Case Report - International Journal of Clinical Rheumatology (2024) Volume 19, Issue 5
TREATMENT OF SECONDARY IMMUNE THROMBOCYTOPENIA UNRESPONSIVE TO STEROIDS IN ADULT-ONSET STILLâ≢S DISEASE: A CASE REPORT SUCCESFULLY TREATED WITH A COMBINATION THERAPY OF RITUXIMAB AND ELTROMBOPAG
Darlo Brono1,2, Elena Rossi3, Annamaria Paglionico1 , Valentina Varriano1,Valerio De Stefano3 and Eliza Gremese1,4*
1Clinical Immunology Unit, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Italy
3Department of Radiological and Haematological Sciences, Catholic University School of Medicine, Italy
4Catholic University of the Sacred Heart, Italy
- *Corresponding Author:
- Eliza Gremese
Clinical Immunology Unit, Fondazione Policlinico Universitario A. Gemelli-IRCCS, Italy,Catholic University of the Sacred Heart, Italy
E-mail: elisa.gremese@policlinicogemelli.it
Received: 10-May-2024, Manuscript No. fmijcr-24-134686; Editor assigned: 16- May-2024, Pre-QC No. fmijcr-24-134686 (PQ); Reviewed: 29-May-2024, QC No. fmijcr-24-134686; Revised: 04-Jun- 2024, Manuscript No. fmijcr-24-134686 (R); Published: 10-Jun-2024, DOI: 10.37532/1758-4272.2024.19 (5).164-169
Abstract
Rationale: Adult-onset Still’s disease (AOSD) is a rare systemic autoinflammatory disease with potentially severe life-threatening complications. Among them, coagulation disorders such as disseminated intravascular coagulopathy and thrombotic microangiopathy may occur. Nevertheless, alterations in platelet count may have several other causes. We present the first case of a patient with AOSD complicated by immune thrombocytopenic purpura (ITP) and the individualized therapeutic approach.
Patient concerns: An 18-year-old female suffering from AOSD on immunosuppressive therapy with methotrexate and anakinra was admitted to our ward due to severe thrombocytopenia, unresponsive to glucocorticoids.
Diagnosis: A bone marrow aspirate showed normal megakariocytes, with no schistocytes, nor hemophagocytosis, thus suggesting increased peripheral destruction as the possible underlying pathological mechanism. The detection of anti-platelet antibodies against Gp Ib/IX epitopes confirmed the ITP hypothesis. In addition, Helicobacter pylori (HP) infection was identified in the gastric biopsy.
Interventions and outcomes: Due to the resistance of thrombocytopenia to high-dose steroids and intravenous immunoglobulins (IVIg), second-line therapy with rituximab (RTX) was started, combined with eltrombopag for persistent recurrent bleeding. HP infection treatment was prescribed and urea breath test (performed after one month) confirmed eradication of HP.
Four months after discharge, platelet counts returned steadily to the normal range; prednisone was tapered to 5 mg/day and eltrombopag continued. Oral cyclosporine was chosen as maintenance immunosuppressive treatment and another course of RTX was administered after six months.
Conclusion: Thrombocytopenia in AOSD can represent a diagnostic and clinical challenge due to many potential differential diagnoses. This is the first reported case of ITP in a patient with AOSD, in which combination therapy with RTX and a thrombopoietin receptor agonist (eltrombopag) was used, with the resolution of thrombocytopenia. Concomitant HP infection was recognized and treated. Abbreviations: AOSD: Adult-onset Still's Disease, ITP: Immune thrombocytopenic purpura, HP: Helicobacter pylori, IVIg: Intravenous immunoglobulins, RTX: Rituximab, TPO-RAs: Thrombopoietin receptor agonists
Abbreviations:
AOSD: Adult-onset Still's Disease, ITP: Immune thrombocytopenic purpura, HP: Helicobacter pylori, IVIg: Intravenous immunoglobulins, RTX: Rituximab, TPO-RAs: Thrombopoietin receptor agonists
Keywords
Immune Thrombocytopenia • Adult-onset Still’s disease • Helicobacter pylori • Rituximab • Eltrombopag
Introduction
