Research Article - International Journal of Clinical Rheumatology (2019) Volume 14, Issue 5
Relation of osteopontine levels in plasma and synovial fluid of patients with knee osteoarthritis to magnetic resonance imaging findings of the knee joint
- Corresponding Author:
- Rasha A Abdel-Magied
Department of Rheumatology and Rehabilitation
Faculty of Medicine
Minia University, Egypt
E-mail: rashahazem@yahoo.com
Abstract
Objective: to detect the levels in plasma and synovial fluid osteopontine (OPN) in patients with primary osteoarthritis (OA) and their relation to progressive joint damage as detected by plain radiography, Magnetic Resonance Imaging (MRI) and its impact on both functional status and disease severity.
Design: 60 patients with primary knee OA and 60 healthy controls were included. Functional status was assessed using Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), pain was assessed using Visual Analogue Scale (VAS). Kellgren-Lawrence grading scale (KL) was used to assess radiological severity. MRI using magnetic resonance imaging osteoarthritis knee score (MOAKS score), levels of plasma and synovial fluid OPN were measured.
Results: Mean synovial fluid and plasma OPN of OA patients was 218.4 ± 37.7 and 136.67 ± 35.1. Plasma OPN was significantly higher in OA than control (p<0.0001). Significant positive correlation between serum and synovial OPN in OA patients (r=0.8, p<0.0001). Significant positive correlation between synovial fluid OPN and patients' age, disease duration, VAS, WOMAC, KL scale and parameters of MOAKS score. Significant positive correlation between plasma OPN and body mass index (BMI), VAS, WOMAC and KL scale parameters of MOAKS score. OPN level were significantly higher in grade ӀV KL than other grades. Synovial fluid OPN and bone marrow lesion (BML) score were the determining predictors for the function status (p=0.003) for both and severity (p=0.004, p<0.0001). Plasma and synovial OPN show high specificity and sensitivity in relation to BML score as parameter of activity and cartilage loss score as parameter of chronicity.
Conclusion: Plasma and synovial fluid OPN correlated with the clinical features, functional status, radiological features of Knee OA and severity MOAKS score. Synovial OPN can predict both functional impact and radiological severity in patients with knee OA.
Keywords
osteoarthritis, osteopontine, functional status, radiological severity
Introduction
Osteoarthritis (OA) is a chronic painful joint disease that is characterized by structural changes to the joint including; articular cartilage loss, osteophytes development, inflammation in the synovium, changes in the subchondral bone, damage of the menisci, and laxity of the ligaments. It results from multiple factors including genetic, metabolic, biomechanical and biochemical factors [1].
Biochemical markers are used for detection of both disease and its severity. Therefore, the extracellular matrix proteins were crucial to the pathogenesis and progress of osteoarthritis. some extracellular matrix proteins such as osteopontine (OPN) was found to play important roles in promoting the inflammatory occurrence of cartilage cells in knee osteoarthritis, OPN can mediate cellular growth, survival, adhesion and migration in osteoarthritis [2,3].
The current reference standard for grading the severity of osteoarthritis (OA) in the knee is the radiography based Kellgren and Lawrence score [4]. This technique only indirectly visualizes the cartilage and is not able to (semi) quantitatively measure cartilage quality [5]. Therefore, quality of cartilage in terms of the sulphated glycosaminoglycan (sGAG), collagen or sodium content of articular cartilage can be measured using magnetic resonance imaging (MRI) techniques [6].
In last decades, MRI had become the most important modality for assessment of pathologic changes in knee cartilage as it allows the manipulation of contrast to highlight different tissue types [7]. The etiopathogenesis of disease as well as structure-function relationships can be detected using semi-quantitative MRI scoring. The magnetic resonance imaging osteoarthritis knee score (MOAKS) score which is a semiquantitative scoring method for MRI assessment of knee OA which can detect scoring of Bone Marrow Lesions (BMLs) (through providing regional delineation and scoring across regions), cartilage (through sub-regional assessment), and the elements of meniscal changes (including meniscal hypertrophy, partial maceration and progressive partial maceration) and subluxation scoring [8].
The aim of the study was to detect the levels in plasma and synovial fluid OPN of patients with primary OA and their relation to progressive joint damage as detected by plain radiography, MRI, and its impact on both functional status and disease severity.
Methods
Sixty patients with primary knee osteoarthritis with effusion candidate for aspiration were enrolled in the study; their diagnosis met the ACR Criteria for diagnosis of knee OA [9]. Sixty apparently healthy persons were also enrolled as controls. Exclusion of patients with either; diabetes mellitus, other causes of arthritis, history of receiving chondroprotective drugs or systemic corticosteroid medication in the last 6 months, recent intra-articular either corticosteroid or hyalouric injection within the last 3 months, history of trauma, or generalized osteoarthritis.
The protocol of the study was approved by the local Ethics Committee of Faculty of Medicine and was in agreement to the World Medical Association Declaration of Helsinki and an informed consent was obtained from all patients.
