Special Report - Interventional Cardiology (2015) Volume 7, Issue 5
Tibial-pedal arterial access & retrograde interventions for advanced peripheral arterial disease & critical limb ischemia
- Corresponding Author:
- Jihad A Mustapha
Metro Health Hospital, 5900 Byron Center Ave SW, Wyoming, MI 49519, USA
E-mail: jihad.mustapha@metrogr.org
Abstract
The incidence of peripheral arterial disease is rising thanks to increasing rates of obesity, diabetes, hypertension and failed attempts at controlling tobacco abuse. Advanced peripheral arterial disease and critical limb ischemia represent the terminal stages and carry high amputation and mortality rates. Evolving technology and understanding of the disease process have introduced newer therapeutic modalities and approaches to peripheral vascular interventions. Among these, retrograde tibialpedal access and minimally invasive retrograde interventions are gaining momentum as new adopters are witnessing the safety and improved outcomes (evidenced by the increased success rate of previously failed attempts at crossing complex occlusions via the traditional antegrade approach). The shift toward less invasive approaches has led to increased endovascular interventions and decreased amputation rates.
Keywords
critical limb ischemia, endovascular, tibial access, tibial-pedal, ultrasound
Scope of the problemPeripheral arterial disease (PAD) affects 12% of the adult population and up to 20% of individuals 70 years or older. Its incidence is on the rise, fueled by the epidemic caused by calorie-rich diets, hypertension, diabetes, tobacco abuse and sedentary lifestyle [1–3].
Advanced PAD refers to patients with symptoms of claudication with minimal effort who have complex anatomy consistent with the Rutherford III classification. Critical limb ischemia (CLI) represents the terminal stage of PAD and it occurs when the restriction to arterial blood flow is so severe that the capillary beds are inadequately perfused and unable to sustain tissue viability. It is defined by the presence of rest pain and/or tissue loss for at least 2 to 4 weeks that can be attributed to occlusive arterial disease. The diagnosis is clinical in nature and refers to patients with pain at rest, minimal tissue loss or frank gangrene, consistent with Rutherford classification IV–VI [4]. The European Consensus Conference included the need for analgesia for more than 2 weeks or ischemic tissue loss with an ankle pressure of less than 50 mmHg as part of the definition [5]. Anatomically, CLI is characterized by multilevel and multivessel infrainguinal and tibial arterial stenoses and chronic total occlusions (CTOs) that create a severe imbalance between supply and demand of oxygen in the affected tissues, compromising its viability and threatening limb loss. It is estimated that 1.5 million patients in Europe and 2 million patients in the USA over 50 years of age manifest symptoms of CLI. Although CLI encompasses <5% of all cases of PAD, its prognosis is poor. The 1-year mortality and major amputation rates range from 20 to 50% [6–8]. It occurs in approximately 1–3% of all PAD cases [9–11] with an incidence between 500 and 1000 persons per million per year in Europe and the USA [12–14]. Many patients do not progress sequentially along predefined stages from claudication to CLI. Select longitudinal studies show that some patients with symptomatic PAD progressively develop CLI [15,16], while others have shown that many patients are asymptomatic prior to developing CLI [17,18]. In less severe circumstances, compensatory mechanisms such as angiogenesis and arteriogenesis [19] are suffi-cient to overcome the increased demand imposed by the lack of adequate tissue perfusion, however in patients with CLI, these mechanisms have been exhausted and/ or are defective. Inadequate perfusion of the skin and surrounding tissues leads to endothelial dysfunction, chronic inflammation [20] and muscle damage [21–23]. The clinical manifestations of these molecular changes are ischemic rest pain, non-healing ulcerations and gangrene. A staggering 20 to 25% of patients with CLI will undergo primary amputation, 50 to 60% revascularization (surgical and/or endovascular) and 25% are treated medically [24]. The 5-year mortality rate is 60% [25]. 40% of CLI patients, who do not undergo revascularization, will undergo leg amputation within 6 months and 20% will die [20].
Therapeutic evolutionEndovascular revascularization of patients with advanced PAD has evolved dramatically over the past decade due to a better understanding of the atherosclerotic process, biomechanical forces and physiopathologic features that characterize the disease in the lower extremities, all of which have led to the development of disruptive technologies, designed to further endovascular interventions (in juxtaposition to the previously established status quo dictated by performance of bypass surgery) [20]. These technologies have been utilized with varying success and without a clear advantage of one device over another in terms of improved clinical outcomes. These include plain balloon angioplasty [26,27]; cryoplasty [28]; cutting balloon angioplasty [29]; bare metal stents [30], drug eluting stents [6,31,32], directional atherectomy [33–35]; laser atherectomy [36], orbital atherectomy [37–39] and most recently, drug-coated balloons [40–42].
Peripheral vascular interventions (PVIs) have traditionally been performed via access in the contralateral common femoral artery (CFA), using the ‘up and over’ approach. This technique has several limitations, which are mainly represented by:
• Lack of equipment that is long enough to approach distal tibial and pedal vessels.
• Inability to treat potential complications such as distal embolization, dissections and perforations.
• Inability to treat the contralateral limb in patients with endovascular aortic aneurysm repairs or aortobifemoral bypass grafts, iliac CTOs and aortoiliac bifurcations with extreme angulations.
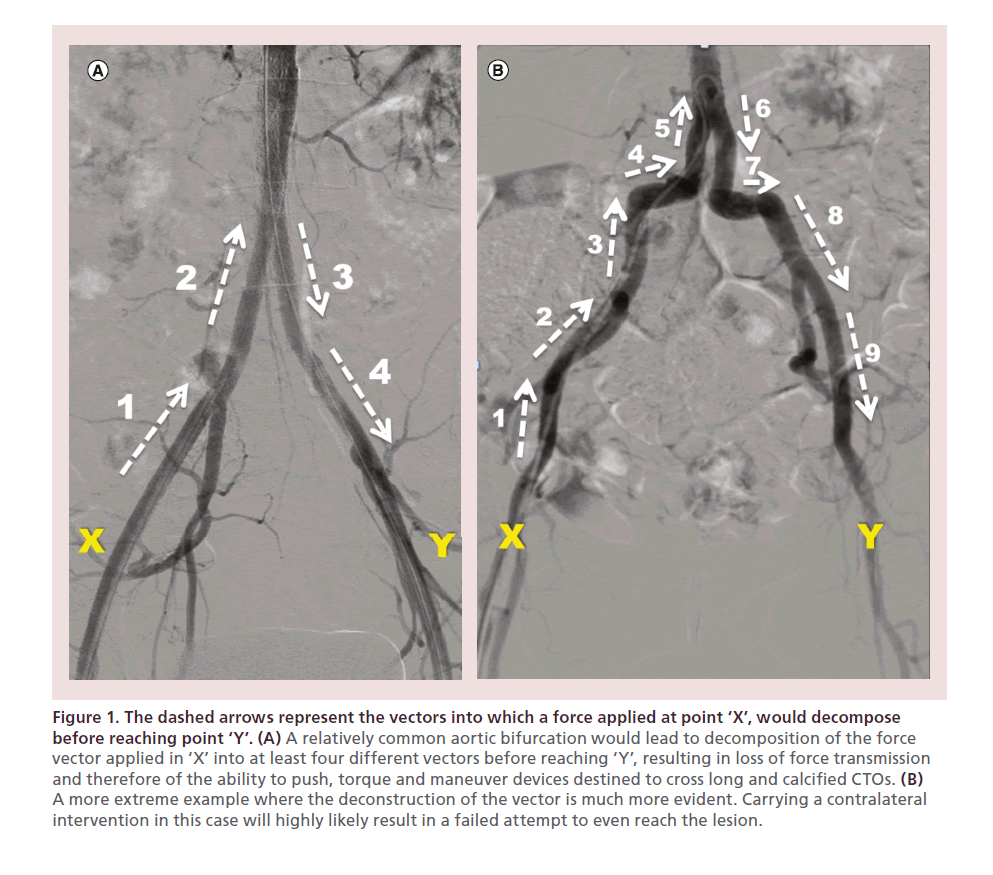
• Decomposition of the force vector applied in the accessed groin with the resulting loss of kinetic energy, pushability, torqueability and maneuverability of the crossing devices when attempting to cross long and calcified CTOs. The force vector that is applied in the contralateral groin has to travel in different and opposite directions to finally be delivered at the tip of the device in the target lesion (Figure 1) resulting in a decreased likelihood of successful crossing.
