Research Article - Clinical Investigation (2022) Volume 12, Issue 12
Treating acute and chronic rhinosinusitis in children using an osmotic, nasal surface cleaning, non-medicated polymeric film
Abstract
Objective: Acute and chronic rhinosinusitis is one of the most common diseases in children, affecting seriously the quality of life of children and parents. The physiopathology is multifactorial involving nasal mucosa inflammation, cellular damage and blocked sinus drainage due to bacterial biofilm on sinus openings. Currently, there are no multi-target treatments available, and nothing is discovered yet to open and drain the blocked sinuses. We evaluated the clinical efficacy of a new generation of multi-target, non-irritant, osmotic, safe, polymeric glycerol film for the treatment of rhinosinusitis in children. Methods: A 15-day, randomized, placebo-controlled, double-blind, efficacy and safety clinical study was conducted on 20 children in a test product group and 10 in a saline solution comparator group. The children were aged between 3 years to 16 years old and had rhinosinusitis symptom severity score>25/50 at baseline. 2 nasal sprays-3 nasal sprays were applied 3 times to 4 times per day consequently for 15 days. Effects on rhinorrhoea or congestion, fever, cough, sleep, and facial pain were recorded at baseline, 2 h after 1st treatment, and on days 1 (visit 1), 3 (visit 2), 6 (visit 3), and 15 (visit 4). The need for antibiotics and adverse effects were also noted. Results: The test product as well as saline solution nasal washes reduced rhinosinusitis symptoms, but the reduction was much faster and stronger in the test group compared to the comparator group. Nearly 50% of symptomatic relief was seen within 3 days with test product vs 10 days to 15 days for saline solution and all the patients in the test product group recovered by day 15. Compared to 40% of children in the comparator group, no patients in the test product group required rescue antibiotics therapy. Both products were non-irritant and safe as no adverse effects were observed. Conclusion: In the absence of any treatment to open and drain the sinuses and act on multiple factors involved in children’s rhinosinusitis; using a topical multi-target, instant nose cleaning, non-irritant, topically applicable, and safe filmogen solution can be considered a breakthrough for the treatment.
Keywords
Acute & chronic rhinosinusitis •Children •Clinical trial • Saline •Polymeric osmotic film •Sinus blockage.
Abbreviations
TP=Test Product, CP=Comparator product, RS=Rhinosinusitis, RSSS=Rhinosinusitis Severity Score, NS=Statistically Not Significant
Introduction
Rhinosinusitis (RSS) and Chronic Rhinosinusitis (CRSS), hereafter referred to as Rhinosinusitis (RS), are inflammatory disorders of paranasal sinuses and lining of the Nasal Mucosa (NM), which is a widely prevalent disease affecting more than 14% of adults and children [1]. Although it may sometimes be of bacterial, fungal, or allergic origin, the disease usually starts as a viral infection, following a common cold involving influenza, parainfluenza, rhino, or coronaviruses [2]. Initial intracellular virus growth causes cell death and millions of free virus particles are liberated on the NM surface [3]. These newly generated virus particles now attack new healthy cells and cause widespread NM damage. The opportunist bacteria such as Streptococcus pneumonia, Hemophilus Influenzae, Moraxella Catarrhalis, and Beta-Hemolytic Streptococcus Pyogenes start multiplying in this highly favorable environment [4]. The microorganisms, particularly bacteria and fungi, act symbiotically to protect themselves against all aggressions: the sessile planktonic bacteria adhere to the sinus surface, secrete protective extracellular matrix, and form 3-dimensional biofilm aggregates of microorganisms [5, 6]. The number of these biofilm aggregates increases with time; enter sinus cavities, hide and grow inside the sinuses; damage sinus mucosa, obstruct sinus clearance, increase intra-sinus pressure, cause pain, causing RSS which becomes CRSS if not treated within a few weeks [7-9]. CRSS may be broadly defined as sinus inflammation and infection that lasts 12 weeks or longer [10]. The physiopathology of RSS and CRSS in children shows that it’s a multifactorial disease where only a multi-target treatment can be effective. As the sinus cavities are poorly vascularized, no drug or antibiotic can fully reach up to the sinuses and treatment becomes extremely difficult
Current treatments of RS include single targetoriented medical or surgical therapy, but being a multi-factorial disease, the medical therapy often requires combining multiple medications including antibiotics, topical nasal steroids, and/or oral steroids as well as saline or sea water irrigation to minimize the concentration of surface pathogens and contaminants [11-13]. These treatments are aimed to provide symptomatic relief, reduce the bacterial load, facilitate nasal drainage, minimize secondary infection, or reduce edema or inflammation, but all these treatments cannot be administered simultaneously, particularly in children. Most of these treatments are chemicals, have poor efficacy because they are usually mono-target, and they can damage sinus mucosa as the children’s nasal cavity is the most sensitive organ in the body.