Full history taking and clinical examination of patients especially for the knee joints was performed. Assessment of function status using Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC); for assessment of pain, stiffness and physical function of patients with knee OA. The scores are summed for items in each subscale as follows: pain=0-20, stiffness=0-8, physical function=0-68 and the total score of WOMAC is calculated by adding the items of the three subscales (0-96) [10]. Joint pain was evaluated using Visual Analogue Scale (VAS) [11]. Radiological severity was assessed using Kellgren-Lawrence grading scale on an antero-posterior view of the knee joint on standing position [4].
MRI was performed for the most affected knee joint using 1.5 Tesla scanners (Philips Medical Systems, Japan). Axial, sagittal and coronal fat saturated, proton density weighted dual spin echo images were from pulse sequence used in the assessment of knee OA features. Presence or absence of cartilage loss and its score, BMLs and its score, marginal osteophytes, meniscal lesion, subchondral bone cyst, subchondral bone sclerosis, ligamentous abnormalities as parameters of magnetic resonance imaging osteoarthritis knee score (MOAKS score) in painful knees were the key for evaluation of tibio-femoral joint (tibial plateau, central weight bearing and posterior portions of femoral condyles), however for statistical purpose, absence of each of these specific feature was graded zero and its presence was graded one.
Laboratory investigation included Erythrocyte Sedimentation Rate (ESR), C-Reactive protein (CRP) and plasma Osteopontine (OPN) level for both patients and controls and in synovial fluid level of OPN for patients only; theses were measured by Enzyme-Linked Immuno Sorbent Assay (ELISA) (Quantkine ELISA kit D 05T00 USA). Plasma samples were collected using EDTA as anticoagulant. The results of both plasma and synovial fluid OPN levels were expressed in ng/ml relative to standards included in test kit (manufactured by Eiiab Company in China). Synovial fluid aspiration from OA patients for assessment of OPN level in synovial fluid, however synovial fluid was not aspirated from controls for ethical considerations.
Statistical analysis
The analysis of data was done using personal computer using SPSS (Statistical program for social science) version 16 (SPSS Inc., Chicago, IL, USA). The categorical and quantitative variables were respectively described in the form of numbers/percentage (%) and mean ± SD. Comparison between variables were done using Mann Whitney or Chi-squared (x2) tests. Comparison of more than 2 means were done using Analysis of Variance test (ANOVA or F-test). Spearman rho correlation coefficient was used to calculate correlation between variables. Regression analysis was performed to find predictors of functional disability and disease severity of OA. Sensitivity and specificity of plasma and synovial OPN levels independent of BMLs score and cartilage loss score by MRI using Receiver Operator Characteristic (ROC) curve. p-values<0.05 were considered significant.
Results
The OA patients' characters; demographic data, laboratory investigation, functional status, radiological grading, MRI findings and MOAKS score (Table 1), Figures 1 and 2 represent plain radiography and MRI finding. In the control the mean ESR was 7.36 ± 1.93 mm/1st hour, the mean level of serum OPN was 55.43 ± 18.56 ng/ ml. Serum OPN show significantly higher level in OA patients than control group (p<0.0001). Mean levels of OPN in plasma and synovial fluid in different OA grading, BML score grading and cartilage loss score grading are presented in Table 2.
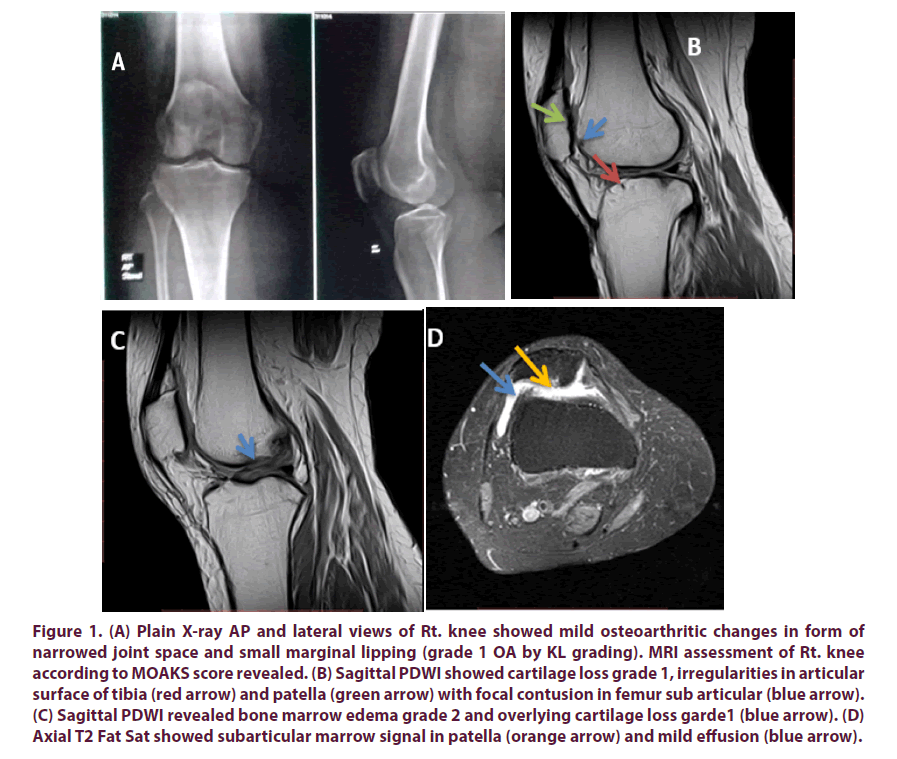
Figure 1: (A) Plain X-ray AP and lateral views of Rt. knee showed mild osteoarthritic changes in form of narrowed joint space and small marginal lipping (grade 1 OA by KL grading). MRI assessment of Rt. knee according to MOAKS score revealed. (B) Sagittal PDWI showed cartilage loss grade 1, irregularities in articular surface of tibia (red arrow) and patella (green arrow) with focal contusion in femur sub articular (blue arrow). (C) Sagittal PDWI revealed bone marrow edema grade 2 and overlying cartilage loss garde1 (blue arrow). (D) Axial T2 Fat Sat showed subarticular marrow signal in patella (orange arrow) and mild effusion (blue arrow).