Figure 1. The dashed arrows represent the vectors into which a force applied at point ‘X’, would decompose before reaching point ‘Y’. (A) A relatively common aortic bifurcation would lead to decomposition of the force vector applied in ‘X’ into at least four different vectors before reaching ‘Y’, resulting in loss of force transmission and therefore of the ability to push, torque and maneuver devices destined to cross long and calcified CTOs. (B) A more extreme example where the deconstruction of the vector is much more evident. Carrying a contralateral intervention in this case will highly likely result in a failed attempt to even reach the lesion.
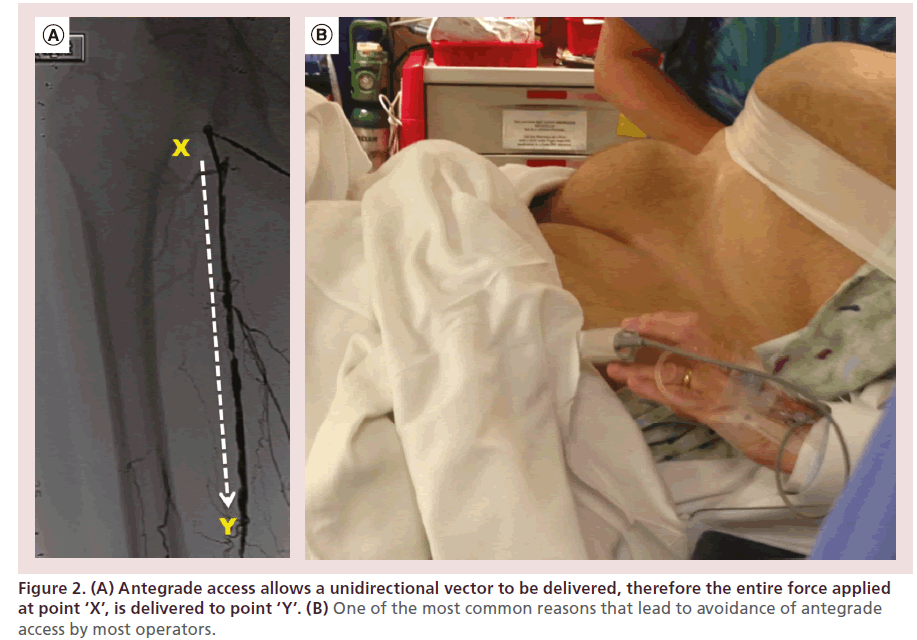
These limitations are overcome by performing CFA antegrade access and interventions. Placement of an antegrade sheath allows for direct and complete transmission of the force applied at the groin to the tip of the crossing device (increasing the ability to push, torque and maneuver these devices), therefore increasing the likelihood of success (Figure 2A), while allowing treatment of potential distal complications. However, this approach is limited by the unfamiliarity of most operators with the technique and the increased risk of access-related complications in patients who are morbidly obese (Figure 2B), and in those unable to lie flat (a common limitation among patients with advanced PAD and CLI who also have lower back issues or advanced forms of chronic obstructive pulmonary disease [COPD] or congestive heart failure [CHF]). Patients with CLI frequently have CTO caps with antegrade convex configurations, which do not favor intraluminal crossing and require a retrograde approach or a combined antegrade-retrograde approach to be successfully crossed and treated.
Figure 2. (A) Antegrade access allows a unidirectional vector to be delivered, therefore the entire force applied at point ‘X’, is delivered to point ‘Y’. (B) One of the most common reasons that lead to avoidance of antegrade access by most operators.
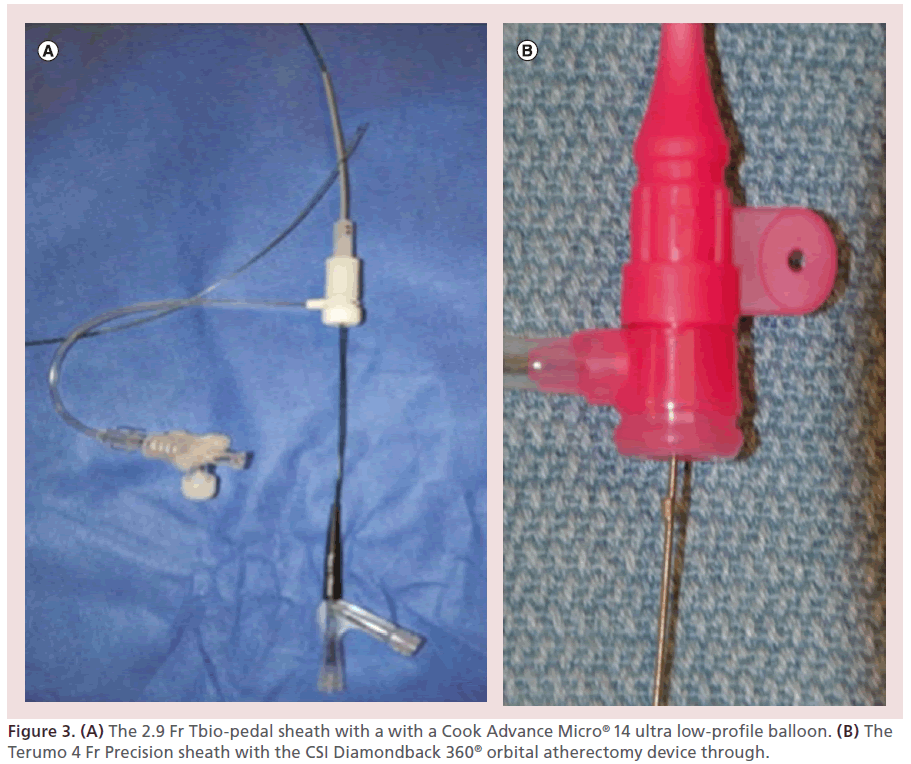
The challenge posed by this subset of patients, resulted in the development of the tibial-pedal arterial minimally invasive (TAMI) retrograde revascularization technique. This technique is based on the use of single or dual retrograde tibial-pedal access under extra vascular ultrasound (EVUS) guidance, to perform diagnostic and interventional procedures in patients that are poor candidates to receive the same therapy from a traditional CFA access. Currently available low-profile devices including sheaths, balloons, atherectomy devices and stents, can be utilized from the retrograde tibial-pedal approach to achieve complete revascularization of the lower extremity (Figure 3) in select patients.