Adult-onset Still's Disease (AOSD) is a rare systemic auto-inflammatory disease, classically described by the "Still's triad" of fever, rash, and arthritis. Other classical clinical manifestations include evanescent rash, sore throat, serositis, lymphadenopathy, hepatosplenomegaly, and common laboratory biomarkers are neutrophilic leukocytosis, elevated acute-phase reactants (ESR, CRP, ferritin) and abnormal liver function tests. A key role in the pathogenesis of AOSD is carried out by the intense activation of innate immune cells, which determines a “cytokine storm”, with an overproduction of several pro-inflammatory cytokines including IL-1, IL-6 and IL-18 [1,2]. The diagnosis is primarily clinical and requires the exclusion of a wide range of mimicking disorders. The severity of organ involvement can vary considerably, representing a wide spectrum from the self-limited to life-threatening. Among the serious and potentially fatal complications, there is the macrophage activation syndrome (MAS) [3,4], caused by excessive activation and expansion of T lymphocytes and macrophages that exhibit hemophagocytic activity, leading to a hyperinflammatory state associated with three cardinal features: cytopenias, liver dysfunction, and coagulopathy resembling disseminated intravascular coagulation (DIC). The treatment here is based on the introduction of high-dose steroids and cytokine-targeted approaches (anti-IL1 and anti-IL-6). Another rare, but quite severe, coagulation disorder is thrombotic microangiopathy (TMA), that should be suspected in case of unexplained multi-organ failure or stroke [1,5,6]. Here we report a case of thrombocytopenia in a young female affected by AOSD, which finally was proven to be a form of secondary immune thrombocytopenia (ITP) plus Helicobacter pylori gastric infection, successfully treated with rituximab (RTX), a thrombopoietin receptor agonist (TPO-RA) and H. pylori eradication.
Case Presentation
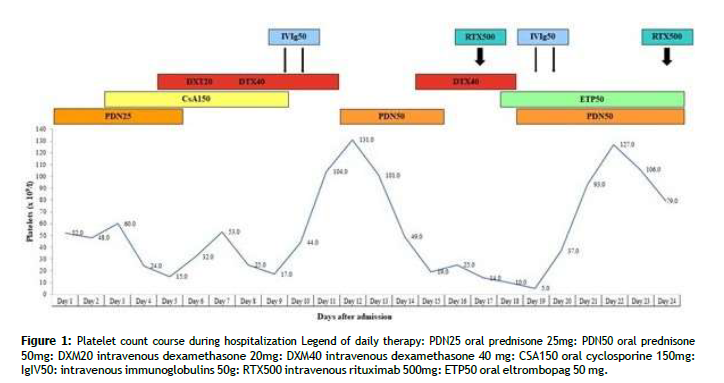
An 18-year-old female (165cm, 50kg) affected by AOSD was admitted to our ward due to severe thrombocytopenia, unresponsive to glucocorticoids. Her clinical history began eighteen months earlier, when she was admitted to the Infectious Disease Unit because of fever (with peaks up to 40°C), sore throat, transient itchy maculopapular rash and arthralgias, mainly at knees, ankles and wrists. Blood tests showed neutrophilic leukocytosis, ALT elevation (>3x ULN), a marked rise of C-reactive protein (CRP) (215.9mg/l) and procalcitonin (PCT) (20.7 ng/mL) and a severe hyperferritinemia (>16500ng/mL). Initially, empiric antibiotic therapy with ceftriaxone and amikacin was started; vancomycin was then added due to persistent fever, still without achieving any clinical improvement. Blood and urine cultures were negative; chest X-rays, heart and abdominal ultrasound did not show suspected sites of infection; bone marrow biopsy showed appropriate cellularity for age with associated normal trilinear hematopoiesis. The autoantibody panel showed positivity of antinuclear antibodies (ANA1/160, fine speckled pattern) and lupus anticoagulant (LAC). After five days, the patient was moved to our department, and according to Yamaguchi criteria [7] a diagnosis of Still’s disease was made (fever>39°C, leukocytosis>10.000/mm3, typical rash, arthralgias, sore throat, liver disfunction), with a Pouchot score [8] of 4/12 (fever, evanescent rash, pharyngitis, elevated liver enzymes). High dose oral prednisone (1mg/kg/day) was started, but after one day a petechial eruption of arms and legs occurred, in association with a reduction of platelet (PLT) count (down to 51×109/l) and fibrinogen blood levels, and with a marked increase of D-dimer (>80,000ng/ml). A diagnosis of MAS with DIC was made, according to Fardet criteria for hemophagocytic syndrome [9], with an H-score of 218 (conferring a probability of having a MAS of 95.8%), and intravenous methylprednisolone at a dosage of 125 mg every 6 hours plus intravenous cyclosporine (CsA) at a dosage of 3mg/kg/day (then increased up to 5mg/kg/day) were administered for five days. Subsequently, antibiotic therapy was withdrawn and steroids were tapered with a concomitant increase of CsA dosage up to 7mg/kg/day. Nevertheless, neutropenia (down to a minimum of 500 cells/mm3) and a reappearance of fever occurred. Glucocorticoid dosage was then increased (intravenous methylprednisolone, 60mg every 6 hours), and CsA was withdrawn due to possible myelotoxicity. In consideration of the persistent hyperferritinemia and arthralgias, treatment with subcutaneous anakinra 100mg/day was also started, enabling to achieve the resolution of fever and gradual clinical and laboratory improvement and to slowly taper glucocorticoids. Shortly before the discharge, oral methotrexate (MTX) at a dosage of 10mg weekly was added, without adverse events, allowing, after four months, to stop glucocorticoids thanks to clinical remission and complete normalization