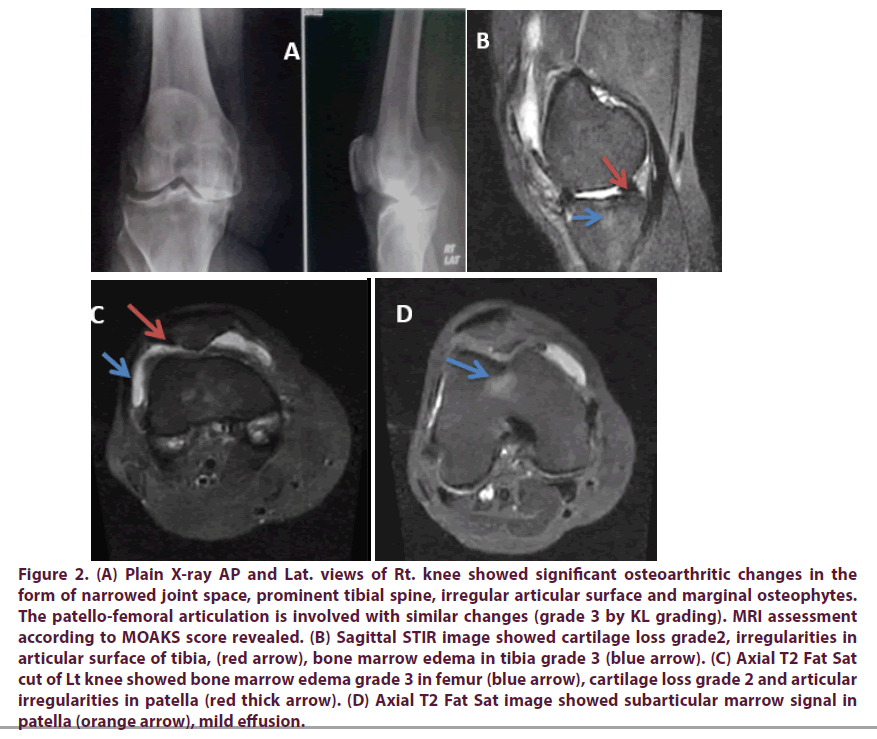
Figure 2: (A) Plain X-ray AP and Lat. views of Rt. knee showed significant osteoarthritic changes in the form of narrowed joint space, prominent tibial spine, irregular articular surface and marginal osteophytes. The patello-femoral articulation is involved with similar changes (grade 3 by KL grading). MRI assessment according to MOAKS score revealed. (B) Sagittal STIR image showed cartilage loss grade2, irregularities in articular surface of tibia, (red arrow), bone marrow edema in tibia grade 3 (blue arrow). (C) Axial T2 Fat Sat cut of Lt knee showed bone marrow edema grade 3 in femur (blue arrow), cartilage loss grade 2 and articular irregularities in patella (red thick arrow). (D) Axial T2 Fat Sat image showed subarticular marrow signal in patella (orange arrow), mild effusion.
Table 1. Characters of OA patients.