Several factors should be taken into consideration in order to decide which patients are ideal candidates for this approach. However, as with any other procedure, the decision-making process needs to be tailored to each individual patient, operator and institution.
Patient factors:
• Advanced PAD or CLI;
• Inability to lie flat for prolonged periods of time (severe osteoarthritis, lower back pain, CHF, COPD);
• Contracted/deformed hip;
• Hostile groins: morbid obesity, infected groins, severely scarred/ fibrotic groins.
Vascular anatomy: The distal third of the tibial arteries should be patent (in order to obtain access and accommodate a tibial-pedal sheath).
• Flush occlusion of the ostium of the superficial femoral artery (SFA) (in patients where the contralateral approach is not feasible);
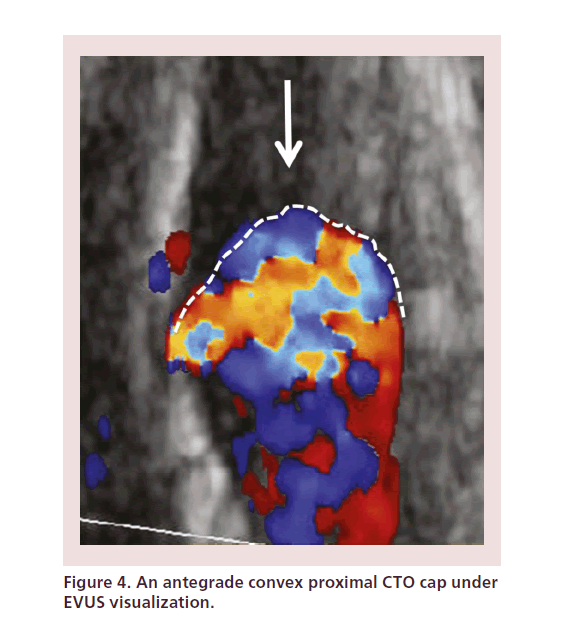
• The proximal CTO cap has an antegrade convex morphology, which increases the likelihood of subintimal penetration and dissection (Figure 4);
• Long suprapopliteal CTO with reconstitution of flow at the P3 segment of the popliteal or the tibial trifurcation, with an antegrade convex cap (which would increase the risk of compromising the distal vessels if crossing from the traditional antegrade approach.
Procedure description: stepwise approach
Preprocedural imaging
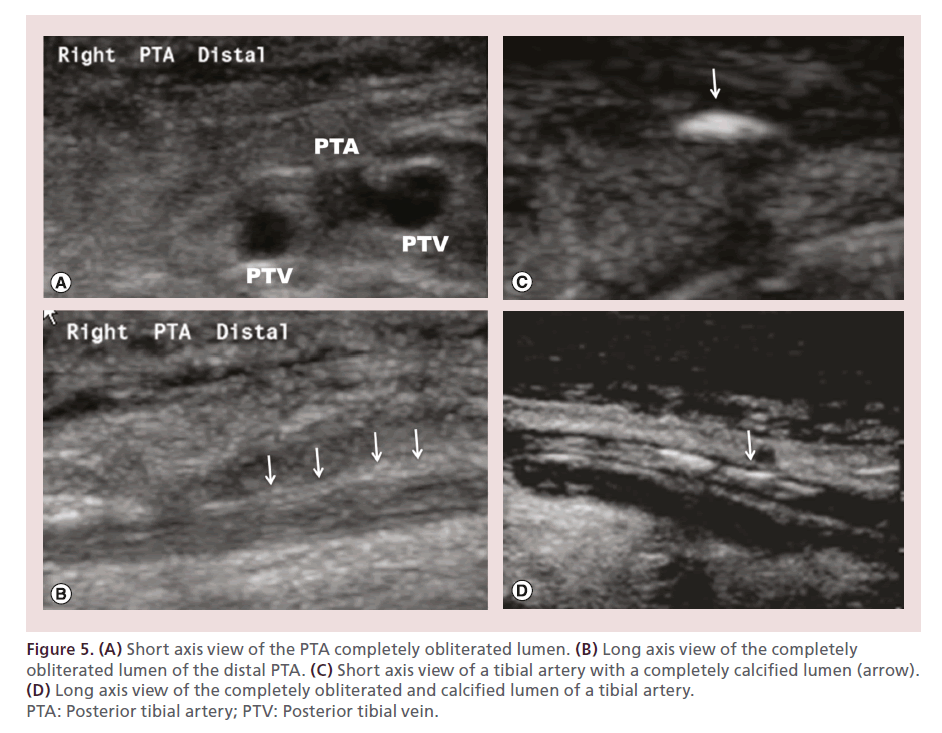
A detailed noninvasive evaluation of the target limb with EVUS allows identification of occlusions and reconstitutions, segments of hibernating lumen (patent arterial segments located between CTO caps, previously thought to be completely occluded due to inability of the antegrade column of contrast to penetrate the vessel beyond the occlusive cap), as well as areas of complete luminal obliteration (‘white stop sign’) (Figure 5). This, in conjunction with selective angiography (performed with the catheter tip as close as possible to the tibials) allows the identification of the true length of the CTO segment [43,44].
Figure 5. (A) Short axis view of the PTA completely obliterated lumen. (B) Long axis view of the completely obliterated lumen of the distal PTA. (C) Short axis view of a tibial artery with a completely calcified lumen (arrow). (D) Long axis view of the completely obliterated and calcified lumen of a tibial artery. PTA: Posterior tibial artery; PTV: Posterior tibial vein.