Therefore, to be effective, the basic treatment strategy should be multi-target, directed at protecting and cleaning the nasal mucosa; minimizing the concentration of bacteria, viruses, inflammatory proteins, and other contaminants from the NM surface along with opening the blocked sinuses to drain the contaminants and to reduce intra-sinus pressure [14]. In addition, such a multi-target treatment should also be free of side effects, nonchemical, and non-irritant to allow rapid healing of the damaged nasal mucosa. Only a treatment that physically cleans the NM can achieve these objectives but except for saline or salt solution nasal wash, there is nothing else that can possess these basic requirements [15]. The saline or salt solutions containing up to 3.2% salt act physically and osmotically to clean the NM but their efficacy is low because they are not very osmotic, not stable, and short-acting to continue cleaning the NM over a longer period.
To achieve these objectives of an ideal treatment for children, we employed the technology of exerting anti-inflammatory and antiviral nasal treatment described by Shrivastava et al [16, 17]. For conceiving a glycerol-based osmotic filmogen solution that can be rendered mechanically resistant by incorporating glycerol molecule binding natural polymers. It was postulated that topical NM application of such a solution should form a stable osmotic film to protect the nasal mucosa against irritation. Secondly, being osmotic, the film should attract hypotonic liquid from NM to continue cleaning all the free-floating contaminant particles, including viruses and undesired inflammatory proteins secreted on the NM surface. The thickness, osmotic power, irritation potential, and absorbance capacity of the film were adjusted, and specific natural polymers were used to direct the film for the treatment of rhinosinusitis pathology in children. Such a film would be capable of exerting strong osmotic pressure on the external surface of the nasal sinus-blocking layer and may also help disrupt and break the sinus-blocking film to open and drain the sinuses, thereby relieving intra-sinus pressure and pain.
The clinical efficacy and safety of this nasal polymeric glycerol film (Nesospray) are evaluated in comparison with saline solution for the treatment of RSS and CRSS in children.
Materials and Methods
Clinical study organizer
The study was sponsored by VITROBIO France and was performed by MUDRA CLINCARE, Koparkhairane, Navi Mumbai-400709, India as per the regulations applicable for studies in children. The protocol was approved by relevant ethics committees (Altezza Institutional Ethics Committee, Shree Ashirwad Hospital, Dombivli, and Maharashtra, India) and institutional review boards. Being a pilot clinical trial on a product that is already approved and marketed as a medical device in Europe, CTR was not required. The authors vouch for the conduct of the trial, adherence to the protocol, the accuracy and completeness of the data, and reporting of adverse events. The trial complied with the International Conference on Harmonization Guidelines for Good Clinical Practice, the principles of the Declaration of Helsinki, and relevant national and local regulations. At the time of screening, the children’s parent(s) signed written informed consent forms. The sponsor provided the trial medication (ISO 13485 certified) and supplied relevant investigation product information.
Test and comparator products
The Test Product (TP), designated as Nesospray-Kid, commercialized in Europe as a medical device, was supplied by Vitrobio Pharma in France (ISO13485 certified) in 15ml plastic containers fitted with a spray for nasal application (± 125 sprays: 120 µl/ spray). The solution contained osmotically active filmogen glycerol as described by Shrivastava et al which was rendered filmogen by adding small quantities of rhinocyanidin-e polymeric-premix derived from the fruit extracts of Vaccinium macrocarpon, Vaccinium myrtillus, Sambucus nigra and Ribes nigrum, HPC (Hydroxypropyl Cellulose) as a thickener, and Potassium sorbate, Sodium benzoate, and Citric acid as stabilizers [18, 19].
Study design and rationales
The study was designed as a comparative, randomized, double-blind, parallel-group, observational clinical trial to evaluate the efficacy and safety of Nesospray-Kid (TP) against 0.9% NaCl saline solution as a Comparator Product (CP), for the treatment of acute or chronic rhinosinusitis in children. 3 sprays to 4 sprays were applied on the nasal surface of 20 children in the TP group and 10 in the CP group, every 30 min at the beginning of the treatment during the first 2 h, and 3 times to 4 times per day afterward up to 15 days. Saline solution (0.9%) was selected as a comparator product based on its close characteristic similarity with the TP. The doses were selected for children (0.4 ml-0.6 ml /application) based on the doses used in adults for similar treatment, the surface area of the nasal cavity in children, the duration of action of the product film, and the mean quantity of a liquid substance required to form a film over the nasal mucosa.
The rationale for selecting children aged between 3 years to 16 years and the duration of the study:
Nesospray-Kid is directed for the treatment of RSS and CRSS in children. Due to difficulties in applying the product spray and obtaining reliable replies in children below the age of three, it was decided to conduct the study in the 3 years to 16 years age group population. As most of the time, rhinosinusitis symptoms in children disappear progressively within about 2 weeks, the duration of the study was kept for 15 days.
Main inclusion criteria: Children, aged between 3 years to 16 years with cooperative and understanding skills, accompanied by at least 1 parent or caretaker, and having clinical manifestations of RSS or CRSS with strong rhinosinusitis symptoms (scored on a scale of 0 to 10 for 5 key symptoms: rhinorrhoea or congestion, fever, cough, lack of good sleep, pain upon facial pressure), with a mean Rhino-sinusitis Severity Score (RSSS)=or>25 on a maximum score of 50. Patients/Parents are ready to abstain from using any drug which may affect the study outcome except in cases when the patient’s condition worsens, other treatments could be allowed upon physicians' recommendations. Enrolled patients accepted not to use facial masks, nasal sprays or lavages, eye drops, and any other local or other herbal or homeopathic treatment during the study period. Both parents and caretakers gave written consent to participate in the study.