| Parameters mean ± SD and/or n (%) | OA patient n.=60 | |
|---|---|---|
| Age (y) | 46-72(56.3 ± 7.95) | |
| DD (y) | 2-10 (5.8 ± 2.33) | |
| BMI | Over weight | 4 (6.7%) |
| Obese Ι | 16 (26.7%) | |
| Obese ΙΙ | 24 (40%) | |
| Obese ΙΙΙ | 16 (26.7%) | |
| VAS | 5-10 (7.53 ± 1.22) | |
| WOMAC total score | 45-96 (74.26 ± 14.07) | |
| KL grading | Ι | 3 (5) |
| ΙΙ | 21(35 %) | |
| ΙΙΙ | 23 (38.33 %) | |
| ΙV | 13 (21.67 %) | |
| MRI findings | BML | 39 (65%) |
| Bone marrow cyst | 36 (60%) | |
| Subchondral bone sclerosis | 36 (60%) | |
| Osteophytes | 43 (71.7%) | |
| Ligamentous lesion | 35 (58.3%) | |
| Meniscal lesions | 47 (78.3%) | |
| Cartilage loss | 38 (63.3%) | |
| BMLs by MRI | 0 | 35% |
| Ι | 38.33% | |
| ΙΙ | 20% | |
| ΙΙΙ | 6.67%. | |
| Cartilage loss score by MRI | 0 | 36.67% |
| Ι | 38.33% | |
| ΙΙ | 20% | |
| ΙΙΙ | 5% | |
| Plasma OPN (ng/ml) | 92-222 (136.67 ± 35.1) | |
| Synovial fluid OPN (ng/ml) | 162-298 ( 218.4 ± 37.7) | |
| ESR mm/ 1st hour | 8-36 (23.97 ± 7.4) | |
| CRP | 53 (88.3%) | |
OA: osteoarthritis; DD: Disease Duration; BMI: Body Mass Index; VAS: Visual Analogue Scale, WOMAC score: Western Ontario and Macmaster; K.L: Kellgran & Laurence grading scale; MRI: Magnetic Resonance Imaging; BML: Bone Marrow Lesion; BMLs: Bone Marrow Lesion score; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; OPN: Osteopontine.
Table 2. Level of plasma and synovial fluid OPN in different K-L OA grading, BML score grading and cartilage loss score grading.
| K-L grading | Grade Ӏ | Grade ӀӀ | Grade ӀӀӀ | Grade ӀV | F | P |
|---|---|---|---|---|---|---|
| Plasma OPN (ng/ml) | 100-132 (115.3 ± 16.04) | 92-164 (119.7 ± 19.7) | 95-222) (139.78 ± 41.6) | 115-213 (163.46 ± 28.1) | 5.667 | 0.002 |
| Synovial fluid OPN (ng/ml) | 170-200 (183.3 ± 15.2) | 162-266 (202.14 ± 29.5) | 170-286 (221.47 ± 39.54) | 200-298 (247.3 ± 30.54) | 5.967 | 0.001 |
| BMLs | Grade 0 | Grade Ӏ | Grade ӀӀ | Grade ӀӀӀ | F | P |
| Plasma OPN (ng/ml) | 92-164 (118.66 ± 19.34) | 95-222 (129.8 ± 29.55) | 102-210 (159 ± 35.8) | 190-213 (203.2 ± 10.4) | 14.348 | <0.0001 |
| Synovial fluid OPN (ng/ml) | 162-266 (197.85±26.15) | 170-298 (220±39.39) | (186-286) (232.16±31.7) | 258-290 (275.75±14.56) | 7.634 | <0.0001 |
| Cartilage loss score | Grade 0 | Grade Ӏ | Grade ӀӀ | Grade ӀӀӀ | F | P |
| Plasma OPN (ng/ml) | 97-178 (130.04 ± 23.07) | 92-213 (124.26 ± 29.6) | 98-222 (156.75 ± 43.4) | 190-210 (200 ± 10) | 7.759 | <0.0001 |
| Synovial fluid OPN (ng/ml) | 162-278 (207.2 ± 28.8) | 168-285 (214.65 ± 38.3) | 178-298 (232 ± 41.4) | 258-286 (274.66 ± 14.7) | 3.99 | 0.01 |
OA: Osteoarthritis; K.L: Kellgran & Laurence grading scale; BMLs: Bone Marrow Lesion score; OPN: Osteopontine.
By using post Hoc analysis for OPN level; in K-L grading, OPN shows significantly higher level in grade IV than other grades, while in comparing the different grades of BML and cartilage loss score by MRI, OPN level were significantly higher in grade III, IV than other grades.
OPN show significantly higher levels in the presence of some MRI features as cartilage loss (t=1.94, p<0.048 for synovial fluid), subchondral cyst (t=2.99, p=0.004 for plasma, t=3.14, p<0.003 for synovial fluid), BMLs (t=3.74, p<0.0001 for plasma, t=3.75, p<0.0001 for synovial fluid) and for subchondral sclerosis (t=3.62, p<0.0001 for plasma, t=3.66, p<0.0001 for synovial fluid), however; there was no significant difference in OPN level was found for the presence of such features as osteophytes, meniscal or ligamentous abnormalities.