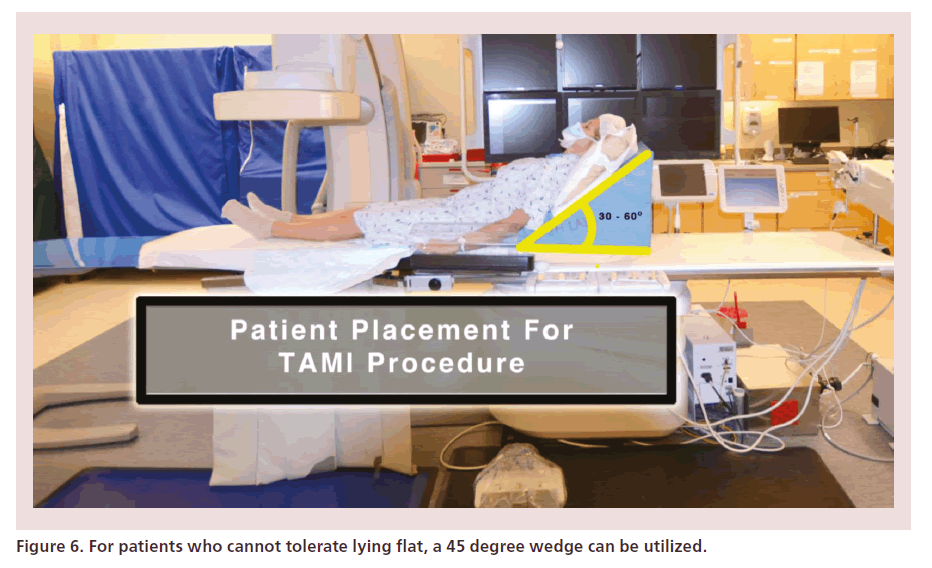
Step 1: patient placement
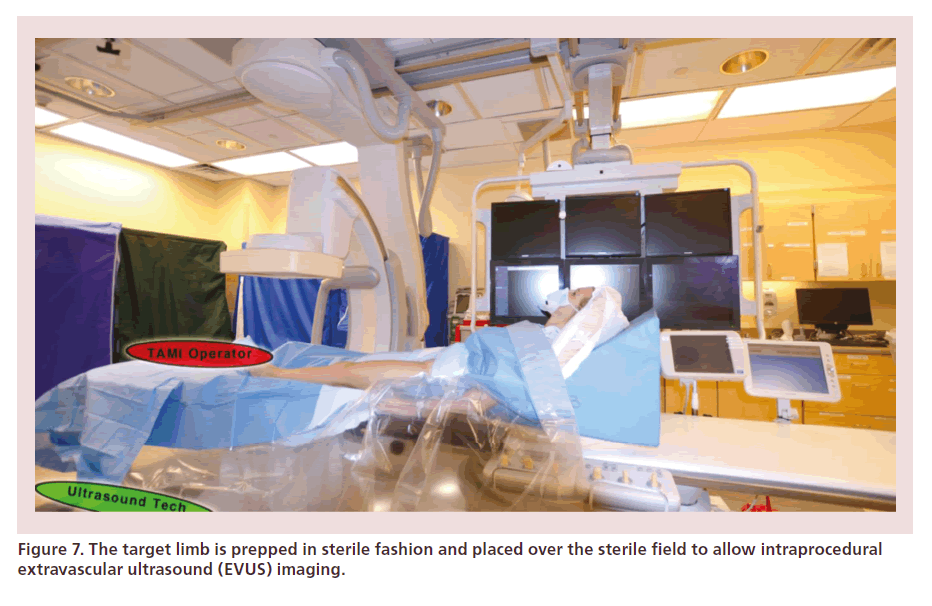
Patients are placed in ‘antegrade fashion.’ If unable to tolerate lying flat, a supporting wedge can allow the patient to virtually sit at 45° (Figure 6). The target limb is prepped in sterile manner with iodine or chlorhexidine painting and is kept fully exposed on top of the sterile field, in order to allow intraprocedural EVUS imaging (Figure 7).
Step 2: EVUS-guided access site selection
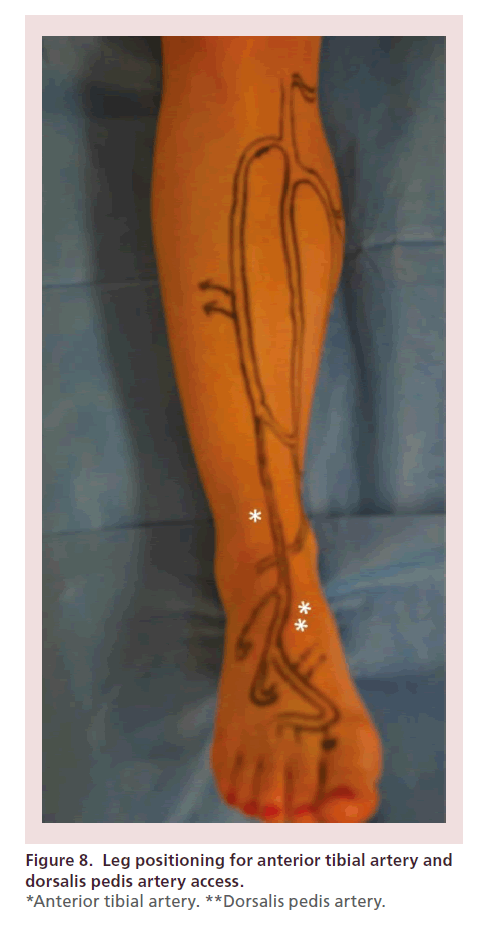
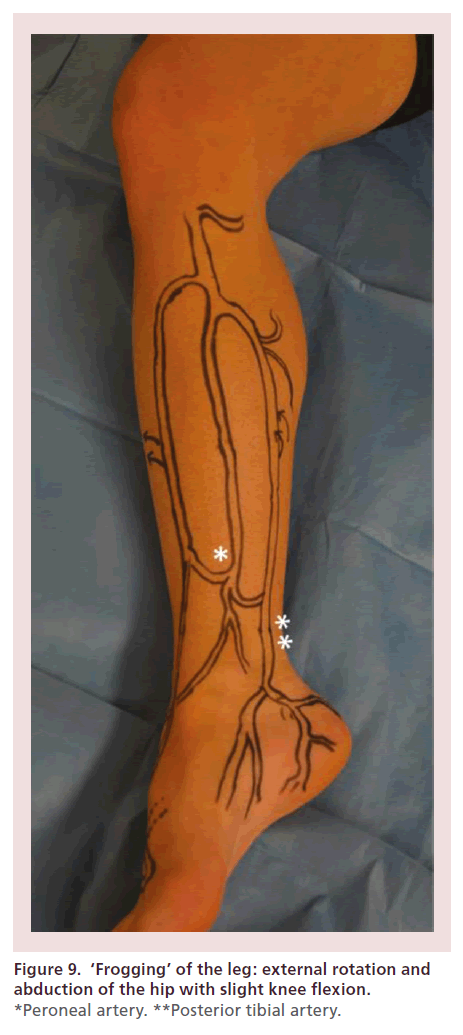
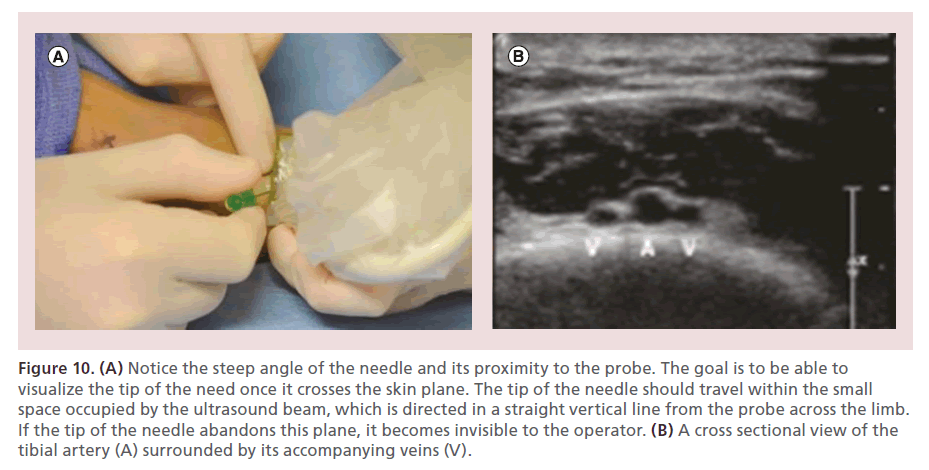
The leg must be adequately positioned in order to facilitate maximal exposure of the target vessel. For the anterior tibial and dorsalis pedis arteries, the leg is maintained in its neutral anteroposterior orientation, with the heel resting on the mattress (Figure 8). In order to access the posterior tibial and peroneal arteries, external rotation and abduction of the hip are performed, as well as slight flexion of the knee (Figure 9). Once the target access point is identified under EVUS (using a 7–15 MHz or ‘hockey stick’ probe) in the short axis view, 0.5 cc’s of subcutaneous lidocaine are injected. The needle is oriented at a 60–75° angle, with its tip located next to the edge of the probe. Once the tip of the needle crosses the skin, every effort is made to clearly see its tip under EVUS, allowing precise arterial puncture, while avoiding complications such as arteriovenous (AV) fistulas (Figure 10).
