothing effect of the TP on the pharyngitis-induced throat irritation.
Figure 5: Throat Irritation: Mean scores (± SD) for irritation severity in CP group (black) versus TP group (gray) pre- treatment (baseline T0) and on Days 1 (+ 5 min & +2h), 2, 3, 4, 7, and 14. *p<0.05, **p<0.01, ***p<0.001 for TP compared to CP at the same time point, and ▪ <0.05 compared to baseline values.
In the TP group, just 5 minutes and 2h after the 1st product application, the mean irritation severity score was lower by 7.66% and by 10.75% respectively than in the CP group, with identical baseline mean values. Although not statistically significant at this early timepoint, due in large part to the fairly limited number of patients, these results have high importance as marked effects were observed within only 5 minutes following 1st product application and this strong and fast amelioration trend was continuously sustained up to the end of the study and significant in comparison to the CP group from 24 h onwards.
The results of this study highlight the efficacy of the TP on throat irritation, as well as pain, studied above, compared to the CP product. This also shows that the TP application provides nearly instant (within 5 minutes) relief in throat irritation. These rapid and durable soothing, anti-irritant properties of the TP may be imputable to its strong osmotically active and filmogen properties, manifested by the attraction of hypotonic liquid from the inner throat tissue. Hydrating the throat surface and draining the contaminants away mechanically with the liquid flow should help reduce throat irritation as well as pain.
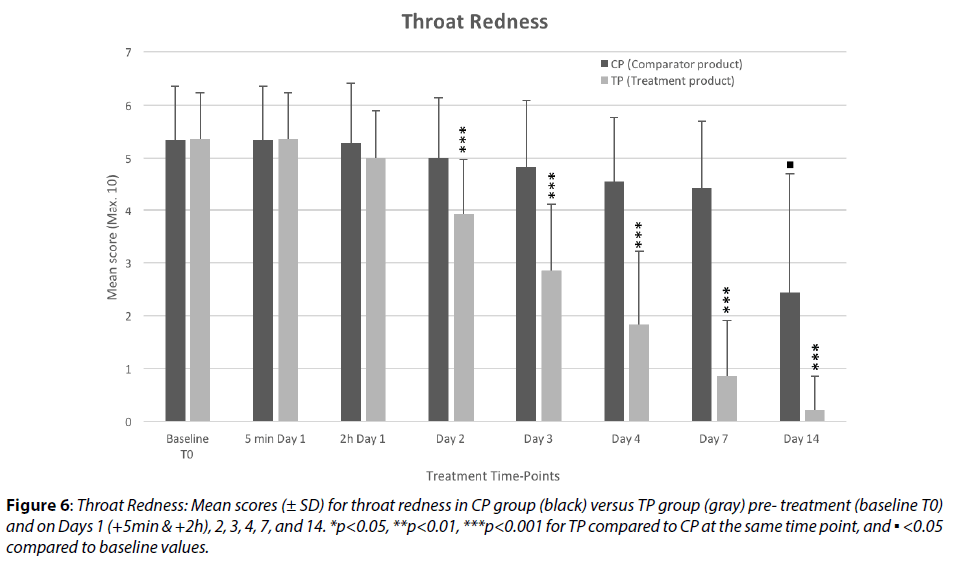
Effect on throat redness
The mean score for throat redness (Figure 6) was not affected in the comparator group during the first 24 h and only a very slight and progressive reduction was observed throughout the study course, with no statistical significant, however, until the Day 14. The mean scores in the CP group were 4.44 and 2.44 on Days 7 and 14 (indicating 16.7%, p=0.60, and 54%, p<0.0001 reduction respectively compared to baseline score: 5.33/10).
Figure 6: Throat Redness: Mean scores (± SD) for throat redness in CP group (black) versus TP group (gray) pre- treatment (baseline T0) and on Days 1 (+5min & +2h), 2, 3, 4, 7, and 14. *p<0.05, **p<0.01, ***p<0.001 for TP compared to CP at the same time point, and ▪ <0.05 compared to baseline values.