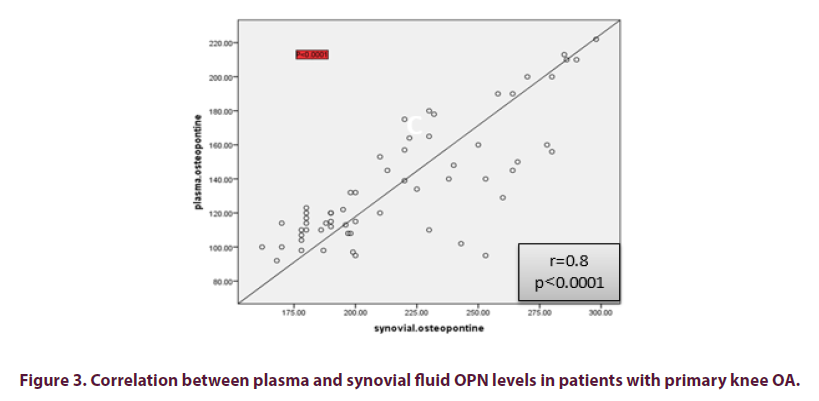
Significantly positive correlation was found between OPN level in plasma with paired synovial fluid OPN level (r=0.8, p<0.0001) (Figure 3). Plasma and synovial fluid levels of OPN correlation with the demographic, clinical, functional assessment score, VAS, laboratory data, radiological grading, and MRI findings of OA patients are presented in Table 3.
Table 3. Plasma and synovial fluid OPN level correlation OPN with demographic, clinical data, functional assessment score, VAS, laboratory data, radiological grading, and MRI findings of knee OA patients.
| Parameters r (p) | Plasma OPN | Synovial fluid OPN |
|---|---|---|
| Age | 0.032 (0.730) | 0.800 (<0.0001) |
| DD | 0.155 (0.236) | 0.390 (0.002) |
| BMI | 0.629 (<0.0001) | 0.074 (0.574) |
| Patient pain VAS | 0.431(0.001) | 0.338 (0.008) |
| WOMAC total score | 0.342 (<0.001) | 0.358 (<0.001) |
| KL grading | 0.358 (<0.001) | 0.680 (<0.001) |
| ESR | 0.362 (0.004) | 0.087 (0.510) |
| CRP | 0.895)) 0.017 | 0.689 (0.455) |
| Subchondral sclerosis | 0.397 (0.002) | 0.405 (0.001) |
| Cartilage loss | 0.145 (0.270) | 0.227 (0.08) |
| Cartilage loss score | 0.420 (0.001) | 0.381 (0.003) |
| BML | 0.379 (0.003) | 0.403 (0.001) |
| BML score | 0.625 (<0.0001) | 0.521(<0.0001) |
| Bone marrow cyst | 0.339 (0.008) | 0.360 (0.005) |
| Osteophytes | 0.039 (0.481) | 0.083 (0.529) |
OA: osteoarthritis; DD: Disease Duration; BMI: Body Mass Index; VAS: Visual Analogue Scale; WOMAC: Western Ontario and Macmaster; K.L: Kellgran & Laurence grading scale; MRI: Magnetic Resonance Imaging; BML: Bone Marrow Lesion; BMLs: Bone Marrow Lesion score; ESR: Erythrocyte Sedimentation Rate; CRP: C-Reactive Protein; OPN: Osteopontine.
For determining the function status (using WOMAC as dependent factor), many independent factors were included; the serum and synovial fluid OPN levels, patients' age, disease duration and BML score, we found that synovial fluid OPN and BML score were the significant predictors (p=0.003) for both of them; while among many independent factors including the serum and synovial fluid OPN levels, patients' age, disease duration and BML score for determining severity (using Kellgren- Lawrence grading score as dependent factor); synovial fluid OPN and BML score were the significant predictors (p=0.004, p<0.0001 respectively).
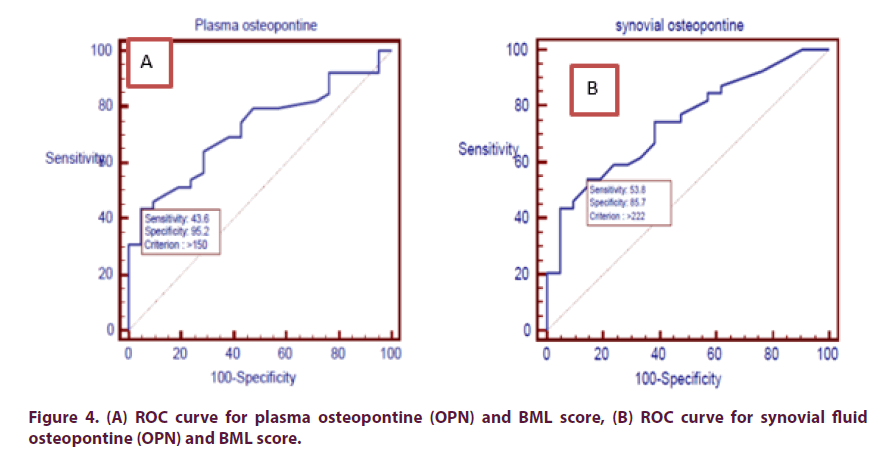
Assessment of sensitivity and specificity of plasma and synovial OPN levels independent of BML score (as parameter of activity) using ROC curve, which revealed that: at plasma OPN level (>150 ng/ml) sensitivity was 43.59% and specificity was 95.24%, accuracy is 72% and above this level BMLs appear in MRI. While at synovial fluid OPN level (>222 ng/ml), sensitivity was 53.85%, specificity was 85.71% and accuracy was 74.1%, and above this level BMLs appear in MRI (Figure 4).