Figure 10. (A) Notice the steep angle of the needle and its proximity to the probe. The goal is to be able to visualize the tip of the need once it crosses the skin plane. The tip of the needle should travel within the small space occupied by the ultrasound beam, which is directed in a straight vertical line from the probe across the limb. If the tip of the needle abandons this plane, it becomes invisible to the operator. (B) A cross sectional view of the tibial artery (A) surrounded by its accompanying veins (V).
Step 3: EVUS-guided arterial cannulation
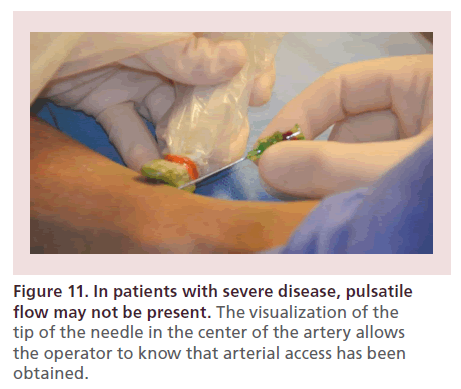
The needle trajectory is monitored under EVUS guidance. Visualization of the tip of the needle in the center of the artery allows the operator to know that access has been gained (as it is not uncommon to find lack of pulsatile blood flow in patients with severe disease) (Figure 11).
Step 4: access wire introduction
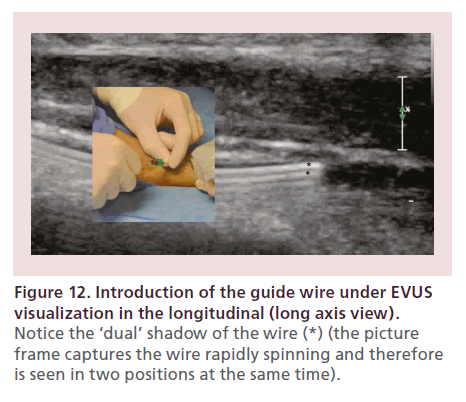
Next, the EVUS probe should be switched to the long axis. The guide wire is slowly introduced into the artery under direct visualization (Figure 12). It is recommended to continuously spin the wire, in order to increase the reverberation artifact (which enhances the wire visibility) and maintain its tip free, decreasing the likelihood of subintimal insertion.
Step 5: sheath placement
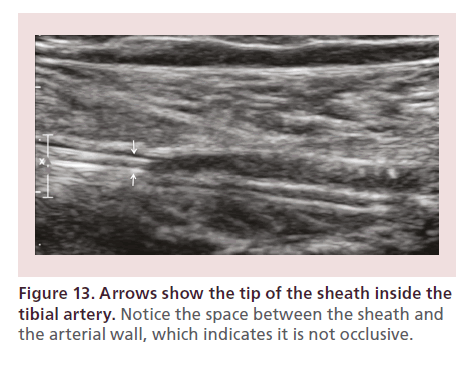
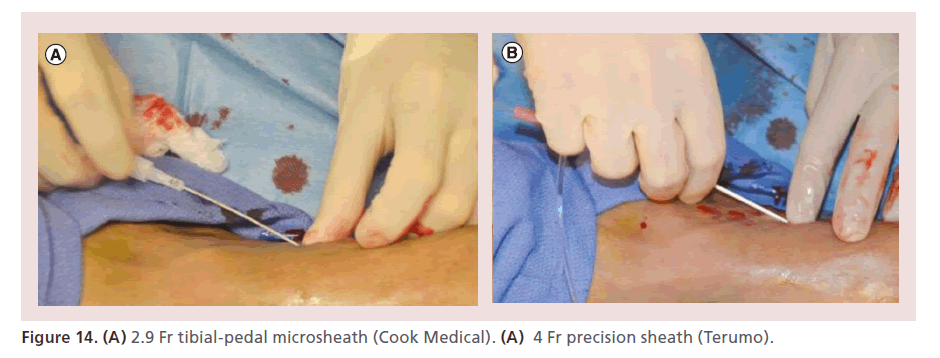
The tibial pedal sheath is then slowly introduced 1 cm at a time, by pushing very close to the skin (to avoid kinking). EVUS also allows visualization of the sheath inside of the tibial artery and its relationship to the vessel wall (Figure 13). Five French (Fr) sheaths are reserved for limb-salvage cases when all other options have been exhausted (in order to use large support catheters or perform retrograde stenting). When 5 Fr sheaths are utilized, the intra-arterial time should be kept to less than 5 min (Figure 14).
Step 6: intra-arterial pharmacotherapy & retrograde angiography
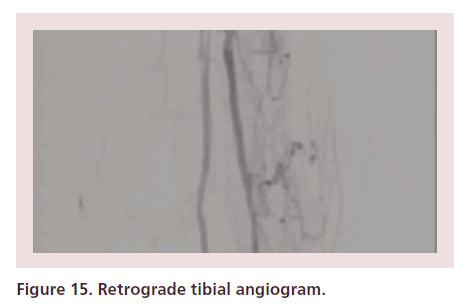
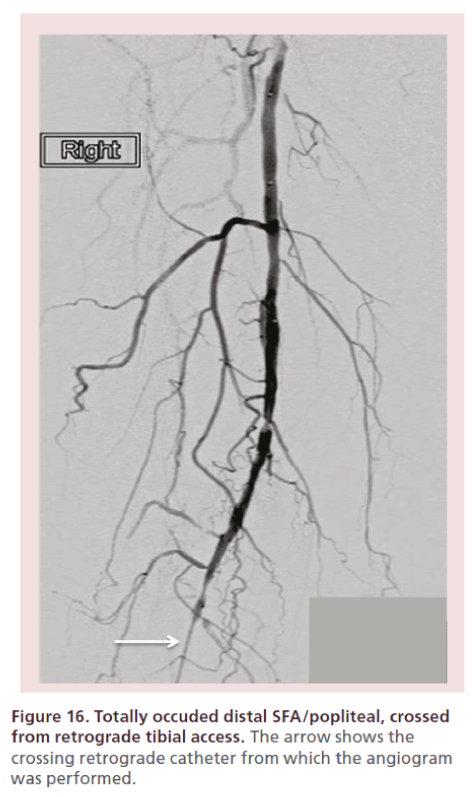
Intra-arterial heparin is administered at 60–80 units/kg bolus, followed by 200–400 μg of intra-arterial nitroglycerin. The activated clotting time (ACT) is checked every 30 min and heparin administered to keep an ACT of 200–250 s. Nitroglycerin is administered every 10–20 min to avoid spasm. Then, a slow, modulated contrast injection (retrograde tibial angiogram) is performed (Figures 15 & 16). Alternatively, the TAMI solution can be administered as a continuous drip through the sheath’s sidearm (Box 1) at a rate of 6–7 cc/min. If the blood pressure is marginal, decrease the rate to 3 cc/ min.
