Research Article - Journal of Experimental Stroke & Translational Medicine (2011) Volume 4, Issue 1
Transcutaneous Therapeutic Ultrasound Reduces Infarct Size in a Rabbit Model of Acute Insoluble Ischemic Stroke
- *Corresponding Author:
- Dr. William C. Culp
Department of Radiology
University of Arkansas for Medical Sciences
4301 W. Markham St., Slot 556
Little Rock, AR 72205-7199
Tel: (501) 686-6910
Fax: (501) 686-6900
E-mail: culpwilliamc@uams.edu
Abstract
Low-frequency ultrasound (US) enhances muscle and myocardial perfusion without lysis of the arterial obstruc-tion. We evaluated transcutaneous therapeutic ultrasound with tissue plasminogen activator (tPA) for decreas-es in cerebral infarct volume in rabbits with insoluble arterial occlusions. We hypothesized that US with tPA is effective at reducing infarcts without lysis of the arterial obstruction. New Zealand White rabbits (n=26; 5.2±0.08 kg) received angiography and three 700 to 900 μm embolic spheres were injected into the internal carotid artery occluding its branches. Treatment groups: control (n=10; embolized only), tPA alone (n=7), and tPA+US (n=9). Rabbits receiving US received pulsed-wave US (1-MHz, 0.8 W/cm2) over 1 hour. Rabbits administered tPA re-ceived intravenous tPA (0.9 mg/kg) over 1 hour. Rabbits were sacrificed 24 hours later and infarct volume was determined following staining with triphenyltetrazolium chloride. Immediate collateral flow was determined with digital subtraction angiography. Percent infarct volume was greater (P=0.035) for control (3.4±1.0%) than for tPA+US (0.9±1.0%) rabbits. Rabbits treated with tPA alone (2.0±1.1%) were intermediate and did not differ (P>0.29) from control and tPA+US rabbits. Collateral flow did not (P=0.93) influence infarct volume. Treatment with tPA+US decreases brain infarct volume in rabbits with permanent arterial occlusions, supporting flow stu-dies in muscle or myocardium.
Keywords
Collateral flow; ischemic stroke; sonothrombolysis; ultrasound;, tPA
Introduction
Ultrasound (US)-facilitated thrombolysis, or sonoth-rombolysis, with tissue plasminogen activator (tPA) and 2-MHz transcranial Doppler ultrasound improves arterial recanalization in patients with acute ischemic stroke (Alexandrov et al. 2004). Animal evidence indicates that low-frequency US improves tissue per-fusion in the rabbit ischemic limb (40 kHz US) and enhances myocardial perfusion in dogs (27 kHz US), even without removing the arterial obstruction (Such-kova et al. 2000; Suchkova et al. 2002; Siegel et al. 2004). As emboli occlude vessels fresh clot forms in stagnant blood upstream and downstream to the point of continued flow. Upstream this is usually to the first major branch that remains open and down-stream it is potentially very extensive and continues until collateral flow is encountered. The mechanism of action of improved tissue perfusion due to US ad-ministration is not clearly understood but may involve redistribution of collateral flow to the area of ischemia and nitric oxide dependent mechanisms operating at the level of very small vessels (Suchkova et al. 2000; Suchkova et al. 2002). Potential novel therapeutic strategies that improve brain tissue perfusion inde-pendent of recanalization would be beneficial in the treatment of acute ischemic stroke, thereby decreas-ing morbidity associated with stroke due to insoluble occlusions such as embolic atheroma.
Although great emphasis has been given to evaluat-ing the effectiveness of combined tPA and US admin-istration on the lysing of freshly formed clot, under-standing the effects of sonothrombolysis with tPA on brain infarct volume following embolization with inso-luble emboli is limited (Behrens et al. 2001). Since atheromata, very common embolic agents in ischem-ic stroke, are insoluble, this has great clinical impact. Perhaps direct effects of ultrasound on collateral ves-sels with increased dilatation and increased flow into ischemic areas are responsible here. In order to faci-litate selection and management strategies of treat-ment protocols utilizing tPA and US, a better under-standing of sonothrombolysis therapy with tPA with respect to insoluble arterial occlusions is needed. Using a rabbit model of acute insoluble ischemic stroke, the objectives of this study were to (1) investi-gate the effect of US-facilitated thrombolysis with tPA on infarct volume in rabbits with insoluble arterial oc-clusions and (2) evaluate the influence of immediate collateral flow on infarct volume using digital subtrac-tion angiography (DSA). The rabbit model of stroke was used because seminal discoveries of the use of tPA which ushered in its use in clinical medicine were made using this model.
Material and Methods
Animals and Surgical Procedures
All animal procedures were approved by the Institu-tional Animal Care and Use Committee. New Zeal-and White rabbits (n=26; mean body weight 5.2±0.08 kg) were used.
The surgical and angiographic procedures were pre-viously described (Culp et al. 2007). Rabbits were sedated with an intramuscular injection of ketamine, 30 mg/kg (Ketaset; Fort Dodge; Fort Dodge, IA) and xylazine, 3 mg/kg (AnaSed; Lloyd Laboratories; She-nandoah, IA) and anesthetized with isoflurane (No-vaplus; Hospira Inc.; Lake Forrest, IL). A femoral artery was exposed, and utilizing a 3F vascular sheath, a modified 65-cm angled-tip 3F catheter (SlipCath; Cook Inc.; Bloomington, IN) was placed into the artery. The 3F catheter was advanced over a 0.025-inch angled-tip guide wire (Glidewire; Terumo Medical Corp.; Elkton, MD) under fluoroscopy to the common carotid artery and subsequently into the in-ternal carotid artery (ICA).
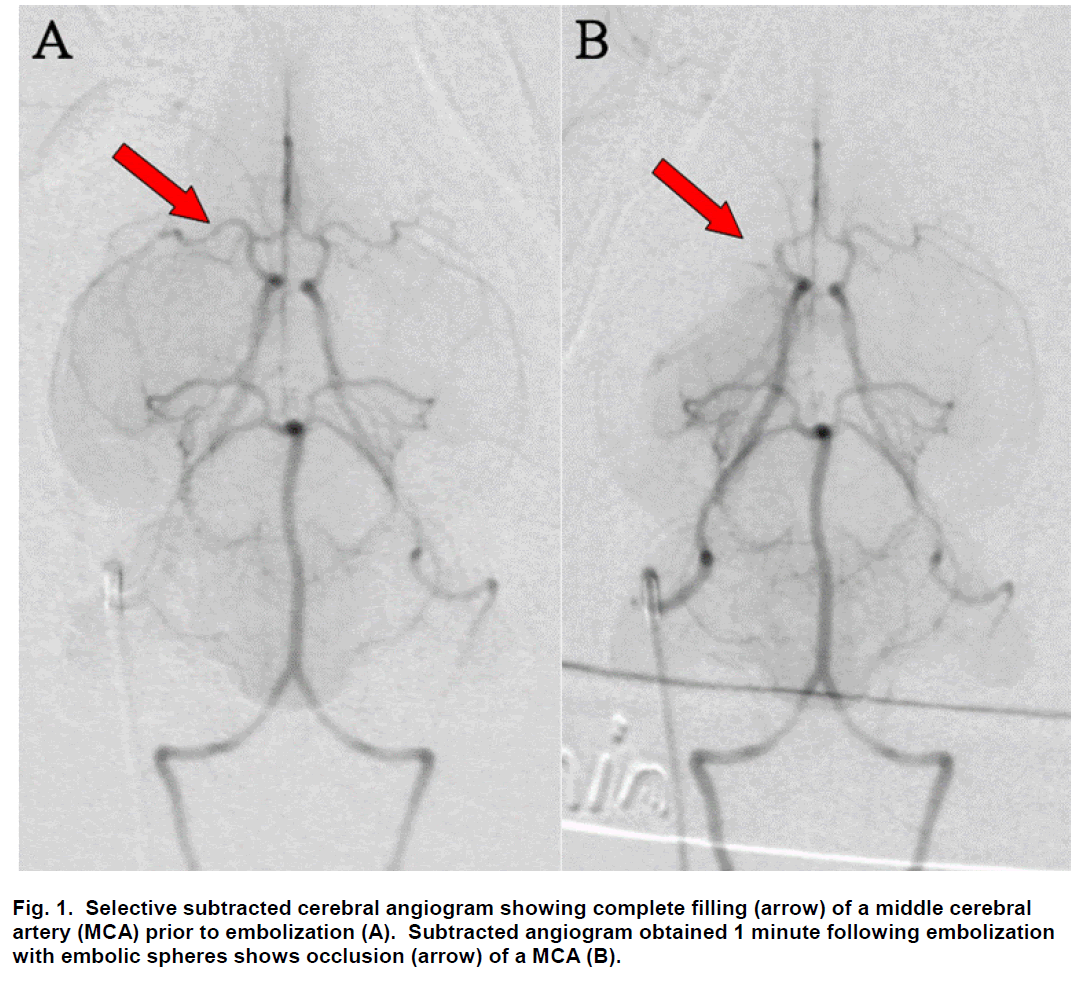
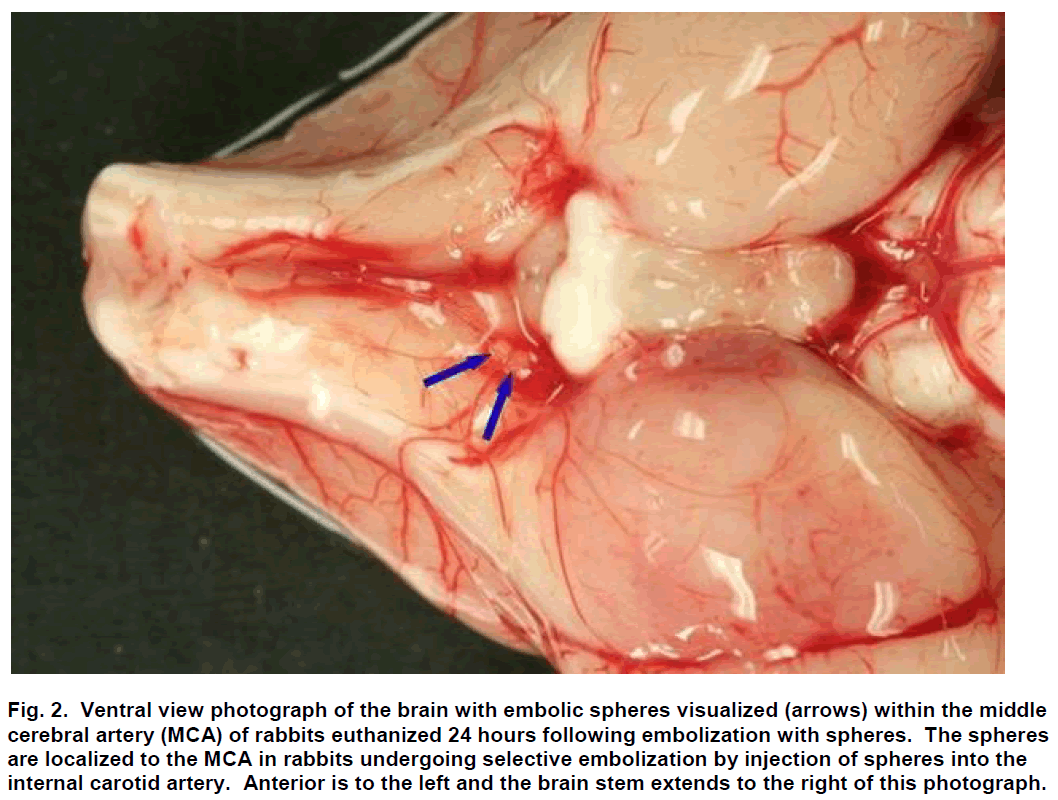
Sub-selective ICA magnification angiography was performed in lateral and frontal projections to docu-ment the cerebral vasculature prior to embolization (Figure 1A). Embolization was accomplished by injec-tion of three 700 to 900 μm embolic spheres (Em-bosphere Microspheres; BioSphere Medical Inc.; Rockland, MA) with 0.7 to 2.0 mL of saline into the ICA. Flow usually carried the embolic spheres to the middle cerebral artery (MCA; Figure 2). One minute following embolization, repeat angiography was per-formed and the location of the arterial occlusion was recorded (Figure 1B) using a single plane C-arm DSA machine (OEC 9800; GE Healthcare; Salt Lake City, UT). Additional repeat angiography was performed 30 minutes following embolization.
Figure 2: Ventral view photograph of the brain with embolic spheres visualized (arrows) within the middle cerebral artery (MCA) of rabbits euthanized 24 hours following embolization with spheres. The spheres are localized to the MCA in rabbits undergoing selective embolization by injection of spheres into the internal carotid artery. Anterior is to the left and the brain stem extends to the right of this photograph.
The images obtained from angiography were eva-luated for the presence or absence of immediate col-lateral flow. Collateral flow pathways that were considered were (1) collateral flow through the anterior communicating artery, resulting in retrograde flow in the occluded side toward the affected MCA and (2) posterior to anterior flow through the ipsilateral post-erior communicating artery and posterior cerebral artery.
One hour following embolization and angiography, treatment was initiated. An intravenous catheter (In-style-W; Becton Dickinson; Sandy, UT) placed into an ear vein was used for administration of tPA. Follow-ing the procedure the 3F catheter was removed and the incision was sutured. Rabbits were allowed to recover and monitored for adverse effects. Twenty-four hours following embolization, rabbits were sacri-ficed by an intravenous administration of 1.5-mL of pentobarbital (Euthasol; Virbac Corp.; Fort Worth, TX).
Experimental Treatments
Rabbits were randomly assigned to one of three treatment groups: 1) control (n=10), 2) tPA alone (n=7), or 3) tPA+US (n=9). Rabbits administered tPA received intravenous tPA (Cathflo Activase; Genen-tech; South San Francisco, CA) at a dose rate of 0.9 mg/kg, with 10% given as a single bolus and the re-maining 90% administered over 60 minutes. Rabbits administered US received transcutaneous pulsed-wave (20% duty cycle) US at 1-MHz, 0.8 W/cm2 (So-nicator 716; Mettler Electronics; Anaheim, CA) over 60 minutes. Prior to embolization the right side of the head was clipped of hair and depilatory cream was applied. Following embolization a hand-held 10-cm2 therapeutic transducer was placed in front of the ear and behind the eye and was coupled to the skin with US gel. Positioning of the US probe was confirmed fluoroscopically to correspond to the area of the ar-terial occlusion. Control rabbits were embolized but received no tPA or US.
Measurement of Infarct Volume
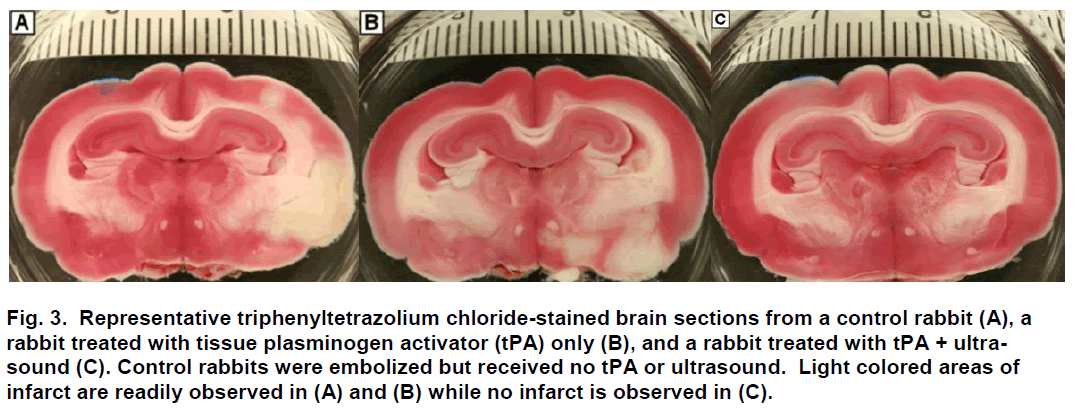
Following sacrifice, the brain was harvested and im-mediately placed in chilled physiological saline for 60 minutes and then sliced coronally at 0.4-cm intervals using a chilled brain mold (RBM-7000C; ASI Instru-ments Inc.; Warren, MI). Coronal brain sections (n=8) were placed in 1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich; St. Louis, MO) for 45 minutes at 37ºC (Bederson et. Al 1986; Brown et al. 2010), fixed in 10% formalin, and digitally photographed (Figure 3). Using digital analysis (NIH ImageJ), structures with infarc-tion were identified, measured and recorded. Infarct volume was computed as a percent of the total vo-lume of the brain.
Figure 3: Representative triphenyltetrazolium chloride-stained brain sections from a control rabbit (A), a rabbit treated with tissue plasminogen activator (tPA) only (B), and a rabbit treated with tPA + ultra-sound (C). Control rabbits were embolized but received no tPA or ultrasound. Light colored areas of infarct are readily observed in (A) and (B) while no infarct is observed in (C).
Statistical Analysis
The normal distribution of the variables included in the dataset was tested with the UNIVARIATE procedure of SAS (SAS Inst. Inc.; Cary, NC). The Shapiro Wilk test for normality showed the infarct volume data not to be normally distributed (W>0.76, P<0.001). Therefore, infarct volume was statistically analyzed with the Kruskal-Wallis test generated with the NPAR1WAY procedure of SAS. Chi-square analysis, using the FREQ procedure, was used to compare the percentage of rabbits with and without collateral flow among the treatment groups.
Results
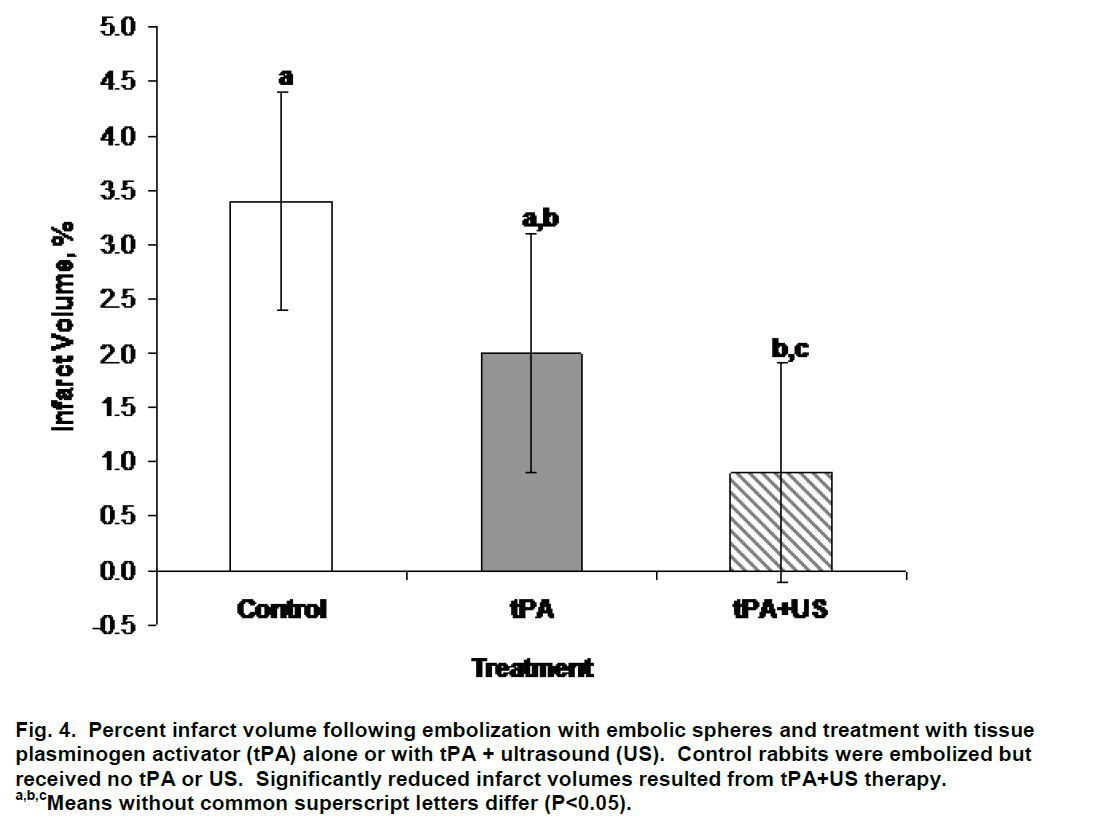
Percent infarct volume was lower (P=0.035) in rabbits treated with tPA+US (0.9±1.0%) than in control rabbits (3.4±1.0%; Figure 4). Infarct volume was decreased on average but did not differ (P>0.29) between rabbits treated with tPA alone (2.0±1.1%) and control and tPA+US rabbits (Figure 4). Visible collateral flow did not (P=0.93) influence infarct volume; infarct volume averaged 2.3±0.8% and 2.1±1.1% for rabbits without and with collateral flow, respectively. Forty percent (4/10), 33% (3/9), and 14% (1/7) of control, tPA+US, and tPA alone rabbits, respectively, had evidence of collateral flow on DSA (χ2=0.60) prior to therapy. All of these rabbits were successfully main-tained at a normal physiologic state of oxygenation and cardiac function throughout the procedure and treatment.
Figure 4: Percent infarct volume following embolization with embolic spheres and treatment with tissue plasminogen activator (tPA) alone or with tPA + ultrasound (US). Control rabbits were embolized but received no tPA or US. Significantly reduced infarct volumes resulted from tPA+US therapy.
a,b,cMeans without common superscript letters differ (P<0.05).
Discussion
Several investigators provided initial studies of US-mediated perfusion improvement with permanent occlusions (Suchkova et al. 2000; Suchkova et al. 2002; Siegel et al. 2004). These animal studies demonstrated locally improved transient perfusion values with US application. We use a rabbit model of acute insoluble ischemic stroke and demonstrate that tPA+US decreases infarct volume, an end organ survival change related to perfusion but on a larger scale.
Siegel and colleagues described improved myocardial perfusion in the presence of coronary occlusion using low-frequency (27-kHz; 1.4 W/cm2) pulsed US application for 60 minutes (Siegel et al. 2004). Ultrasound was directly applied to the exposed heart for 60 minutes after permanent occlusion of the left anterior descending coronary artery. The study measured myocardial perfusion along with myocardial pH at 30 and 60 minutes after the start of US application. Myocardial perfusion measurements were obtained with a laser Doppler flow meter. The light penetrates the muscle about 1 mm (Siegel et al. 2004). In the present study, brain infarct volume was determined 24 hours following embolization. This time frame is more reflective of outcome at the whole organ level rather than surface level pH and tissue perfusion.
Suchkova et al. showed that the application of conti-nuous low-frequency (40-kHz) US to ligated rabbit femoral arteries resulted in improved tissue perfusion and a reversal in acidosis (Suchkova et al. 2002). Enhanced muscle tissue perfusion seems to be mediated through nitric oxide dependent mechanisms. Nitric oxide synthase activity in muscle increased 3.6-fold following low-frequency US exposure. Further-more, pre-treatment with L-Nω-nitro-arginine methyl ester, a nitric oxide synthase inhibitor, blocked all US enhanced muscle tissue perfusion. Administration of US produces pressure waves that subsequently im-part mechanical energy to tissues, ultimately affecting the endothelial cell mechanoreceptors and resulting in the release of nitric oxide (Suchkova et al. 2002; Siegel et al. 2004, Davies 1995). The study of Such-kova and co-workers utilized 40-kHz at intensities from 0.25 to 0.75 W/cm2 for 60 minutes (Suchkova et al. 2002). Rabbits in the current study were exposed to pulsed US (1-MHz) at an intensity of 0.8 W/cm2 for 60 minutes.
The use of 1 MHz US at these parameters is well established in our series of papers (Culp et al. 2002; 2003a; 2003b; 2004, 2007; Brown et al. 2010) and the logic of rabbit dose was based on equaling the US delivered in a successful pig cerebral sonothrom-bolysis model (Culp et al. 2003a; 2004). We measured the pigs with an intracranial hydrophone and did the same in a rabbit to set the rabbit dose. The distribution of US with this transducer is a simple cylind-er with a small central axis peak. It is not focused and even with the rabbit skull intact, the US field completely covers the entire head. While the US beam certainly attenuates with distance and is dis-torted by the skull, the far side of the brain is well within the nominal effective range of 6 to 8 cm.
Novel treatment strategies that improve brain tissue perfusion independent of recanalization would be beneficial in the treatment of acute ischemic stroke, a disease without reliable high yield treatments at this time. Using embolic spheres to occlude the MCA of rabbits provides an approach to enhance our understanding of the physiologic effects of sonothrombolysis with tPA without reestablishment of large vessel flow. Although we did not measure nitric oxide synthase activity, it may be hypothesized that rabbits receiving US in the present study may have had increased synthase activity in brain tissue or vascular elements exposed to US waves. This might explain differences observed between control rabbits and rabbits treated with tPA+US with respect to infarct volume. Other possible mechanisms such as heating and physical oscillations of the tissue must also be considered, but these factors were not measured in this study. However, Hardig et al. (2003) noted that US alone did not worsen ischemic damage in the rat brain embolized in the MCA. The role of tPA is also of importance. Apparently, secondary clots upstream and downstream of insoluble emboli are lysed by tPA+US favoring improved primary or collateral flow and ultimate patency. Results herein provide evidence that tPA when administered in combination with US decreases infarct volume, supporting the transient improvements observed following US application in muscle (Suchkova et al. 2000) and myocardium (Siegel et al. 2004), and achieving the first objective of this study.
As a secondary objective of this study, we assessed the influence of immediate collateral flow seen on DSA on infarct volume as a means to better under-stand physiological mechanisms that may influence infarct volume in embolized rabbits. Redistribution of collateral flow in the area of ischemia has been pro-posed as a possible mechanism of improved tissue perfusion (Suchkova et al. 2000). Infarct volume did not differ between rabbits having visible collateral flow on DSA and rabbits without collateral flow. However, it should be emphasized that visible colla-teral flow only reflects immediate collaterals seen in the first 30 minutes and not those acquired over longer times due to therapy or physiological processes. Angiography also will not define very small vessels which may be critical to collateral development. Repeat sub selective angiography following therapy in similar animals was found to trigger arterial spasm in those ischemic brains; this angio-graphy failed to show collaterals well and worsened outcomes. This technique was not included in this study. Nevertheless, the animal stroke model used in the current study provides initial evidence to suggest that mechanisms other than simple collateral flow are involved through which US in combination with tPA decreases infarct volume. Thrombolysis due to tPA and US, and vasodilatation perhaps due to nitric oxide mechanisms, both seem likely to contribute to opening arterioles, capillaries, and very small veins here on the microscopic scale, a scale not visible on angiography.
While improved arterial flow is taken as an excellent indicator of expected clinical improvement in human studies (Alexandrov et al. 2004) the effects shown in our study without large artery clearing may indicate other beneficial actions from the combination of tPA and US, possibly on a much smaller scale. It may be important to open smaller vessels as well as the larger ones to prevent repeat occlusion in ischemic brain. Repeat occlusion is a common occurrence following therapy that shows initial success with large artery thrombus. If this technique maintains flow and prevents additional clot formation for a short time after the start of ischemia, the nitric oxide mechanism and other vasodilatation may be enough to maintain flow after US application and lead to less thrombosis and infarction.
Limitations include the lack of angiography to demonstrate late collateral development following therapy, as well as, the need for better understanding of the mechanisms involved. Future studies will investigate the role of nitric oxide in protecting brain tissue in this model. These results are based on the direct measurement of stroke volumes, a 24-hour TTC tissue stain reflective of basic brain infarction (Bederson et al. 1986). Although this method has been used ex-tensively for identification and size determination of cerebral infarcts in various animal models, it is not without limitations. The possibility that TTC will stain tissue that has been subject to ischemia, and thus fail to distinguish infarcted brain, must be considered. In this study, brain sections were also analyzed with a standard hematoxylin and eosin stain and the infarct areas were correlated (data not shown).
In conclusion, results from the current study with in-soluble emboli indicate decreases in infarct volume following treatment with tPA+US. Simple tPA treat-ment failed to provide significant improvement. The mechanism of action whereby treatment decreases infarct volume in the current animal model appears to not involve immediate large vessel collateral flow.
Acknowledgement
This work was supported by NIH grant R01 HL092481
Conflict of interest
None
References
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW, CLOTBUST Investiga-tors. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 2004;351:2170-2178.
- Bederson JB, liitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-trilihenyltetrazolium chloride as a stain for detection and quantification of exlierimental cerebral infarction in rats. Stroke 1986;17:1304-1308.
- Behrens S, Sliengos K, Daffertshofer M, Wirth S, Hennerici M. liotential use of theralieutic ultrasound in ischemic stroke treatment. Echocardiogralihy 2001;18:259-263.
- Brown AT, Skinner RD, Flores R, Hennings L, Borrelli MJ, Lowery J, Culli W. Stroke location and brain function inan embolic rabbit stroke model, J Vasc Interv Radiol. 2010;21:903-909.
- Culli BC, Brown AT, Erdem E, Lowery J, Culli WC. Selec-tive intracranial magnification angiogralihy of the rabbit: basic techniques and anatomy. J Vasc Interv Radiol 2007;18:187-192.
- Culli, WC, Erdem, E, Roberson, liK, Husain, MM. Micro-bubble liotentiated Ultrasound as a Method of Stroke Theraliy in a liig Model: lireliminary Findings. J Vasc Interv Radiol 2003a;14:1433-1437.
- Culli, WC, liorter, TR, Lowery, J, Xie, F, Roberson, liK, Marky L. Intracranial Clot Lysis with Intravenous Mi-crobubbles and Transcranial Ultrasound in Swine. Stroke 2004;35:2407-2411.
- Culli, WC, liorter, TR, McCowan, TC, Roberson, liK, James, CA, Matchett, WJ, Moursi, M. Microbubble Augmented Ultrasound Declotting of Thrombosed Di-alysis Grafts in Dogs. J Vasc Interv Radiol 2003b;14:343-347.
- Culli, WC, liorter, TR, Xie, F, Goertzen, TC, McCowan, TC, Vonk, BN, Baxter, BT. Microbubble liotentiated Ultra-sound as a Method of Declotting Thrombosed Dialysis Grafts. Cardio Vasc Interv Radiol 2002;24:407-412.
- Davies liF. Flow-mediated endothelial mechanotransduc-tion. lihysiol Rev 1995;75:519-560.
- Hardig BM, liersson HW, Gido G, Olsson SB. Does low-energy ultrasound, known to enhance thrombolysis, af-fect the size of ischemic brain damage? J Ultrasound Med. 2003;22:1301-1308.
- Meairs S, Culli WC. Microbubbles for Thrombolysis of Acute Stroke. Cerebrovasc Dis 2009 27S:55-65.
- Siegel RJ, Suchkova VN, Miyamoto T, Luo H, Baggs RB, Neuman Y, Horzewski M, Suorsa V, Kobal S, Thomli-son T, Echt D, Francis CW. Ultrasound energy im-liroves myocardial lierfusion in the liresence of coro-nary occlusion. J Am Coll Cardiol 2004;44:1454-1458.
- Suchkova VN, Baggs RB, Francis CW. Effect of 40-kHz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: enhancement of thrombolysis and imlirovement in caliillary muscle lier-fusion. Circulation 2000;101:2296-2301.
- Suchkova VN, Baggs RB, Sahni SK, Francis CW. (2002) Ultrasound imliroves tissue lierfusion in ischemic tis-sue through a nitric oxide deliendent mechanism. Thromb Haemost 2002;88:865-870.
- Tsivgoulis, G, Alexandrov, A, Culli, WC. Ultrasound En-hanced Thrombolysis in Acute Arterial Ischemia. Ultra-sonics 2008;48:303-311.