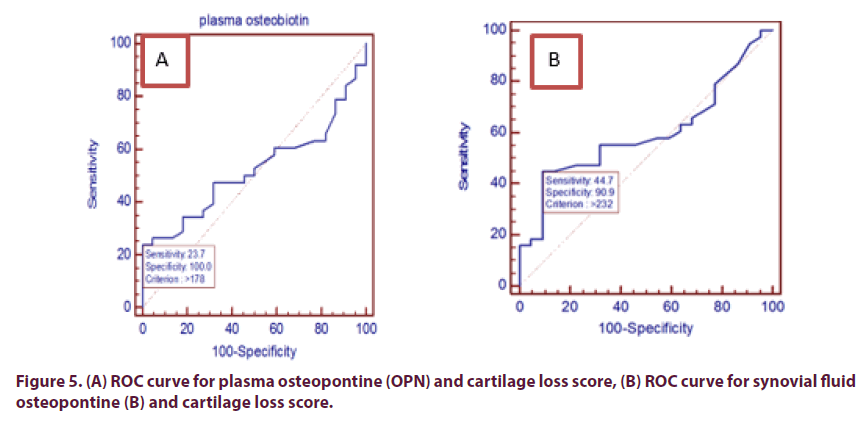
Also by assessment of sensitivity and specificity of plasma and synovial OPN levels independent of cartilage loss score (as parameter of chronicity) using ROC curve, which revealed that: at plasma OPN level (>178 ng/ml) sensitivity was 23.68% and specificity was 100%, accuracy was 52.2% and above this level cartilage loss appear in MRI. While at synovial fluid OPN level (>232 ng/ml), sensitivity was 44.74%, specificity was 90.9% and accuracy was 59.7% and above this level cartilage loss appear in MRI (Figure 5).
Discussion
Osteoarthritis (OA) is a low-grade inflammatory disease involving synovial joints and is considered the most common form of arthritis that cause chronic pain and physical disability [12]. The changes that are observed in both articular cartilage and subchondral bone in OA include sclerotic changes, articular cartilage thinning, subchondral plate thickening and thickness subchondral cortex, formation of osteophyte, vascular invasion of the calcified cartilage leading to both bone marrow lesions and bone cysts in the subchondral compartment development [13,14].
Bone marrow lesions (BMLs) which are focal changes in the subchondral bone are features that can be detected by magnetic resonance imaging (MRI) and associated with the severity features of OA including symptoms such as pain and degeneration of osteo-chondral unit which occur in both early asymptomatic OA and in severe late-stage OA [15-18].
Osteopontine is a multifunctional phosphoprotein which is considered as a critical intrinsic regulator which plays an important role in the progression of OA [19,20]. The increased expression of OPN has been observed to be related to the severity of joint involvement and the inflammatory status in OA [21,22].
This aim of the study was to detect OPN levels in both plasma and synovial fluid in patients with primary knee OA, and to determine the relation between these levels and both disease severity as detected MRI of osteoarthritic knee using MOAKS score (for cartilage loss and BML) and functional status. 60 patients with knee OA were included and 60 apparently healthy individuals were matched as regard age and sex and served as a control group.
In this study WOMAC score and K-L grading show positive correlation with both plasma and synovial OPN levels. These results were in agreement with many previous studies as; Mohammed et al. [23] Lee et al. [24] and Kim et al. [25] who found positive correlation between plasma OPN level with WOMAC index and K-L grades, while synovial fluid OPN level positively correlated with K-L scale.
In our study plasma osteopontine show statistically significant higher level in OA patients than control group and OPN levels in synovial fluid of OA patients were significantly higher with respect to paired plasma level. Plasma OPN level show significant positive correlation with paired synovial fluid OPN level and both of these levels were correlated with VAS of pain. These results were previously proven in many studies as detected by Honsawek, et al. [21], Mohammed et al. [23], Qin et al. [3] and Haider et al. [26].
Although these findings were inconsistent with Hasegawa et al. [2] as they proved that no statistically significant difference between the levels of non-thrombin-cleaved osteopontine (OPN full-length) in OA knees and controls. In this study statistically significant positive correlation between plasma OPN and body mass index, also a statistically significant positive correlation was found between synovial fluid OPN and age and disease duration. Mohammed et al. [23] found positive correlation between plasma OPN with age and disease duration. While a positive correlation between synovial fluid OPN and disease duration was detected in their study. Elsebaie et al. [27], Haider et al. [26] and Honsawek et al. [21] disagree with our result; they did not found such significant correlations.
In this study plasma and synovial fluid OPN levels show significant positive correlation with K-L grading. Plasma & synovial fluid OPN levels were significantly higher in grade IV K-L than other grades. These results were previously proven in the studies of Honsawek et al. [21], Gao et al. [28], Hasegawa et al. [2], Honsawek et al. [29], Elsebaie et al. [27], Mohammed et al. [23] and, Haider et al. [26].