In the TP group, on the other hand, throat redness showed significant reduction from Day 2 (-27%, p=0.01), continuing decreasing steadily and significantly (both compared to baseline and to CP) throughout the study period, with severity approximately 40-60-80 and 90% lower than in CP group (p<0.0001) on Days 3-4-7-and 14, respectively. These results clearly show a significant and very marked beneficial effect exerted by the TP on throat redness, a sign directly linked to throat inflammation.
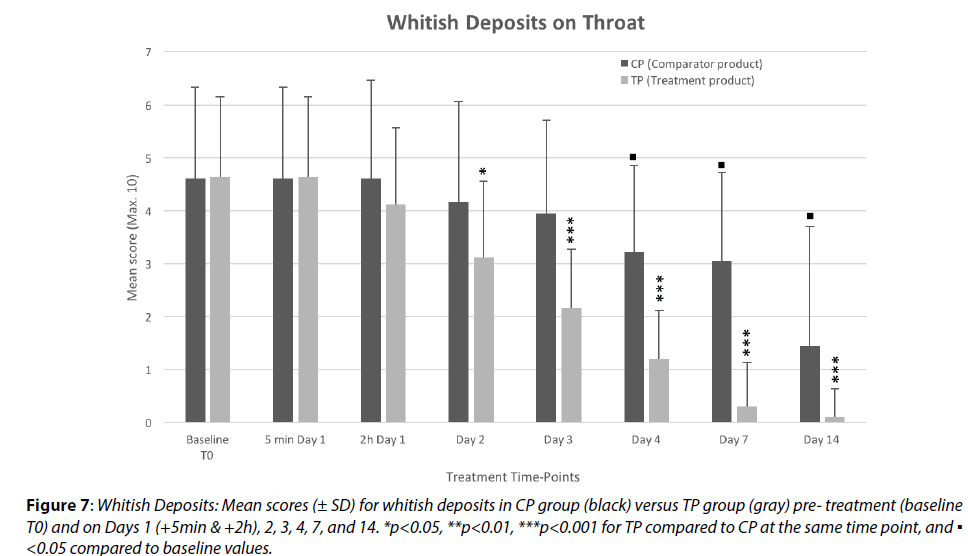
Effect on bacterial deposits on throat
Whitish deposits on the throat (or yellow-green mucus expectorations) can be a clinical sign of bacterial infection (Figure 7). As could be reasonably expected,none of the treatments had any effect on the whitish deposits on the throat surface, during the observation performed 5 minutes after 1st application of the investigational products. One of the most interesting findings of this study resides in the results obtained 2 h after the 1st application in the TP group, with 11% reduction in the mean score compared to the CP group. The reduction in whitish deposits then became statistically significant on Day 2, when clinical observation 24 h after first treatment showed a 33% (p<0.01) reduction of bacterial whitish deposits on the throat surface (25%, p<0.05, difference with CP group). As no product can act so rapidly unless through a mechanical cleaning effect, it is postulated that the strong outward liquid flow generated by TP treatment helps to detach and drain the contaminants present on the throat surface, including bacterial whitish deposits. The TP can therefore be considered as a mechanical or physical antiseptic.
Figure 7: Whitish Deposits: Mean scores (± SD) for whitish deposits in CP group (black) versus TP group (gray) pre- treatment (baseline T0) and on Days 1 (+5min & +2h), 2, 3, 4, 7, and 14. *p<0.05, **p<0.01, ***p<0.001 for TP compared to CP at the same time point, and ▪ <0.05 compared to baseline values.
The subsequent results obtained during the study also clearly show that the comparator product is not very active in reducing bacterial load over the throat surface as the slow and progressive reduction of mean values seen up to Day 14 is more likely related to the activation of the body’s natural defenses and to the use of antibiotics in several patients in this group.
In the TP group, the reduction of whitish deposits on the pharynx is as fast as the reduction observed in other parameters (e. g. throat pain, throat irritation, and swelling). The reduction was as high as 74% on Day 4 and 93% on Day 7 compared to baseline values, indicating that the TP had a very strong effect on this parameter and leading to suppose that the reduction in the bacterial load on the throat surface may probably be the primary mode of action of the TP.