of blood tests. The patient continued combination therapy with MTX and anakinra for eleven consecutive months, when isolated thrombocytopenia occurred (PLT 62×109/l, confirmed in citrate sample to exclude pseudo-thrombocytopenia), in the absence of other laboratory abnormalities. A progressive worsening of the PLT count followed, and, not being able to exclude an adverse event to immunosuppressants, MTX and anakinra were stopped. After two weeks, having reached a PLT count of 17×109/l, oral prednisone at a dosage of 25mg/day (0.5mg/kg/day, body weight 50kg) was started. However, having no improvement in the PLT count after several days of treatment, the patient was hospitalized for further investigations. At admission, we found an increase of acute phase reactants (ESR 61 mm/1st hour, CRP 7mg/l, ferritin 345ng/mL) and a reappearance of arthralgias and transient itchy rash at trunk and legs, probably as the result of the interruption of the maintenance immunosuppressive treatment. No clinical and laboratory signs suggestive of MAS or TMA were noted. The abdominal ultrasound did not show hepatosplenomegaly. The autoantibody panel resulted negative, not confirming the previous finding of ANA and LAC positivity. Initially, we started oral CsA at a dosage of 3mg/kg/day. After three days, due to worsening of PLT count (15×109/l), an urgent bone marrow aspirate was performed, showing a discrete amount of megakaryocytes in all maturation phases in the absence of any sign of hemophagocytosis. Intravenous dexamethasone at a dosage of 20mg/day was then administered for four days, with an initial improvement in PLT count (Figure 1) and a resolution of signs of Still’s reactivation. However, after a new acute reduction of the PLT count and occurrence of epistaxis, dexamethasone dosage was increased to 40mg/day for further four days and intravenous human normal immunoglobulins (IVIg) at a dosage of 1g/kg/day were administered for 2 days, obtaining a peak response at 36-48 hours (PLT 131×109/l); oral CsA was withdrawn (Figure 1). However, quickly after switching to the maintenance glucocorticoid regimen (oral prednisone 1mg/kg/day), a worsening of thrombocytopenia (PLT 19×109/l) occurred, and a new cycle of intravenous dexamethasone at a dosage of 40 mg/day was administered for four days, with no response on PLT count (Figure 1). Therefore, treatment with intravenous RTX at a dosage of 375mg/m2 weekly for four weeks was started and, due to the persistence of episodes of profuse epistaxis, eltrombopag 50mg/day was added. After two days, severe epistaxis occurred, in conjunction with the nadir of PLT count of 5×109/l, and we received the report of presence of anti-platelet antibodies against Gp Ib/IX epitopes. A second cycle of IVIg at a dosage of 1g/kg/day for two days was administered, again achieving a rapid response (PLT 127×109/l after 72 hours) (Figure 1). After excluding infections from the most common infectious agents, an esophagogastroduodenoscopy with biopsies was performed showing a histological picture consistent with active chronic gastritis and Helicobacter pylori (HP) positive infection. Accordingly, a course of eradicating therapy with amoxicillin and metronidazole plus esomeprazole for ten days was prescribed. The patient was discharged after 24 days with a PLT count of 79×109/l, after the resolution of epistaxis. No major bleedings were observed during hospitalization. After two weeks from the completion of the RTX course (4 weeks from the discharge), another reduction of PLT count was observed (16×109/l) and eltrombopag dosage was increased to 75mg/day, with normalization of PLT count (231×109/l) after one week. Urea breath test, performed after one month from the end of eradicating therapy, resulted negative for signs of HP infection, confirming a successful eradication. At four months after the discharge, PLT count was normal and stable (216×109/l). Prednisone dosage was tapered to 5mg/day and eltrombopag dosage was reduced to 50mg/day. Oral CsA at a dosage of 3mg/kg/day was introduced as a maintenance immunosuppressive treatment. At the sixth month a new course of RTX as maintenance therapy was administered and prednisone was withdrawn. For the following six months PLT count remained stable and no flares of AOSD were registered. Informed written consent was obtained from the patient for publication of this case report.