However, our results disagree with Matsui et al. [22] who noticed that severity OA changes in mice was the result of OPN deficiency and not increases. In this study a statistically significant correlation was found between age and BML, bone marrow cyst, cartilage loss, subchondral scelerosis, and meniscal lesion. These findings not studied before. Our results revealed that total WOMAC score and K-L scale was positively correlated with subchondral bone sclerosis, BML, BML score, meniscal lesion.
In this study we found near similar percentage of different grades of K-L score for knee OA to the results of the studies of Quatman et al. [30] and Haider et al. [26]. However as regarding MRI done for the same patients, Quatman et al. [30] found lower percentage than ours regarding articular cartilaginous defects (37.1%), meniscal lesions (32.3%), and ACL lesions (38.7%), BME-like lesions (45.2%), osteophytes (45.2%) and joint effusions (67.7%) of knees, however they found synovitis in 100% of knees. Haider et al. [26] found higher percentage than our results regarding the cartilage loss in medial tibiofemoral compartment (80%) and Marginal osteophytes (74%), the other MRI finding were at lower percentage than ours; subchondral cyst (52%), Bone marrow edema (44%), subchondral sclerosis (32%), meniscal abnormalities (12%), ligamentous abnormalities (4%) and synovial effusion (24%).
In this study; the plasma and synovial fluid OPN show significantly higher level in OA patients with grades III, IV according to BML classification in MOAKS score by MRI when compared to patients with grades I and II. Also they show significantly higher level in grade III in comparison with other grades according to cartilage loss classification in MOAKS score by MRI. The presence of some MRI features as cartilage loss, subchondral cyst, BMLs and subchondral sclerosis were associated with ignificantly higher OPN levels. However, the presence of osteophytes or meniscal or ligamentous abnormalities was not associated statistical significant difference in OPN level.
There was significant positive correlation between plasma and synovial fluid OPN and BML, BML score, subchondral sclerosis, cartilage loss score and bone marrow cysts, however no such significant correlation were found between plasma, synovial fluid OPN levels and meniscal lesion, ligamentous lesions, cartilage loss and osteophyte. This was in agreement with Haider et al. [26] apart from significant results regarding marginal osteophytes and synovial effusion and non-statistical significant difference regarding subchondral sclerosis.
Our study revealed that K-L scale show significant positive correlation with subchondral bone sclerosis, BML, BML score, meniscal lesion. This finding was previously proven in the studies of Joshi et al. [31] and Schiphof et al. [32].
In our study MRI lesions was found in older age group, and both cartilage loss and marginal osteophytes were the commonest MRI findings in OA patients and subchondral bone cyst and bone marrow edema were found in about half of patients. There was a significant positive correlation between BML and VAS. These were in agreement with the studies of Guermazi et al. [5] and Kim et al. [33].
Assessment of sensitivity and specificity of plasma and synovial OPN levels independent of BML score by using ROC curve, the cut-off value for plasma OPN level (>150 ng/ml) and for synovial fluid OPN level was (>222 ng/ml). For cartilage loss score the cut-off value for plasma OPN level (>178 ng/ml), for synovial fluid OPN level (>232 ng/ml).
Honsawek et al. [21] found that the determination of an accurate cut-off value for circulating osteopontine level is one critical issue in its clinical application. The documented normal circulating osteopontine levels in previous reports are highly variable, with a range from 31 ng/mL to 200 ng/mL. The exact reason is unclear but it could be attributed to the different assay systems and conditions of sample collection utilized in those studies.
Conclusion
In conclusion Plasma and synovial fluid OPN correlated with the clinical features, functional status, radiological features of Knee OA and severity MOAKS score. Synovial OPN can predict both functional impact and radiological severity in patients with knee OA.
Conflict of interest
The author(s) declared no potential conflicts of interest.
Role of the funding source
None
Acknowledgments
None.
References
- Zhang Y, Nevitt M, Niu J et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis. Rheum. 63(3), 691–699 (2011).
- Hasegawa M, Segawa T, Maeda M et al. Thrombin-cleaved Osteopontin Levels in Synovial Fluid Correlate with Disease Severity of Knee Osteoarthritis. J. Rheumatol. 38(1), 129–134 (2011).
- Qin LF, Wang WC, Fang H et al. Expression of NF-κB and osteopontin of synovial fluid of patients with knee osteoarthritis. Asian. Pac. J. Trop. Med. 6(5), 379–382 (2013).
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16(4), 494 (1957).
- Guermazi, A, Niu, J, Hayashi D et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 345, e5339 (2012).
- Felson DT, Lawrence RC, Dieppe PA et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann. Intern. Med. 133(8), 635–646 (2000).
- Felson DT. Osteoarthritis of the knee. N. Engl. J. Med. 354(8), 841–848 (2006).
- Hunter DJ, Guermazi A, Lo GH et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 19(8), 990–1002 (2011).
- Altman R, Alarcon G, Appelrouth D et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis. Rheum. 34(5), 505–514 (1991).