Effect on the need for antibiotics
Antibiotics could be added to the treatment, at the investigator’s discretion, when lack of improvement, aggravation of symptoms of throat infection, or persistence of whitish deposits on the throat surface were noted, or when bacterial cultures remained positive.
These results show that the requirement of antibiotics was strongly diminished in the TP group (11.11% patients) compared to the CP group (38.89%). Antibiotherapy was initiated between Days 2 and 4 for all patients in each group save for 1 patient in the CP group who first began taking antibiotics only from the last day of the study (D14) and continued for 10 days. The mean duration of antibiotic treatment was not widely different between the two groups (Table 2).
| Antibiotherapy | Control group | Test product group |
|---|---|---|
| Nbr of patients | 7/18 | 4/36 |
| % | 38.89 % | 11.11% |
| Mean duration (within the study) | 7.50 days | 6.25 days |
Table 2: Summary of antibiotherapy needs
These results correspond to the progressive decrease, during the study period, in the presence of bacteria on the throat surface in the TP group. Drastic reduction in the bacterial count during the 1st 3 days of treatment (-52.78%, Table 3) indicates that the test product’s mechanical properties confer it a considerably effective action to detach and drain bacteria from the throat surface.
| Results Test product Group (n=36) |
Baseline T0 (before treatment) | +2 hours after 1st application | Day 3 | Day 7 | Day 14 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive Swabs (n) | 36 | 36 | 17 | 14 | 2 | |||||
| Positive Swabs (%) | 100% | 100% | 47.22% | 38.89% | 5.56% | |||||
| Negative Swabs (n) | 0 | 0 | 19 | 22 | 34 | |||||
| Negative Swabs (%) | 0% | 0% | 52.78% | 61.11% | 94.44% | |||||
Table 3: Evolution of bacterial presence in throat swabs – Test group
Time to complete recovery
On Day 14/visit 6, in the CP group, only 1 patient out of 18 showed complete disappearance of clinical symptoms (overall score: 0) with normal bacterial count. Another patient showed nearly complete disappearance of symptoms (overall score: 0.14 i.e. all symptoms scored 0 except for one with score 1/10) with normal bacterial count. It can therefore be concluded that a total of 2 patients or 11% patients in the CP group had fully recovered by Day 14.
Among the remaining patients in the CP group, 9 (50%) remained positive for bacterial presence in their throat swabs (Table 3) and their symptoms had been only moderately or slightly reduced.
In the TP group, by Day 14/visit 6, 20 patients out of 36 showed complete disappearance of clinical symptoms (overall score: 0) with normal bacterial count. Another 9 patients showed nearly complete disappearance of symptoms (overall score: 0.14 i.e. all symptoms scored 0 except for one with score 1/10) with normal bacterial count. It can therefore be concluded that a total of 29 patients or 80% patients in the TP group had fully recovered by Day 14.
Among the remaining patients in the TP group, only two remained positive for bacterial presence in their throat swabs or had reduced but still lingering symptoms at the end of the study (Table 3).
Safety parameters
A few patients reported a headache related to their condition, at one visit with resolution at the next visit, but no treatment-related undesirable or adverse effect was observed in any of the patients, in either group.
Immediately following application of Investigational Products, some patients reported a warm sensation on the throat, sometimes accompanied by some slight but temporary (lasting only the first couple of minutes after application) irritation. This brief, transient effect is considered entirely normal and is related to the osmotic properties of the TP.
Investigational Products tolerability, acceptability and overall satisfaction
The CP treatment was found to be fair by 100% of participants, but no one found the treatment good or very good, nor excellent. In the TP group, product was scored as very good (19.44%) or excellent (80.55%) indicating a very high satisfaction rate. Strong amelioration was reported very soon in the treatment course by most patients, further resulting in fast recovery, which is rather remarkable for a product acting only topically. There was no clinically significant difference in vital signs, laboratory test values or general/systemic examination results during study period compared to the baseline data.
Discussion
Throat infection or pharyngitis is among the most common diseases affecting populations of all ages worldwide [20-22]. It is usually initiated by a viral infection [23], and as the virus attacks and invades its host, it damages the upper respiratory tract mucosa cells, opening the door to the proliferation of opportunistic bacteria, such as Streptococcus [24]. The infectious processes lead to irritation and inflammation of the airway mucosa, resulting in acute throat pain.
In some patients, pharyngitis may manifest as mild to moderate pain and symptoms, and although representing a significant economic cost [25] in rather inefficacious over-the-counter healthcare products [16,26,27] as well as lost productivity, it is not a life-threatening disease. It becomes a more worrisome issue when patients experience severe infection and impairing symptoms, as when caused by the influenza virus, with possible serious complications [28-30]. Although antibiotics can be necessary in case of secondary bacterial colonization [31], they are still too often systematically resorted to, despite local governmental sensitization campaigns [32,33] to promote more appropriate antibiotic use, which has led to the worldwide bacterial resistance crisis [34-36].
In view of the common yet increasingly problematic situation, it has become urgent to find more pertinent, effective and safe means of treating such widely spread conditions.
The ideal treatment should not give rise to antimicrobial resistance and be free of side effects [37]. Systemic antivirals are poorly effective in such upper respiratory tract infections, therefore a topical treatment that would be able to mechanically clean away viruses, bacteria, and all other contaminants without entering into systemic circulation or interacting with the underlying live cells, would seem the perfect therapeutic agent. Unfortunately, so far only drugs or remedies, including local antiseptics, saline gargling, honey and other traditional plant remedies, providing partial symptomatic relief but allowing the condition to linger, were available, since combining all therapeutic prerequisites into a single molecule or solution had not been achievable. Those treatments, although fairly to very safe, have poor or limited efficacy.
The efficacy and safety profile of a new hyperosmotic solution, with filmogen capacity, mechanically acting as antiseptic and cleaning solution, detaching and removing, through the exudation of liquid generated, all contaminants present on the throat surface, including bacteria and free virus particles, and alleviating the pain and irritation, was therefore evaluated on various signs and symptoms of sore throat and pharyngitis.
The results obtained in this study show that the test solution produced significant, strong, rapid and durable effects with regard to all parameters studied.
The comparator product, saline solution, was also found effective in reducing symptomatic manifestation of sore throat but the efficacy was relatively poor and slow compared to the test product. The rapidity of the test solution to produce significant beneficial effects is due to its mechanical mode of action, since patients reported significant reduction in throat pain and swallowing difficulty as early as on the first day of treatment (within just 5 min and 2 h, respectively), whereas other symptoms: throat irritation, swollenness and redness (the latter two being signs associated with local inflammation) as well as the pharyngeal bacterial load decreased drastically and with statistically significance from just the second day of treatment (p<0.0001). Those quick-obtained results led to a reduced need for antibiotherapy (by about 70%) compared to the comparator group, indicating that TP exerts some remarkable antimicrobial effect by cleaning the throat surface of pathogens and contaminants. The amelioration was not only quickly observed but also proved durable, as all patients had completely or close to completely recovered within the study period. These findings demonstrate the fast and high efficacy, and total, side effect-free, safety of the Septicyanidin-containing F-VB-Gy solution, and confirm the novel therapeutic approach that a mechanical, non-discriminating, osmotic drainage-based, antiseptic activity not only provides fast symptomatic relief, but evidently helps stop pathogenic infection in its tracks fairly early in the treatment period. The sustained exudation of liquid prevents virus particles and bacteria from attaching and to host mucosa and infecting the epithelium cells. As the infectious attack is thus repelled, the body’s normal defense functions are permitted to kick in more promptly and efficiently, the combination of beneficial actions resulting in fast recovery.
Although the study spanned over two weeks, the results obtained in the test group lead to surmise that even a shorter treatment course would be sufficient in most, uncomplicated, pharyngitis cases. Those observations allow to conclude that this very promising new generation of products, conceived based on a more holistic, scientifically solid, therapeutic approach shall offer new, remarkably safe, high efficacy options for the treatment of uncomplicated pharyngitis and common sore or strep throat infections.