Step 7: retrograde revascularization strategy
The choice of endovascular tools must be individualized for each lesion. The techniques utilized to cross and treat stenoses and occlusions from the retrograde approach include:
• Wire/catheter technique;
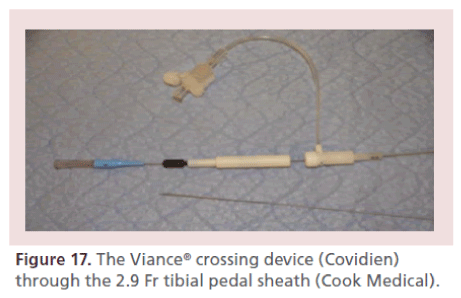
• CTO crossing devices: TruePath™ (Boston Scientific, MA, USA); Crosser™ (Bard, AZ, USA); TurboElite Laser™ (Spectranetics, CO, USA); Viance™ (Covidien, MN, USA) (Figure 17);
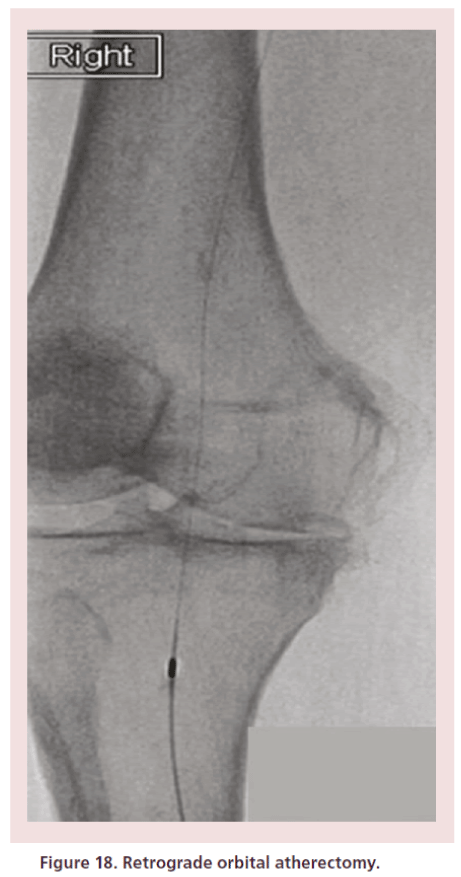
• Atherectomy devices: DiamondBack™ (CSI, MN, USA) (Figure 18); ablative laser atherectomy (Spectranetics, CO, USA);
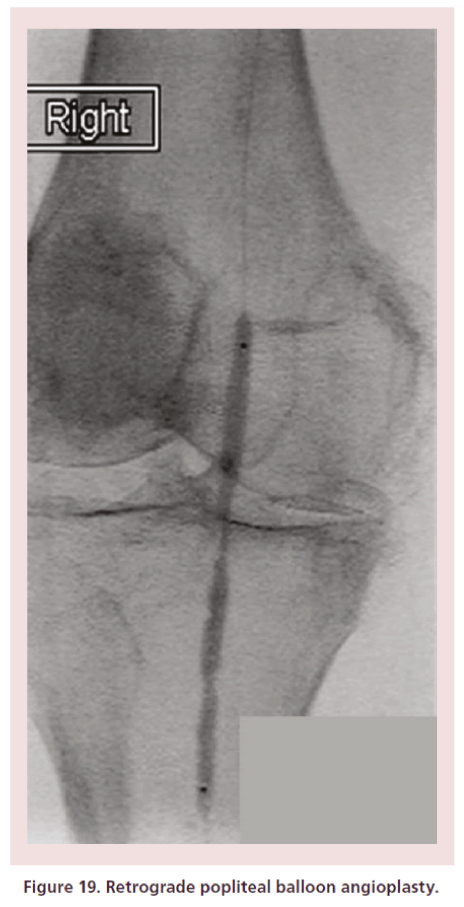
• Balloon angioplasty (Figure 19) and self-expanding stents (for SFA and popliteal lesions) can also be delivered.
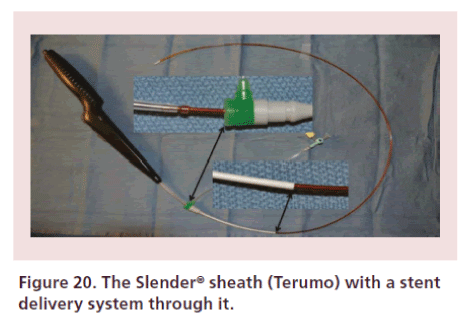
Depending on the size of the balloons being used, a sheathless approach can be used. The authors however recommend against the sheathless insertion of selfexpanding stents for popliteal or SFA applications (due to increased risk of arterial laceration). If stenting is necessary, a 5 Fr sheath should be used expeditiously, minimizing its intra-arterial time. All equipment should be on the table and ‘ready to go’ to rapidly deliver therapies and exchange back to the smaller sheath to complete the case. Recently, the Slender Sheath™ (Terumo Medical, NJ, USA) was launched, featuring an outer 5 Fr with an inner 6 Fr diameter, which allows delivery of 6 Fr devices from a tibial-pedal position (Figure 20).
Step 8: post-intervention angiographic assessment
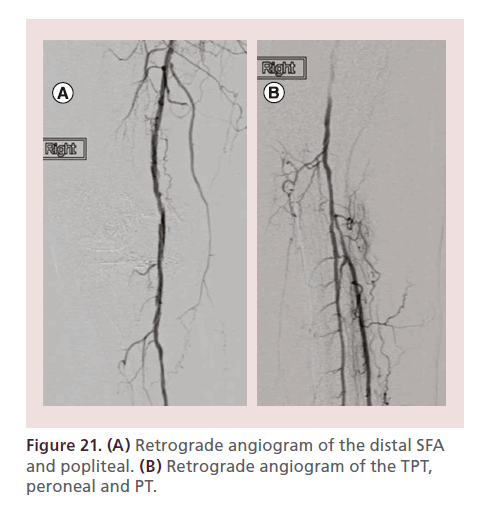
It is recommended to maintain a 0.014” wire in the main vessel and advance a catheter beyond the treated segment. Angiography is then performed in a stepwise fashion from supra to infrapopliteal vessels (Figure 21). The last angiogram should be performed through the dilator of the microsheath after intra-arterial nitroglycerin is administered, to visualize the pedal circulation distal to the access point.
Step 9: hemostasis
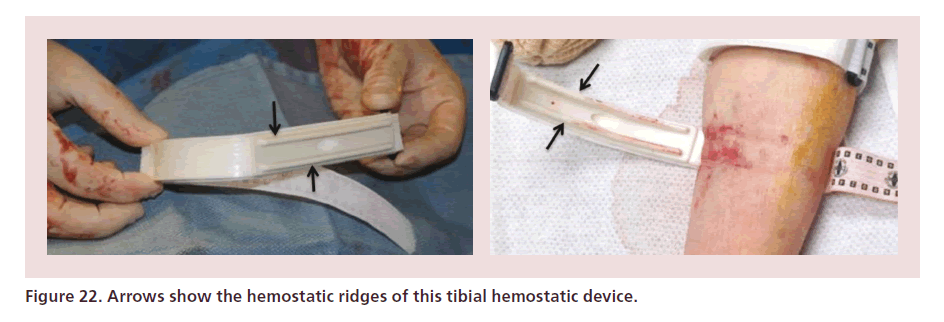
At the end of the procedure the final ACT is documented, and the sheath is removed immediately. Tibial compression devices such as the Boa™ (Lakeshore Medical, MI, USA), or those designed for radial access can be used to achieve hemostasis (Figure 22). The site is checked every 15 min. Once there is no bleeding, the device is removed. Patients can ambulate 30 min after hemostasis is achieved. In patients with edema, the site should be carefully checked after compression device removal as subcutaneous bleeding may still occur.