- Ebrahimzadeh MH, Makhmalbaf H, Birjandinejad A et al. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) in Persian Speaking Patients with Knee Osteoarthritis. Arch. Bone. Jt. Surg. 2(1), 57–62 (2014).
- Bruce B, Fries J. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress and documentation. J. Rheumatol. 30(1), 167–178 (2003).
- Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 21(1), 16–21 (2013).
- Tanamas SK, Wluka AE, Pelletier JP et al. The association between subchondral bone cysts and tibial cartilage volume and risk of joint replacement in people with knee osteoarthritis: a longitudinal study. Arthritis. Res. Ther. 12(2), 58 (2010).
- Roman-Blas JA, Herrero-Beaumont G. Targeting subchondral bone in osteoporotic osteoarthritis. Arthritis. Res. Ther. 16(6), 494 (2014).
- Zubler V, Mengiardi B, Pfirrmann CW et al. Bone marrow changes on STIR MR images of asymptomatic feet and ankles. Eur. Radiol. 17(12), 3066–3072 (2007).
- Davies-Tuck ML, Wluka AE, Wang Y et al. The natural history of bone marrow lesions in community-based adults with no clinical knee osteoarthritis. Ann. Rheum. Dis. 68(6), 904–908 (2009).
- Wluka AE, Hanna F, Davies-Tuck M et al. Bone marrow lesions predict increase in knee cartilage defects and loss of cartilage volume in middle-aged women without knee pain over 2 years. Ann. Rheum. Dis. 68(6), 850–855 (2009).
- Roemer FW, Neogi T, Nevitt MC et al. Subchondral bone marrow lesions are highly associated with, and predict subchondral bone attrition longitudinally: the MOST study. Osteoarthr. Cartil. 18(1), 47–53 (2010).
- Gao SG, Li KH, Zeng KB et al. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr. Cartil. 18(1), 82–87 (2010).
- Yamaga M, Tsuji K, Miyatake K et al. Osteopontin level in synovial fluid is associated with the severity of joint pain and cartilage degradation after anterior cruciate ligament rupture. PloS one. 7(11), e49014 (2012).
- Honsawek S, Tanavalee A, Sakdinakiattikoon M et al. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin. Biochem. 42(9), 808–812 (2009).
- Matsui Y, Iwasaki N, Kon S et al. Accelerated development of aging‐associated and instability‐induced osteoarthritis in osteopontin‐deficient mice. Arthritis. Rheum. 60(8), 2362–2371 (2009).
- Mohammed FI, El-Azeem MIA, Kamal ElDin AM. Plasma and synovial fluid osteopontin levels in patients with knee osteoarthritis: relation to radiological grade. Egypt. Rheumatol. 34(3), 131–136 (2012).
- Lee SH, Kim HR, Kim HY. Synovial Fluid Vascular Endothelial Growth Factor Could Predict Progression Of Osteoarthritis According To Radiological and Ultrasonographic Findings. Arthritis. Rheum. 65(1), 482 (2013).
- Kim HR, Lee JH, Kim KW et al. The relationship between synovial fluid VEGF and serum leptin with ultrasonographic findings in knee osteoarthritis. Int. J. Rheum. Dis. 19(1), 233–240 (2016).
- Haider HM, Amin IR, Ahmad KA. Plasma and synovial osteopontin levels, are they associated with disease severity of primary knee osteoarthritis in Egyptian patients? Egypt. Rheumatol. 37(1), 29–34 (2015).
- Elsebaie H, Elchamy HA, Kaddah EA et al. Osteopontin in Patients with Primary Knee Osteoarthritis: Relation to Disease Severity. Life Sci. J. 9(4), 3902–3909 (2012).
- Gao SG, Li KH, Zeng KB et al. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr. Cartil. 18(1), 82–87 (2010).
- Honsawek S, Yuktanandana P, Tanavalee A et al. Correlation between plasma and synovial fluid basic fibroblast growth factor with radiographic severity in primary knee osteoarthritis. Int. Orthop. 36(5), 981–985 (2012).
- Quatman CE, Hettrich CM, Schmitt LC et al. The clinical utility and diagnostic performance of magnetic resonance imaging for identification of early and advanced knee osteoarthritis: a systematic review. Am. J. Sports Med. 39(7), 1557–1568 (2011).
- Joshi V, Singh R, Kohli N et al. Evaluation of osteoarthritis of the knee with magnetic resonance imaging and correlating it with radiological findings in the Indian population. Internet. J. Orthop. Surg. 14(1) (2008).
- Schiphof D, Oei EHG, Hofman A et al. Sensitivity and associations with pain and body weight of an MRI definition of knee osteoarthritis compared with radiographic Kellgren and Lawrence criteria: a population-based study in middle-aged females. Osteoarthr. Cartil. 22(3), 440–446 (2014).
- Kim IJ, Kim DH, Jung JY et al. Association between bone marrow lesions detected by magnetic resonance imaging and knee pain in community residents in Korea. Osteoarthr. Cartil. 21(9), 1207–1213 (2013).