Acknowledgements
The authors would like to thank Mrs Marjorie Georges and Mr Rémi Shrivastava for their appreciable assistance in handling statistical analysis of the data and providing editorial support.
Funding
This clinical study was commissioned and funded by the Naturveda Laboratory.
Conflict of Interest
The authors have declared that no conflict of interest exists.
References
- Kenealy T. Sore Throat. AmFam Physician 91: 689-690 (2015).
- Gereige R, Cunill-De Sautu B. Throat Infections. Pediatr Rev 32: 459-468 (2011).
- Renner B, Mueller CA, Shephard A. Environmental and non-infectious factors in the aetiology of pharyngitis (sore throat). Inflamm Res61: 1041-1052 (2012).
- Martin JM. Pharyngitis and streptococcal throat infections.Pediatr Ann 39: 22-27 (2010).
- Rousse M, Cucuat N, Janicot C, Shrivastava R. Innovative Scientific Concept of Topical Virus Glycoprotein Inhibitors Incorporated in Hyperosmotic Glycerol Revolutionizes Future Prospects in the Treatment of Viral and Bacterial Throat Infections.Int. J. Pharm. Sci. Drug Res 6: 01-11 (2014).
- Watanabe T, Watanabe S, Kawaoka Y. Cellular networks involved in the influenza virus life cycle.Cell Host Microbe 7: 427-439 (2010).
- König R, Stertz S, Zhou Y, et al.Human host factors required for influenza virus replication. Nature 463: 813-817 (2010).
- Frensing T, KupkeSY, Bachmann M, et al.Influenza virus intracellular replication dynamics, release kinetics, and particle morphology during propagation in MDCK cells. ApplMicrobiolBiotechnol 100: 7181-7192 (2016).
- Esposito S, Blasi F, Bosis S, et al.Aetiology of acute pharyngitis: the role of atypical bacteria.J Med Microbiol53: 645-651 (2004).
- Anjos LM, Marcondes MB, Lima MF, Mondelli AL, OkoshiMP. Streptococcal acute pharyngitis.Rev Soc Bras Med Trop 47: 409-413 (2014).
- Langlois DM, Andreae M. Group A streptococcal infections. Pediatr Rev32:423-429 (2011).
- Hashigucci K, Matsunobu T. Etiology of acute pharyngitis in adults: the presence of viruses and bacteria.Nihon JibiinkokaGakkaiKaiho106: 532-539 (2003).
- Tan TW, Chen BC, Tan HL, Chang CM. Effectiveness of amylmetacresol and 2,4-dichlorobenzyl alcohol throat lozenges in patients with acute sore throat due to upper respiratory tract infection: a systematic review protocol. JBI Database System Rev Implement Rep4: 862-872 (2017).
- Di Pierro F, Zanvit A, Colombo M. Role of a proprietary propolis-based product on the wait-and-see approach in acute otitis media and in preventing evolution to tracheitis, bronchitis, or rhinosinusitis from nonstreptococcal pharyngitis. Int J Gen Med 11: 409-414 (2016).
- Rehman H, Naveed S, Usmanghani K. Efficacy and safety of Linkus, Aminophylline diphenhydramine and acefyllinpiperazine for the treatment of cough in children. Pak J Pharm Sci 29: 1027-1032 (2016).
- de Mey C, Koelsch S, Richter E, Pohlmann T, Sousa R. Efficacy and Safety of Ambroxol Lozenges in the Treatment of Acute Uncomplicated Sore Throat - a Pooled Analysis. Drug Res (Stuttg)66:384-392 (2016).
- Van Driel ML, De Sutter Al, Habraken H, et al. Different antibiotic treatments for group A streptococcal pharyngitis. Cochrane Database Syst Rev 11: CD004406 (2016).
- Wessels MR. Pharyngitis and Scarlet Fever. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes : Basic Biology to Clinical Manifestations [Internet]. Oklahoma City (OK): University of Oklahoma Health Sciences Center(2016).