Discussion
Historically, PVIs have been carried via CFA access and operators have used the femoral head as the ideal landmark to puncture the CFA in order to avoid complications in patients undergoing endovascular procedures. Fluoroscopic identification of the femoral head has been shown to decrease access-related complications; however, anatomical variations that contribute to these complications are often not recognized by this method [45–50]. As endovascular technologies continue to evolve, we face ever-increasing challenging cases in sicker patients with more complex lesions and a multitude of co-morbidities. We have learned that the ‘up and over’ approach has limitations that lead to failed revascularization attempts in a significant percentage of patients with advanced PAD and CLI, and an increasing proportion of these patients are morbidly obese, which inherently increases the risk of complications if the more efficacious antegrade ipsilateral approach is selected. The TAMI technique has filled a void in the armamentarium of interventionists who treat these patients by allowing operators to selectively engage and treat the target vessel from a retrograde tibial-pedal access, thus completely eliminating the potential groin site complications frequently seen in obese elderly patients with calcified vessels, who have a large pannus and are unable to lie flat due to severe low back pain, COPD, CHF and sleep apnea. The technique was evaluated in a singlecenter study, which reported successful EVUS-guided access and successful retrograde crossing and treatment in 100 and 95% of the patients, respectively [51]. Patients with advanced PAD often have multiple comorbidities that can impact the length of a procedure, leading to the need for multiple staged interventions as shown in the BASIL trial (patients underwent an average of 1.9–2.4 procedures) [52], which increases the likelihood of complications. EVUS-guided retrograde access and interventions, decrease the amount of contrast and radiation utilized, while increasing the success rate of revascularization procedures performed in one stage due to the fact that the lesion is closer to the access site (allowing for improved torqueability, device pushability and maneuverability, as well as for expedited device transit time), and that the distal CTO cap typically shows a retrograde concave configuration which is more easily and successfully traversed from a retrograde approach [51,53,54].
Conclusion
The TAMI technique offers an alternative revascularization method for patients in whom a traditional revascularization approach is not feasible or has failed. Currently available endovascular devices are shifting toward a lower crossing profile, allowing the vascular specialist to deliver a broad spectrum of therapies through the low-profile retrograde tibial-pedal access. The increased use of EVUS guidance can potentially improve patient safety and decrease the amount of contrast and radiation utilized, which translates into benefits for the patients, staff and operators.
Future perspective
The field of endovascular interventions for advanced PAD and CLI has evolved dramatically over the last 10 years, thanks to disruptive technologies and to our enhanced understanding of the atherosclerotic process that affects the lower extremities. Current limitations are related to our ability to properly treat heavily calcified, long chronic total occlusions and to obtain positive, durable outcomes. Future iterations of current technologies should bring even lower profile access and treatment tools, with improved engineering design to allow increased success in crossing and treating lesions. Stent technology for infrapopliteal lesions should bring self-expanding platforms with enough radial strength to resist fractures in superficial locations such as the distal tibials and pedal arteries, where improved bioabsorbable platforms may be an option, although early results have been disappointing. Further development of cell- and gene-based therapies is also likely to evolve in the near future, however efforts to this point have failed to come to fruition. Last but not least, development of nanobiotechnologies will likely provide us with the ability to deliver site-specific therapies, which could maximize efficacy while decreasing or eliminating the risk for systemic side effects.
Financial & competing interests disclosure
JA Mustapha and F Saab are consultants to Cardiovascular Systems, Inc., Cook Medical, Covidien and Terumo. LJ Diaz- Sandoval is a consultant to Cardiovascular Systems, Inc. and Terumo. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Scope of the problem
• Patients with advanced peripheral arterial disease and critical limb ischemia have multilevel and multivessel disease with complex, long and heavily calcified stenoses and occlusions, which have traditionally been approached in antegrade fashion with an unacceptable failure rate of 20–40% in the published literature.
Therapeutic evolution
• The contralateral ‘up and over’ technique limits the ability to directly transmit energy to the tip of the crossing devices, resulting in lost pushability, torqueability and maneuverability, all of which are potential explanations for the failure rates of this approach.
Patient selection
• The antegrade ipsilateral approach circumvents many of these issues, however, a significant proportion of these patients tend to have multiple co-morbidities including morbid obesity, end-stage renal disease and conditions that limit their ability to lie flat, and increase the risk of access and procedure-related complications.
Conclusion
• The use of ultrasound-guided tibial-pedal arterial access and interventions offers a new, safe and efficacious approach to treat these patients, while decreasing the amount of contrast and radiation used. It also decreases the potential groin complications that could ensue from attempts at groin access in many of these patients with hostile groins.
References
- Brazeau NF, Pinto EG, Harvey HB et al. Critical limb ischemia: an update for interventional radiologists. Diagn. Interv. Radiol. 19, 173–180 (2013).
- Egorova NN, Guillerme S, Gelijns A et al. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J. Vasc. Surg. 51, 878–885 (2010).
- Bashir R, Cooper CJ. Evaluation and medical treatment of peripheral arterial disease. Curr. Opin. Cardiol. 18, 436–443 (2003).
- Rutherford RB, Baker JD, Ernst C et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J. Vasc. Surg. 26(3), 517–538 (1997).
- European Working Group on Critical Limb Ischemia. Second European Consensus Document on chronic critical leg ischaemia. Eur. J. Vasc. Surg. 6(Suppl. A), 1–32 (1992).
- Bosiers M, Scheinert D, Peeters P et al. Randomized comparison of everolimus-eluting versus bare metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. The DESTINY study. J. Vasc. Surg. 55, 390–399 (2012).
- Balzer J, Zeller T, Rastan A et al. Percutaneous interventions below the knee in patients with critical limb ischemia using drug eluting stents. J. Cardiovasc. Surg. 51, 183–191 (2010).
- Siablis D, Karnabatidis D, Katsanos K et al. Infrapopliteal application of Paclitaxel-eluting stents for critical limb ischemia: Midterm angiographic and clinical results. J. Vasc. Interv. Radiol. 18, 1351–1361 (2007).
- Lambert MA, Belch JJF. Medical management of critical limb ischemia: where do we stand today? J. Intern. Med. 274, 295–307 (2013).
- Hirsch AT, Haskal ZJ, Hertzer NR et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter Society Consensus; and Vascular Disease Foundation. Circulation 113, e463–e654 (2006).
- Gottsater A. Managing risk factors for atherosclerosis in critical limb ischaemia. Eur. J. Vasc. Endovasc. Surg. 32, 478–483 (2006).
- Norgren L. Pharmacotherapy for critical limb ischaemia. Diabetes Metab. Res. Rev. 16(Suppl. 1), S37–S41 (2000).
- European Work Group on Study of Severe Peripheral Ischemia. Second European consensus document on chronic critical leg ischemia. Circulation 84(4 Suppl.), IV1–IV26 (1991).
- Misra S, Lookstein R, Rundback J et al. Proceedings from the Society of Interventional Radiology Research consensus panel on critical limb ischemia. J. Vasc. Interv. Radiol. 24(4), 451–458 (2013).