Figure 1: Platelet count course during hospitalization Legend of daily therapy: PDN25 oral prednisone 25mg: PDN50 oral prednisone 50mg: DXM20 intravenous dexamethasone 20mg: DXM40 intravenous dexamethasone 40 mg: CSA150 oral cyclosporine 150mg: IgIV50: intravenous immunoglobulins 50g: RTX500 intravenous rituximab 500mg: ETP50 oral eltrombopag 50 mg.
Discussion
We presented a complex case of thrombocytopenia with several potential alternative diagnoses. First, we hypothesized a drug-induced adverse event, since the patient was in combination therapy with MTX, though at a low dosage, and anakinra. Nevertheless, this therapy was stable in the last 12 months, and thrombocytopenia is a very rare side effect related to IL-1 blockade. It was first described in AOSD in 2007 [10]: in that report, there was a temporal relationship between the institution of therapy with anakinra and development of thrombocytopenia, which resolved soon after IL-1Ra discontinuation, even if the causality was not confirmed by a rechallenge [10]. In an observational cohort of 146 patients with rheumatoid arthritis (RA) treated with anakinra, only one episode of thrombocytopenia related to treatment was reported, a side effect already experienced on adalimumab by the same subject [11]. Possible explanations of this adverse event are the inhibition of megakaryocytopoiesis, in which IL-1 seems to play a central role together with other cytokines [12], or an idiosyncratic reaction in genetically predisposed patients, as for the thrombocytopenia rarely induced by tumor necrosis factor-α blocking [13]. Against the initial suspicion of anakinra-related thrombocytopenia, it was the observation that no improvement was seen after drug interruption, which would be expected given the short half-life of anakinra. Therefore, we admitted the patient to our ward in order to search for other possible causes of thrombocytopenia, which could be serious complications of AOSD (i.e. MAS with DIC or TMA). Flares of AOSD and development of MAS can be clinically similar and share the same alterations, such as anemia and marked elevation of serum ferritin and CRP. Laboratory hallmarks of MAS are leukopenia and thrombocytopenia, very high levels of serum triglycerides and, in the case of concomitant DIC, low levels of haptoglobin and fibrinogen [14-16]. Coexistence of TMA as thrombotic thrombocytopenic purpura (TTP) and AOSD is extremely rare. In this eventuality, as well as thrombocytopenia, renal failure, marked elevation of lactate dehydrogenase and red cell fragmentation on peripheral blood smear are usually observed [17]. In our case, the first blood exams allowed to exclude these life-threatening complications, because of only slight serum ferritin increase and absence of schistocytes and coagulation disorders. Moreover, anti-nuclear and antiphospholipid antibodies were absent, making coexistent systemic lupus erythematosus and antiphospholipid antibody syndrome unlikely. A clinically significant splenic sequestration was not consistent with the abdominal ultrasonography picture, showing the absence of hepatosplenomegaly. In order to investigate the central or peripheral genesis of thrombocytopenia, a bone marrow aspirate was performed. Cytological examination showed no alterations of the megakaryocytic series, indicating an increased peripheral destruction as the possible underlying pathological mechanism. Therefore, once excluded a decreased bone marrow platelet production, we searched the causes of increased peripheral destruction, immune or non-immune mediated. The detection of anti-platelet antibodies against Gp Ib/IX epitopes confirmed the hypothesis of immune thrombocytopenic purpura (ITP). Accordingly, diagnosis of ITP secondary to AOSD was made [18]. It has been reported that anti-GPIb/IX antibodies are associated with a lower platelet count and inadequate responses to glucocorticoids [19] and lower response rate to IVIg [20], although they were less prevalent than anti-GPIIb/IIIa antibodies in ITP patients. Moreover, ITP patients with anti-GPIIb/IIIa autoantibodies were more responsive to rituximab treatment [21]. Therefore, antibody subtyping might be useful as a predictor of treatment response in ITP, in order to optimize rescue therapy in resistant patients. As shown, the thrombocytopenia of our patient was unresponsive to high dose intravenous glucocorticoids, while the response to IVIg, even if marked, was surprisingly limited in terms of duration (no more than 72 hours). Therefore, second-line