The main exclusion criteria: Children below the age of 3 or above the age of 16, having hypersensitivity/ history of allergy to any of the investigational product’s components and unwilling to participate in the study; patients with the abnormal nasal passage, polyps, recent nasal surgery, bronchopneumonia, chronic allergy, clinical evidence of immunosuppression, and RS of known fungal or allergic origin. Patients treated with any other medications for rhinosinusitis including antibacterial/antiviral treatment, antihistamine, and steroids within 2 weeks before screening.
Randomization
After screening, patients satisfying all the inclusion criteria were enrolled and randomly allocated in a 2:1 ratio as per the randomization schedule. Treatments were allocated to patients by carrying out randomization using SAS Version 9.1.3 following a randomization schedule. Block Randomization methodology was employed for generating the list.
Parameters recorded and study endpoints
Primary study parameters included rhinosinusitis severity symptom scores consisting of rhinorrhoea or congestion, fever, cough, lack of good sleep, and pain upon facial pressure at baseline, 2 h after 1st treatment, and on days 1 (visit 1), 3 (visit 2), 6 (visit 3), and 15 (visit 4) after the start of treatment.
Secondary study parameters comprised all adverse events, eventual need for antibiotics or other medications as well as global assessment with product acceptability by patient/parents and physician
Statistical analysis
Statistical analysis was performed by unpaired two-tailed Student’s test, for comparisons between two groups and the one-way or two-way ANOVA followed by the post hoc Bonferroni’s test for comparisons of multiple groups. p<0.1 was considered statistically significant (GraphPad Prism version 8.4.2, La Jolla, USA).
Results
Demographics
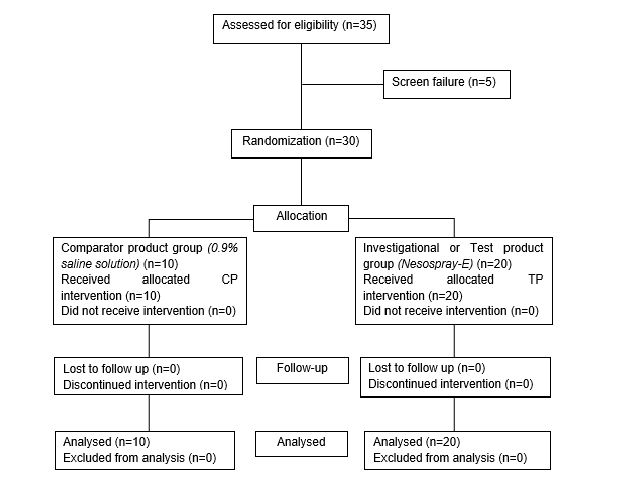
As shown in the Consort chart, 22 boys and 13 girls (total of 35) between the age group of 3 to 16 years, with a mean age of boys and girls between 8.33 to 7.77 years respectively, confirming all the inclusion and exclusion criteria, were enrolled in the study. Among these patients, 13 boys and 07 girls (n=20) were allocated to the TP group and 08 boys & 02 girls, to the CP group.
There were relatively more girls in the TP vs CP group but as rhinosinusitis physiopathology remains identical in boys and girls, it was assumed that distribution has a negligible effect on the study parameters and therefore, the distribution pattern was not modified (Figure 1).
Effect on rhinorrhoea/ nasal congestion
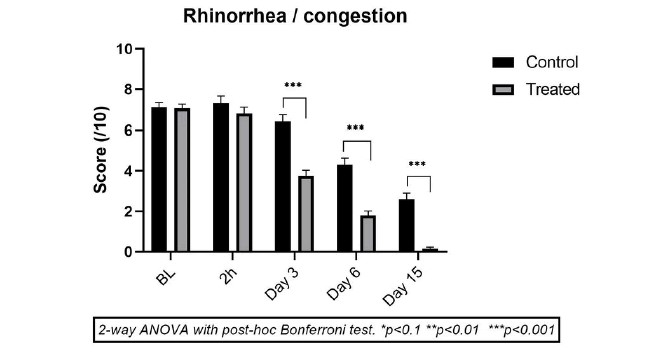
The mean scores of rhinorrhoea &/or nasal congestion at baseline were close in both the CP (7.10 ± 0.74) and TP (7.05 ± 0.95) groups, indicating homogeneity of symptomatic manifestation of RS in both groups. 2h after 1st treatment, there was not much change in mean scores in both groups (between 6.8 ± 1.39 and 7.30 ± 1.16, NS vs baseline) showing that no treatment provides instant relief. In the CP, the mean rhinorrhoea/nasal congestion score also did not change significantly until day 3 (mean score 6.40 ± 1.07; -9.33% vs baseline, NS) but started decreasing progressively on day 6 (4.3 ± 1.06; -37.3% vs baseline, p<0.001) and diminished significantly on day 15 (2.60 ± 0.97; 60.0% vs baseline, p<0.001). These results demonstrate that saline solution nasal wash offers symptomatic relief but the effects are slow and progressive, without complete relief up to day 15.
In the TP group (n=20) there was no specific improvement in rhinosinusitis symptoms within 1st 2h of treatment but a strong reduction vs baseline was observed on day 3 (-49.3%; p<0.001). Thereafter, the reduction was near -76.0% (p<0.001) on day 6, and complete recovery was observed in almost all the children on day 15 (mean reduction of -98.0%; p<0.001 vs baseline). The difference in efficacy between the two groups is statistically significant (p<0.001) in favor of TP from day 3 onwards (Figure 2).
These results indicate that although regular nasal wash with saline solution slightly improves rhinosinusitis symptoms, the CP treatment doesn’t offer complete healing within 15 days of treatment The mean residual score on day 15 in this group was 2.60 ± 0.97 vs only 0.15 ± 0.36 in the TP group at the end. The TP treatment already achieved a mean score of 1.8 ± 1.0 on day 6, a score which is even lower compared to the score with CP on day 15. It can be concluded that the TP treatment is twice more effective compared to CP in reducing rhinosinusitis-induced rhinorrhoea &/or nasal congestion in children.
Effect on cough
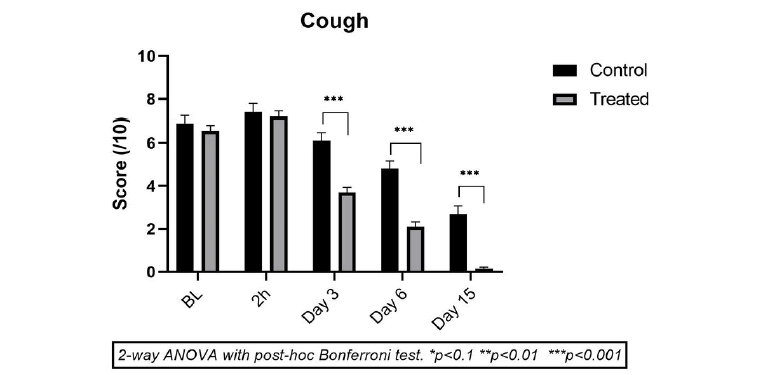
Coughing is a very common symptom of RSS/ CRSS and one of the most important factors for disturbing the quality of life in children. At the start of the treatment, baseline cough severity scores were relatively high in both groups (6.90 ± 1.10 in CP vs 6.55 ± 1.19 in TP). A very slight increase in cough intensity was noticed 2 h after the 1st drug administration in both groups (NS), showing that none of the products have an instant anti-coughing property. Thereafter, compared to baseline, a progressive but very slight reduction in the mean cough severity was observed in the CP group on day 3 (-12.2%, p<0.001), which progressed further on day 6 (-32.06%, p<0.001) up to day 15 (-64, 1% p<0.001) (Figure 3).
TP was highly effective in suppressing child cough symptoms compared to CP as the mean reductions in coughing compared to baseline were very strong on day 3 (-43.5%, p<0.001), which progressed further until day 6 (-67.94%, p<0.001), and day 15 (-97.7%, p<0.001). These results indicate that TP is much more active in stopping coughing in children compared to CP and the cough-suppressing properties of TP are very fast.
Effect on sleep
Unrest and bad sleep during RSS or CRSS have a strong impact on sleep, the most important quality of life parameter for children as well as for parents.
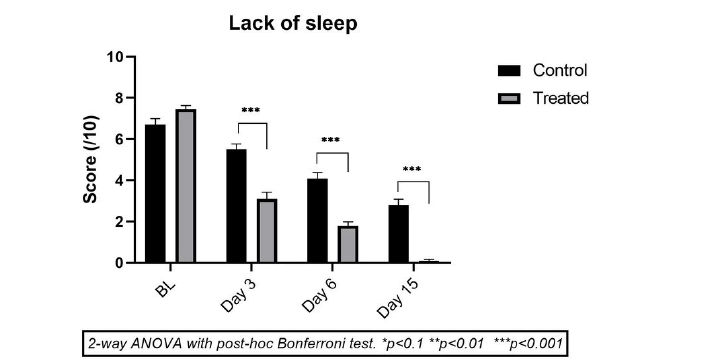
Treatment with saline solution CP was relatively effective as the mean improvement in sleep parameters was 16.10%, 34.9%, and 52.35% after 3, 6, and 15 days of treatment (p<0.001 on vs baseline on days 6 and 15). This improvement looks good but is probably not very pertinent as the rhinosinusitis symptoms naturally fade with time and complete recovery may be expected within 15 days to 20 days.
In the TP group children, a drastic improvement in sleep parameters was noticed, as early as day 3, with a mean improvement of 58.4% (p<0.001 vs baseline). This improvement on day 3 is even better than the sleep enhancement effect of CP on day 15 (Figure 4). The mean improvement in night rest with TP on day 6 was 75.8% (p<0.001) and no child in this group complained about bad sleep after 1 week to 2 weeks of treatment. When compared with the CP group, the difference in sleep improvement in the TP group is statistically significant (p<0.00) from day 3 onwards. These results confirm the concomitant improvement of other RSS symptoms observed in children, which allows for better sleep.
Figure 4: Mean scores (± SD) for Effect on sleep in TP (light grey) vs. CP group (dark grey) just before treatment (baseline T0) and on Day 1, Day 3, Day 6 and Day 15. *p<0.1, **p<0.01, ***p<0.001 for TP compared to CP at the same time point. Sleep disturbances were dissipated much faster in TP vs CP group.
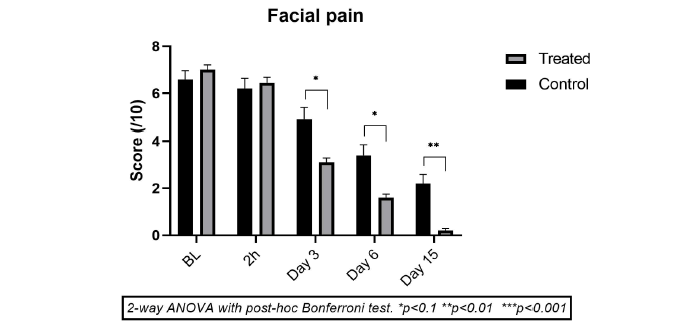
Effect on facial pain
In RSS and CRSS, the cause of facial pain is blockage of sinus drainage leading to increased intra-sinuses pressure, which develops inflammation and pain in the front part of the face and around the nose and may lead to chronic sinusitis if acute rhinosinusitis is not treated.
The mean facial pain intensity score in the CP group reduced progressively from day 3 (-24.3%, p<0.003), and improved further on day 6 (-45.7%, p<0.001) and day 15 (-62.9%, p<0.001) compared to baseline score (Figure 5).
Figure 1: Mean scores (± SD) for effect on facial pain intensity in test product group (light grey) vs. Comparator Product group (dark grey) just before treatment (baseline T0) and on Day 1 (2 h after 1st application), Day 3, Day 6 and Day 15. *p<0.1, **p<0.01, ***p<0.001 for TP compared to CP at the same time point.
Whereas in the TP group, compared to baseline values, the facial pain had subsided strongly and significantly on day 3 (-55.7%, p<0.001) with further decrease on day 6 (-77.1%, p<0.001) and day 15 (-97.1%, p<0.001). The reduction in facial pain is only slightly better in TP vs CP (p<0.1 up to day 6 and p<0.01 on day 15).
Being an osmotically active, strongly hypertonic solution, the TP probably exerts osmotic pressure over the membrane blocking the sinuses and helping in membrane disintegration, and sinus drainage thereby reducing intra-sinus pressure and consequently sinus pain.
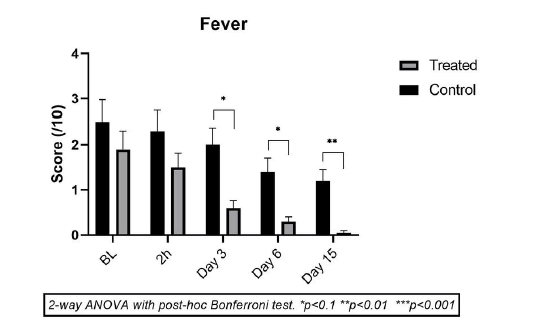
Effect on fever
In the CP group, 8/10 children had some fever at the start of the study. The maximum score of fever was 4/10 in 3 children, 3/10 in 3 children, and 2/10 in 2 children. The number and the intensity of fever in these children remained nearly the same up to day 3. Only 4/10 children had mild to moderate fever by day 6 and 3/10 by day 15. In the TP group, the number of children having mild to moderate fever was 12/20 at the start, which reduced progressively to 9/20 on day 3, 6/20 on day 6, and 1/20 on day 15 (Figure 6). The fever reduction is better in the TP group and follows an overall reduction in RSS observed in this group.
Figure 6: Mean scores (± SD) for Effect on Fever Intensity in Test Product group (light grey) vs. Comparator Product group (dark grey) just before treatment (baseline T0) and on Day 1 (2 h after 1st application), Day 3, Day 6 and Day 15. *p<0.1, **p<0.01, ***p<0.001 for TP compared to CP at the same time point
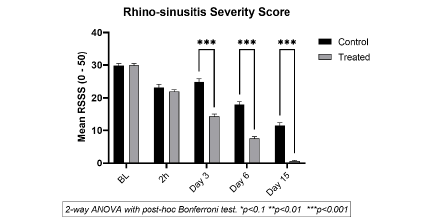
Mean Rhinosinusitis Severity Symptom Score (RSSS)
The RSSS is calculated on a scale of 0 to 10, on 5 key RSS parameters and averaged at each time point. Comparing mean scores of CP vs TP shows the difference in RSS symptom reduction, whether statistically significant or not, between the groups. The results in Figure 7 clearly show that the effects of TP are much faster with a statistically significant improvement in TP vs CP, just 3 days after the start of treatment. Although CP treatment helps minimize RSS symptoms in children, progressively over 15 days, TP efficacy is much faster and stronger than CP and all the patients in the TP group recovered completely after 15 days of treatment in TP.
Figure 7: Mean Rhinosinusitis severity score ( ± SD) bases on the effects on rhinorrhoea, nasal congestion, cough, facial pain, effect on sleep and fever, indicative of the quality of life of the patients in the TP (light grey) vs. CP (dark grey) group at each time point for TP compared to CP. Note a gradual reduction in RSSS in both groups where the effects of TP are statistically significant (p<0.001 vs CP) from day 3 onwards
Effect on the need for antibiotics
For ethical reasons, investigators were authorized to use antibiotics if the patient’s condition does not improve or worsen during the study duration. In the CP group, 4/10 children were given short-term antibiotics but in the TP group, no child required antibiotic treatment as their recovery was stronger and faster compared to CP treatment. These results indicate that the TP helps minimize the need for the use of antibiotics in children suffering from RSS.
Product efficacy, adverse effects, and safety profile
The TP showed much better efficacy on all the rhinosinusitis parameters evaluated in this study which was nearly twice as better as compared to CP treatment in reducing the symptomatic manifestation of the disease without the use of other concomitant drugs.
Safety was assessed by evaluating the number of patients reporting incidences of Adverse Events (AE) and/or Serious Adverse Events (SAE) arising during the study and their assessment concerning the intensity, duration, pattern, and causal relationship to the investigational product. There were no adverse effects observed in both the TP or CP groups during the study. The TP was well tolerated and there were no significant or test product-related changes in vital signs, physical examination, or systemic parameters. The absence of any effects on the systemic parameters, even after 15 days of consecutive use of the products, proves that they are not absorbed, do not interact with cell surface receptors, and have no pharmacological, metabolic, or immunological interactions with the body cells. Both products are therefore considered safe for topical application as a nasal spray for the treatment of RSS &/or CRSS, however, the TP is much more effective, fast acting, and as safe as the saline solution.
Discussion
Children have on average 6 episodes to 8 episodes of colds per year and 0.5% to 5% of these develop into rhinosinusitis which can be classified according to the duration of symptoms in acute, up to four weeks: subacute, from 4 weeks to 12 weeks, and chronic, greater than 12 weeks. There is also recurrent acute rhinosinusitis in which each episode lasts less than 30 days. Rhinitis evolves towards sinusitis when the infection enters the nasal sinuses which usually becomes chronic as sinus infections are extremely difficult to dislodge [2, 20].
RSS and CRSS are multifactorial diseases because they involve viral infection, secondary bacterial infection, NM and sinus inflammation, NM damage, secretion of multiple pro-inflammatory cytokines on the NM, and corresponding symptomatic manifestations such as rhinorrhea and/or nasal congestion, cough, pain, fever, and sleep disturbances [21]. To curb RSS rapidly, as per the stage of symptom development, only a specific treatment for each symptom or a multi-target treatment that acts on different factors simultaneously can help ease the disease rapidly [22].
Recent findings prove that bacterial biofilm, which obstructs sinus openings in as many as 75% of CRS patients, is the main cause of persistent rhinosinusitis [23, 24]. Microorganisms enter the sinuses, grow and protect themselves by forming a biofilm at the sinus opening, which contains a specialized community of adherent microorganisms surrounded by an extracellular polymeric substance. No drug can reach therapeutic or antimicrobial concentrations inside the sinuses because sinuses are empty and poorly vascularized cavities. It is also not easy to disintegrate sinus-blocking biofilm because the film gets stronger with time, is resistant to any topical or systemic treatment, and cannot be removed except by using a mechanical cleaner. Unfortunately, no mechanical biofilm disintegrator has yet been discovered and therefore, there is no treatment to open and drain the sinuses [7]. All current and even emerging treatment options are intended to ease one or two clinical symptoms, often in association with other therapies including saline nasal washes, topical or oral antibiotics, nasal decongestants, steroids, and anti-inflammatory drugs [11]. New emerging options include antimycotics, anti-IgE, anti-IL5, new antihistamines, complementary medicine, immunosuppressant medications, leukotriene inhibitors, phytotherapy, probiotics, and proton pump inhibitors [15-21]. Despite the abundance of treatments available, one wonders why none of them is working and why, even today, we don’t have a cure for RSS and CRSS. In theory, it would be sufficient to open and drain the sinuses and keep the NM clean, which should reduce inflammation, and pain, as well as rebuilt NM, to enhance natural defense. In the absence of any treatment, saline solution, used as a nasal wash, is still considered one of the best and safest treatments to get some symptomatic relief as saline solution is safe and has a slightly positive benefit/risk ratio [22, 23]. Regular and frequent nasal washes help clean the NM, improving respiration and reducing NM surface microbial load, thereby alleviating CRS symptoms but saline wash does not affect pressurized, inflamed, and infected sinuses [2, 3].
In this study, when the saline solution was used as a comparator product for 15 consecutive days, it provided reasonably good symptomatic relief by minimizing rhinorrhea, nasal congestion, cough, and facial pain but the improvement was limited to a maximum of 50% of baseline values and the disease was not alleviated. This is comprehensible as saline solution is poorly osmotic, cannot disrupt the biofilm, and ease intra-sinus pressure [25].
An effective treatment should be multi-target, safe, non-irritant, chemical free, and should be capable of exerting enough positive osmotic pressure over sinus-blocking biofilm membranes to disrupt the membrane and drain the sinus contents. Additional antiseptic, antibiotic, &/or anti-inflammatory activities would be excellent.
The results of this study show that NesosprayKid spray possesses all the above-mentioned key properties, essential for an effective RSS or CRSS treatment. Glycerol is a highly osmotic and cellfriendly solution but an irritant to the NM and easily degradable with the nasal flow. As certain specific polymeric tannins bind to glycerol molecules and render glycerol film open with increased resistance to dilution and improved duration of action, we rendered the glycerol film Rogen and stable by incorporating selected glycerol molecule binding natural polymers [19]. Furthermore, the irritation potential of glycerol was reduced by adding specific jellifying absorbent ingredients. Tannins are large, inert, and highly branched molecules which do not interact with the cellular structures, are noncytotoxic even at higher concentrations, and are easily expelled with the hypotonic liquid flow once the glycerol film is disintegrated after 4 h to 6 h. In this study, the osmotic glycerol film was applied directly onto the nasal mucosa for 15 consecutive days but most of the key symptoms of RSS &/or CRSS were reduced by about 50% just within 3-days and by 80%-90% between 10 days to 15 days. This quick efficacy is attributable to the formation of a stable osmotic film on the nasal mucosa and on the nasal sinus openings. During a period of 4 h to 6 h, the film continuously exerts osmotic pressure and attracts hypotonic liquid from the inner parts of the NM, thereby removing all the free-floating contaminants and inflammatory cytokine particles towards the film where they are trapped. Concomitantly, constant positive pressure on the sinus opening, hydrates and breaks the sinus-blocking biofilm, thereby opening and draining the sinus contents and relieving intrasinus pressure. This physical action of cleaning the NM and sinuses helps reduce NM inflammation, open sinuses, reduce pain, and reconstitute the natural NM barrier. There were no undesired effects or changes in any of the systemic parameters that were noticed during the trial, proving solely the topical mode of action of TP. This polymeric osmotic filmogen technology has already been used for the treatment of other multifactorial topical diseases involving tissue damage, lesion contamination, multiple disease specific and inflammatory cytokines, and inflammation. This technology was found highly effective and safe for the treatment of chemotherapy-induced oral mucositis, severe dry cough, external and internal hemorrhoids, and even early-stage COVID-19 infection, while research on the use of polymeric film for the prevention of asthma and migraine is in progress [26-28].
It should be noted that cleaning the nasal sinuses is required before treating RSS and CRSS, but there was no completely safe mechanical, chemical, biological, or physiological treatment available that could provide nearly instant relief until now. Several efforts have been made (application of N-acetylcysteine, antibiotics, steroids, and sea water, saline with betamethasone, to find an efficient RSS treatment, but without success [29-33].
Conclusion
hinosinusitis in children is an acute or chronic multifactorial disease involving not only viral and/or bacterial infection but also inflamed and damaged nasal mucosa and blocked sinus passages, which are responsible for severe facial pain. Because no treatment can act on all of the factors at the same time, multiple chemical treatments with varying side effects are currently used to treat this disease. The clinical efficacy and safety results presented in this paper show that using an external and topical approach of keeping the nasal surface clean and opening the sinuses with osmotic pressure, represents a highly effective, multi-target and safe approach to treating rhinosinusitis in children.
Acknowledgements
The design and conduct of the trial and data analysis were supported by a grant from VITROBIO & NATURVEDA SAS (ZAC de Lavaur 63500 Issoire, France), the inventor of MIG SPRAY.
We thank the patients who participated in this trial and their families; all investigators, site personnel and coordinating investigators. We also strongly thank Dr. Xavier MOISSET (CHU Clermont-Ferrand, France) for his precious help in writing the article and Dr. Christelle GREMEAU-RICHARD (Neurodol INSERM 1107, France) for her corrections to the protocol.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
References
- Shahid SK. Rhinosinusitis in children. ISRN Otolaryngol. (2012).
[Google Scholar] [Crossref]
- Bachert C, Holtappels G. Pathophysiology of chronic rhinosinusitis, pharmaceutical therapy options. GMS Curr Top Otorhinolaryngol Head Neck Surg. 14:(2015)
[Google Scholar] [Crossref] [Crossref]
- Shrivastava R. A new therapeutic approach to neutralize throat surface proteases and virus glycoproteins simultaneously for the treatment of influenza virus infection. Int J Virol. 7(2):53-63(2011).
[Google Scholar] [Crossref]
- Tomassen P, Van Zele T, Zhang N, et al. Pathophysiology of chronic rhinosinusitis. Proc Am Thorac Soc. 8(1):115-20(2011).
[Google Scholar] [Crossref]
- Dlugaszewska J, Leszczynska M, Lenkowski M, et al. The pathophysiological role of bacterial biofilms in chronic sinusitis. Eur Arch Otorhinolaryngol. 273:1989-94(2016).
[Google Scholar] [Crossref]
- Ferguson BJ, Stolz DB. Demonstration of biofilm in human bacterial CRS. Am J Rhinol. 19(5):452-57(2005).
- Sharma GK, Lofgren DH, Taliaferro HG. Recurrent Acute Rhinosinusitis. StatPearls Publ. (2022).
[Google Scholar] [Crossref]
- Rezende CEB, Pasqual IM, Hiroshima LT, et al. Chronic rhinosinusitis in children: therapeutical update. J Otolaryngol ENT Res. 10(4):224â29(2018).
- Shariati A, Vesal S, Khoshbayan A, et al. Novel strategies for inhibition of bacterial biofilm in chronic rhinosinusitis. J Appl Microbiol. 132(4):2531-46(2022).
- Heath J, Hartzell L, Putt C, et al. Chronic rhinosinusitis in Children: Pathophysiology, Evaluation, and Medical Management. Curr Allergy Asthma Rep. 18(7):37(2018).
[Google Scholar] [Crossref]
- Badr DT, Gaffin JM, Phipatanakul W. Pediatric rhinosinusitis. Curr Treat Options Allergy. 3(3):268-81(2016).
[Google Scholar] [Crossref]
- Torretta S, Drago L, Marchisio P, et al. Review of Systemic antibiotic treatments in children with rhinosinusitis. J Clin Med. 8(8):1162(2019).
- Hitsuthipakorn W, Kanjanawasee D, Hoang MP, et al. Optimal device and Regimen of nasal saline treatment for Sinonasal Diseases: Systematic review. OTO Open. 6(2):1-17(2022).
- Ge M, Liu DH, Ference EH. Pediatric chronic sinusitis: diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 30(1):68-77(2022).
- Cai S, Xu S, Lou H, et al. Comparison of different biologics for treating chronic rhinosinusitis with nasal Polyps: A network analysis. J Allergy Clin Immunol Pract. 10(7):1876-86(2022).
- Shrivastava RM, Shrivastava R, Johansen B, et al. Anti-Inflammatory and antiviral osmotic polymeric film to treat covid-19 early-stage infection. J Inflamm Res. 14:1195-1206(2021).
- Shrivastava RM, Maneby N, Girgoir G et al. Conception and clinical efficacy of an osmotic, polymeric, stable nasal filmogen barrier for preventive treatment of allergen and pollution induced rhinitis and respiratory symptoms. J Clinical Research and Reports. 10(1) (2022).
- Shrivastava R. Non-solid composition for local application. Int. publ. (2000).
[Google Scholar] [Crossref]
- Shrivastava RM, Shrivastava R. A filmogen glycerol for topical application. Int. PCT pat PCT. (2014)
[Google Scholar] [Crossref]
- Dieck LQ, Lam DJ. Chronic rhinosinusitis in Children. Curr Treat Options diatr. 4(4):413-24(2018).
[Google Scholar] [Crossref]
- Lee S, Lane AP. Chronic rhinosinusitis as a multifactorial inflammatory disorder. Curr Infect Dis Rep. 13(2):159-68(2011).
[Google Scholar] [Crossref]
- Richards N, Tiedeken SD, Chang CC. Medical Management of Acute Rhinosinusitis in Children and Adults. Dis Sinuses. 359-71(2013).
[Google Scholar] [Crossref]
- Singh P, Mehta R, Agarwal S, et al. Bacterial biofilm on the sinus mucosa of healthy subjects and patients with chronic rhinosinusitis (with or without nasal polyposis). J Laryngol Otol. 129(1):46-9(2015).
- Bhattacharyya N. Contemporary assessment of the disease burden of sinusitis. Am J Rhinol Allergy. 23(4):392-5(2009).
- Liu L, Pan M, Li Y, et al. Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis. Braz J Otorhinolaryngol. 86(5):639-46(2020).
- Shrivastava R, Deshmukh S.A new therapeutic approach to treat oral mucositis using specific MMP blockers in an osmotically active solution. J Cancer Res & Treatment. 1(1):4-11(2013).
- Shrivastava R, Carrois F, Pisak M, et al. Clinical efficacy of novel filmogen, antimicrobial, cleaning, fluidizing cough treatment. J Clin Trials. 7(4):1-8(2017).
- Georges M, Trouiller R, Shrivastava R, et al. Clinical efficacy of a new generation of multi-target, anti-edematous, anti-inflammatory, tissue repairing topical polymeric liquid bandage for the treatment of internal hemorrhoids. J Clin Exp Dermatol Res. 8(4)1-9(2017).
- Wang YH, Yang CP, Ku MS, et al. Efficacy of nasal irrigation in the treatment of acute sinusitis in children. Int J Pediatr Otorhinolaryngol. 73(12):1696-701(2009).
- Bahtouee M, Monavarsadegh G, Ahmadipour M, et al. Acetylcysteine in the treatment of subacute sinusitis: A double-blind placebo-controlled clinical trial. Ear Nose Throat J. 96(1):7-11(2017).
- Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 10(1)(2020).
[Google Scholar] [Crossref]
- Culig J, Leppée M, Vceva A, et al. Efficiency of hypertonic and isotonic seawater solutions in chronic rhinosinusitis. Med Glas. 7(2):116-23(2010).
[Google Scholar] [Crossref]
- Dawson B, Gutteridge I, Cervin A, et al. The effects of nasal lavage with betamethasone cream post-endoscopic sinus surgery: clinical trial. J Laryngol Otol. 132(2):143-9(2018).
[Google Scholar] [Crossref]