- Dormandy J, Heeck L, Vig S. The natural history of claudication: risk to life and limb. Semin. Vasc. Surg. 12(2), 123–137 (1999).
- Muluk SC, Muluk VS, Kelley ME et al. Outcome events in patients with claudication: a 15-year study in 2777 patients. J. Vasc. Surg. 33(2), 251–257; discussion 257–258 (2001).
- Dormandy JA, Charbonnel B, Eckland DJ et al. Secondary prevention of macrovascular events in patients with Type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366(9493), 1279–1289 (2005).
- Nehler M, Peyton B. Is revascularization and limb salvage always the treatment for critical limb ischemia? J. Cardiovasc. Surg. (Torino)45(3), 177–184 (2004).
- Varu V, Hogg M, Kibbe M. Critical limb ischemia. J. Vasc. Surg. 51, 230–241 (2010).
- Norgren L, Hiatt WR, Dormandy JA et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur. J. Vasc. Endovasc. Surg. 33(Suppl. 1), S1–S75 (2007).
- Pipinos I, Judge A, Selsby J et al. The myopathy of peripheral arterial occlusive disease: part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc. Endovascular Surg. 41(6), 481–489 (2008).
- Pipinos I, Judge A, Selsby J et al. The myopathy of peripheral arterial occlusive disease: part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc. Endovascular Surg. 42(2), 101–112 (2008).
- Rogers RK, Hiatt WR. Pathophysiology and treatment of critical limb ischemia. 2013. www.vascularmed.org/clinical_ archive/Pathophysiology-Treatment-of-CLI_11Feb2013.pdf
- Becker F, Robert-Ebadi H, Ricco JB et al. Chapter 1: definitions, epidemiology, clinical presentation and prognosis. Eur. J. Vasc. Endovasc. Surg. 42(S2), S4–S12 (2011)
- Nehler MR, Hiatt WR, Taylor LM. Is revascularization and limb salvage always the best treatment for critical limb ischemia? J. Vasc. Surg. 37(3), 704–708 (2003).
- Romiti M, Albers M, Brochado-Neto N et al. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J. Vasc. Surg. 47, 975–981 (2008).
- Jaffery Z, Grant AG, White CJ. Critical limb ischemia: does long-term patency matter? Vasc. Med. 15(6), 439–441 (2010).
- Das TS, McNamara T, Gray BH et al. Primary cryoplasty therapy provides durable support limb salvage in critical limb ischemia patients with infrapopliteal lesions: 12-month follow-up results from the BTK Chill Trial. J. Endovasc. Ther. 16, II19–II30 (2009).
- Ansel GM, Sample NS, Botti III CF Jr et al. Cutting balloon angioplasty of the popliteal and infrapopliteal vessels for symptomatic limb ischemia. Catheter Cardiovasc. Interv.61(1), 1–4 (2004).
- Rocha-Singh,, Jaff M, Joye J et al. Major adverse limb events and wound healing following infrapopliteal artery stent implantation in patients with critical limb ischemia: The XCELL trial. Catheter Cardiovasc. Interv. 80(6), 1042–1051 (2012).
- Rastan A, Tepe G, Krankenberg H et al. Sirolimus-eluting stents vs bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur. Heart J.32(18), 2274–2281 (2011).
- Sheinert D, Katsanos K, Zeller T et al. A prospective, randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J. Am. Coll. Cardiol. 60, 2290–2295 (2012).
- Zeller T, Sixt S, Schwarzwalder U et al. Two-year results after directional atherectomy of infrapopliteal arteries with the SilverHawk device. J. Endovasc. Ther. 14, 232–240 (2007).
- McKinsey JF, Goldstein L, Khan HU et al. Novel treatment of patients with lower extremity ischemia: use of percutaneous atherectomy in 579 lesions. Ann. Surg. 248(4), 519–528 (2008).
- Kandzari DE, Kiesz RS, Allie D et al. Procedural and clinical outcomes with catheter-based plaque excision in critical limb ischemia. J. Endovasc. Ther. 13(1), 12–22 (2006).
- Laird JR, Zeller T, Gray BH et al. Limb salvage following laser-assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J. Endovasc. Ther. 13(1), 1–11 (2006).
- Shammas NW, Lam R, Mustapha J et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J.Endovasc. Ther. 19, 480–488 (2012).
- Dattilo R. 12 month results of Compliance 360: a prospective, multicenter, randomized trial comparing orbital atherectomy to balloon angioplasty for calcified femoropopliteal lesions. J. Am. Coll. Cardiol. 59(13) E2085 (2012).
- Das T, Mustapha J, Indes J. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities: the CONFIRM series, a prospective multicenter registry. Catheter Cardiovasc. Interv. 83(1), 115–122 (2014).
- Schmidt A, Piorkowski M, Werner M et al. First experience with drug-eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. J. Am. Coll. Cardiol. 58, 1105–1109 (2011).
- Liistro F, Porto I, Angioli P et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation 128, 615–621 (2013).
- Zeller T, Baumgartner I, Scheinert D et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia. J. Am. Coll. Cardiol. 64, 1568–1576 (2014).
- Mustapha JA, Diaz-Sandoval LJ. Management of infrapopliteal arterial disease: critical limb ischemia. Intervent. Cardiol. Clin. 3, 573–592 (2014).
- Mustapha JA, Diaz-Sandoval LJ, Saab F. Extravascular ultrasound imaging of infrainguinal vessels and CLI devices. In: Critical Limb Ischemia: CLI Diagnostics and Interventions.Mustapha JA (Ed.). HMP Communications LLC, PA, USA. 101–118 (2015).
- Grier D, Hartnell G. Percutaneous femoral artery puncture: practice and anatomy. Br. J. Radiol. 63(752), 602–604 (1990).
- Spector KS, Lawson WE. Optimizing safe femoral access during cardiac catheterization. Catheter Cardiovasc. Interv. 53(2), 209–212 (2001).
- Grossman M. How to miss the profunda femoris. Radiology 111(2), 482 (1974).
- Dotter CT, Rosch J, Robinson M. Fluoroscopic guidance in femoral artery puncture. Radiology 127(1), 266–267 (1978).
- Spijkerboer AM, Scholten FG, Mali WP, van Schaik JP. Antegrade puncture of the femoral artery: morphologic study. Radiology 176(1), 57–60 (1990).
- Garrett PD, Eckart RE, Bauch TD, Thompson CM, Stajduhar KC. Fluoroscopic localization of the femoral head as a landmark for common femoral artery cannulation. Catheter Cardiovasc. Interv. 65(2), 205–207 (2005).
- Mustapha JA, Saab F, McGoff T et al. Tibio-pedal arterial minimally invasive retrograde revascularization in patients with advanced peripheral vascular disease: the TAMI technique, original case series. Catheter Cardiovasc. Interv. 83, 987–994 (2014).
- Adam DJ, Beard JD, Cleveland T et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 366(9501), 1925–1934 (2005).
- Montero-Baker M, Schmidt A, Braunlich S et al. Retrograde approach for complex popliteal and tibioperoneal occlusions. J. Endovasc. Ther. 15, 594–604 (2008).
- Venkatachalam S, Bunte M, Monteleone P et al. Combined antegrade-retrograde intervention to improve chronic total occlusion recanalization in high risk critical limb ischemia. Ann. Vasc. Surg. 28(6), 1439–1448 (2014).