- Shrivastava R. A Pilot Clinical Trial to Evaluate the Efficacy of a Topical Antiviral Osmotically Active Hypertonic Solution for the Treatment of Influenza Virus Induced Sore Throat. J Clinic Trials 1:102 (2011).
- Di Muzio F, Barucco M, Guerriero F. Diagnosis and treatment of acute pharyngitis/tonsillitis: a preliminary observational study in General Medicine. Eur Rev Med PharmacolSci20:4950-4954 (2016).
- Chiappini E, Bortone B, Di Mauro G, et al.Choosing Wisely: The Top-5 Recommendations from the Italian Panel of the National Guidelines for the Management of Acute Pharyngitis in Children. ClinTher 39:646-649 (2017).
- CotsJM, Alós JI, Bárcena M, et al.Recommendations for management of acute pharyngitis in adults. EnfermInfeccMicrobiolClin 34: 585-594 (2016).
- Ivaska L, Niemelä J, Lempainen J, et al. Aetiology of febrile pharyngitis in children: Potential of myxovirus resistance protein A (MxA) as a biomarker of viral infection.J Infect 74: 385-392 (2017).
- Marchello C, Ebell MH. Prevalence of Group C Streptococcus and FusobacteriumNecrophorum in Patients with Sore Throat: A Meta-Analysis.Ann Fam Med14:567-574 (2016).
- Llor C, Moragas A, Cots JM, et al. Happy Audit Study Group.Estimated saving of antibiotics in pharyngitis and lower respiratory tract infections if general practitioners used rapid tests and followed guidelines. AtenPrimaria 6567: 30399-30397 (2016).
- Radetsky M. Hostage to History: The Duration of Antimicrobial Treatment for Acute Streptococcal Pharyngitis. Pediatr Infect Dis J (2016).
- Klimek L, Sperl A. Pharmacy based sore throat therapy according to current guidelines. Med Monatsschr Pharm 38:503-208 (2015).
- Hung IF, Zhang AJ, To KK, et al. Unexpectedly Higher Morbidity and Mortality of Hospitalized Elderly Patients Associated with Rhinovirus Compared with Influenza Virus Respiratory Tract Infection. Int J Mol Sci 18: 259 (2017).
- Gulliford MC, Moore MV, Little P, et al. Safety of reduced antibiotic prescribing for self-limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ354:3410 (2016).
- Carter RR, Sun J, Jump RL. A Survey and Analysis of the American Public's Perceptions and Knowledge About Antibiotic Resistance. Open Forum Infect Dis 3: 112 (2016).
- Harris AM, Hicks LA, Qaseem A. High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention.Appropriate Antibiotic Use for Acute Respiratory Tract Infection in Adults: Advice for High-Value Care From the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med164:425-434 (2016).
- Llor C, Vilaseca I, Lehrer-Coriat E, et al. Survey of Spanish general practitioners' attitudes toward management of sore throat: an internet-based questionnaire study. BMC FamPract 18:21 (2017).
- Khamsarn S, Nampoonsak Y, Busamaro S, et al.Epidemiology of Antibiotic Use and Antimicrobial Resistance in Selected Communities in Thailand.J Med Assoc Thai 99:270-275 (2016).
- Tomas A, PautKusturica M, Tomic Z, et al.Self-medication with antibiotics in Serbian households: a case for action? Int J Clin Pharm (2017).
- Zhao SR, Griffin MR, Patterson BL, et al.Risk Factors for Outpatient Use of Antibiotics in Children with Acute Respiratory Illnesses.South Med J110:172-180 (2017).
- DeMuriGP, SterkelAK, KubicaPA,et al.Macrolide and Clindamycin Resistance in Group a Streptococci Isolated From Children With Pharyngitis. Pediatr Infect Dis J 36: 342-344 (2017).
- Kimura M.Late-onset Rash in Patients with Group A Beta-hemolytic Streptococcal Pharyngitis Treated with Amoxicillin. Pediatr Rep 7:5951 (2015).