therapies were considered: treatment with RTX was started and, for the persistence of recurrent bleeding events, eltrombopag was added. These drugs were well tolerated, and no adverse events were registered. Rituximab has already been used as a treatment option for refractory AOSD [22] since lymphadenopathy is seen in 65% of patients and a role of B cells in the pathogenesis could be hypothesized. Moreover, it has been administered in acute refractory and relapsing secondary TMA [23]. The rationale of TPO-RAs romiplostim and eltrombopag use in ITP is supported by pathophysiologic studies demonstrating both increased platelet destruction as well as inappropriately low platelet production, secondary to the proapoptotic action of glycoprotein-specific platelet autoantibodies and cytotoxic lymphocytes on megakaryocytes [24,25]. TPO-RAs can thus be used as part of combination therapy in treatment-resistant patients, as their unique mechanism of action of increasing platelet production may synergize with agents that diminish platelet destruction, either via decreased platelet clearance (i.e. glucocorticoids) or reduction in platelet autoantibody production (i.e. RTX). Eltrombopag is an oral, nonâpeptide TPOâRA that binds to a transmembrane site on the thrombopoietin receptor and increases the number of platelets. It is currently approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of primary ITP, of thrombocytopenia secondary to aplastic anaemia, and thrombocytopenia secondary to hepatitis C infection. However, its use in secondary ITP is promising: in a retrospective series of 87 patients with secondary ITP (46 with ITP secondary to autoimmune syndromes, 23 with ITP secondary to lymphoproliferative disorders, and 18 with ITP secondary to viral infections), 38% of patients had a platelet response; in the immune group 63% had a platelet response including 28 (61%) with a complete response ≥100×109 /l [26]. Indeed, in case of ITP unresponsive to single-drug treatments, combining an immunosuppressant therapy with TPO-RAs may be a good strategy, since the overall response rate is of 70% in patients who received this treatment [27]. In a series of 13 patients with newly diagnosed ITP, complete response rate at day 28 was 84.6% (overall response: 100%) after a combined treatment with eltrombopag 50 mg/day for 28 days, oral dexamethasone 40 mg/day for 4 days, and low dose rituximab 100 mg weekly for 4 weeks [28]. This schedule is similar in principle to the combination that we employed in our patient, based on the contemporary administration of eltrombopag, prednisone, and rituximab. Finally, HP infection acting as a “trigger” was suspected and identified in the gastric biopsy. Several studies found an association between HP infection and the incidence of ITP and it has been demonstrated that HP eradication significantly increases platelet counts [29]. In order to explain the pathogenic role of HP infection in the development of ITP, mechanism of molecular mimicry has been suggested, together with platelet activation mediated by HP-bound vWF interacting with GPIb and supported by IgG [29-31]. Thus, it is advisable to investigate for HP infection in patients with resistant ITP, even in the absence of gastrointestinal symptoms.
Conclusion
Thrombocytopenia may be multifactorial in AOSD and awareness of many differential diagnoses is critical in order to establish early and proper treatment. To our knowledge, this is the first reported case of AOSD with secondary ITP unresponsive to steroids in which RTX and the TPO-RA eltrombopag have proven as a safe and effective treatment option. We also identified and treated a concurrent HP infection as a possible unfavourable “trigger” factor.
References
- Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nat Rev Rheumatol 14: 603-618(2018).
- Macovei LA, Burlui A, Bratoiu I, et al. Adult-Onset Still's Disease-A Complex Disease, a Challenging Treatment. Int J Mol Sci 23: 12810(2022).
- Tsuboi H, Segawa S, Yagishita M, et al. Activation mechanisms of monocytes/macrophages in adult-onset Still disease. Front Immunol 13: 953730(2022).
- Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun 93: 24-36(2018).
- Schulert GS, Grom AA. Macrophage activation syndrome and cytokine-directed therapies. Best Pract Res Clin Rheumatol 28: 277-92(2014).
- Ananthaneni A, Shimkus G, Weis F, et al. Adult-onset Still's disease with concurrent thrombotic microangiopathy: Observations from pooled analysis for an uncommon finding. Eur J Haematol (2023).
- Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification of adult Still's disease. J Rheumatol 19: 424-30(1992).
- Pouchot J, Sampalis JS, Beaudet F, et al. Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 70: 118-36(1991).
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 66: 2613-20(2014).
- Quartuccio L, De Vita S. Interleukin 1 receptor antagonist therapy-induced thrombocytopenia in adult onset Still's disease. J Rheumatol 34: 892-3(2007).
- den Broeder, de Jong, Franssen MJ, et al. Observational study on efficacy, safety, and drug survival of anakinra in rheumatoid arthritis patients in clinical practice. Ann Rheum Dis 65: 760-2(2006).
- Oudenrijn S, Haas M, Calafat J, et al. A combination of megakaryocyte growth and development factor and interleukin-1 is sufficient to culture large numbers of megakaryocytic progenitors and megakaryocytes for transfusion purposes. Br J Haematol 106: 553-63(1999).
- Pathare SK, Heycock C, Hamilton J. TNFalpha blocker-induced thrombocytopenia. Rheumatology (Oxford) 45: 1313-4(2006).
- Mitrovic S, Fautrel B. Complications of adult-onset Still's disease and their management. Expert Rev Clin Immunol 14:351-365(2018).
- Lenert A, Yao Q. Macrophage activation syndrome complicating adult onset Still's disease: A single center case series and comparison with literature. Semin Arthritis Rheum 45:711-6(2016).
- Parisi F, Paglionico A, Varriano V, et al. Refractory adult-onset Still disease complicated by macrophage activation syndrome and acute myocarditis: A case report treated with high doses (8âmg/kg/d) of anakinra. Medicine (Baltimore) 96: e6656(2017).
- Sayarlioglu M, Sayarlioglu H, Ozkaya M, et al. Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome and adult onset Still's disease: case report and review of the literature. Mod Rheumatol 18:403-6(2008).
- Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 113: 2386-93(2009).
- Zeng Q, Zhu L, Tao L, et al. Relative efficacy of steroid therapy in immune thrombocytopenia mediated by anti-platelet GPIIbIIIa versus GPIbα antibodies. Am J Hematol 87: 206-8(2012).
- Peng J, Ma SH, Liu J, et al. Association of autoantibody specificity and response to intravenous immunoglobulin G therapy in immune thrombocytopenia: a multicenter cohort study. J Thromb Haemost 12: 497-504(2014).
- Feng R, Liu X, Zhao Y, et al. GPIIb/IIIa autoantibody predicts better rituximab response in ITP. Br J Haematol 182: 305-307(2018).
- Ahmadi-Simab K, Lamprecht P, Jankowiak C, et al. Successful treatment of refractory adult onset Still’s disease with rituximab. Ann Rheum Dis 65: 1117-1118(2006).
- Lee WS, Yoo WH. Rituximab for refractory adult-onset Still's disease with thrombotic microangiopathy. Rheumatology (Oxford) 53: 1717-8(2014).
- Al-Samkari H, Kuter DJ. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther Adv Hematol 10: 2040620719841735(2019).
- Zufferey A, Kapur R, Semple JW. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J Clin Med 6: E16(2017).
- González-López TJ, Alvarez-Román MT, Pascual C, et al. Use of eltrombopag for secondary immune thrombocytopenia in clinical practice. Br J Haematol 178: 959-970(2017).
- Mahévas M, Gerfaud-Valentin M, Moulis G, et al. Characteristics, outcome, and response to therapy of multirefractory chronic immune thrombocytopenia. Blood 128: 1625-30(2016).
- Gómez-Almaguer D, Colunga-Pedraza PR, Gómez-De A, et al. Eltrombopag, low-dose rituximab, and dexamethasone combination as frontline treatment of newly diagnosed immune thrombocytopaenia. Br J Haematol 184: 288-290(2019).
- Aljarad S, Alhamid A, Sankari A, et al. The impact of helicobacter pylori eradication on platelet counts of adult patients with idiopathic thrombocytopenic purpura. BMC Hematol18: 28(2018).
- Lee A, Hong J, Chung H, et al. Helicobacter pylori eradication affects platelet count recovery in immune thrombocytopenia. Sci Rep 10: 18198(2020).
- Zain MA, Zafar F, Ashfaq A, et al. Helicobacter pylori: An Underrated Cause of Immune Thrombocytopenic Purpura. A Comprehensive Review. Cureus 11: e5551(2019).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref