Research Article - International Journal of Clinical Rheumatology (2025) Volume 20, Issue 4
Racial Disparities in the Medical Management of Rheumatoid Arthritis
Jaide Cotton1,2*, Ayana Crawl-Bey1,2, Samrawit Zinabu2 and Miriam Michael2
1College of Medicine, Howard University, USA
2Department of Internal Medicine, Howard University, USA
- *Corresponding Author:
- Jaide Cotton
Department of Internal Medicine, Howard University, USA
E-mail: jaidejcotton@gmail.com
Received: 02-Apr-2025, Manuscript No. fmijcr-25-164518; Editor assigned: 04- Apr-2025, Pre-QC No. fmijcr-25-164518 (PQ); Reviewed: 18-Apr-2025, QC No. fmijcr-25-164518; Revised: 23-Apr-2025, Manuscript No. fmijcr-25-164518 (R); Published: 30-Apr-2025, DOI: 10.37532/1758- 4272.2025.20(4).414-420
Abstract
Background: Rheumatoid arthritis (RA) is a chronic, inflammatory polyarthritis characterized by erosive joint destruction and systemic complications. Early detection and prompt treatment with disease-modifying anti-rheumatic drugs (DMARDs) are essential to mitigate joint damage, prevent disability, and reduce the risk of comorbidities such as cardiovascular disease, osteoporosis, and lymphoma. Prior research has identified significant racial and socioeconomic disparities in RA management, with evidence suggesting that African American patients experience delays in treatment initiation and are under-represented in clinical research compared to their Caucasian counterparts. This study aimed to evaluate treatment utilization disparities between white and Black patients with RA. Methods: We conducted a retrospective cohort study using the TriNetX Global Network, a deidentified electronic health record-based database encompassing approximately 160 million patients from 143 healthcare organizations. RA patients were identified using International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Two cohorts were defined based on racial identification white and African American and were matched 1:1 using propensity score matching for demographics, comorbidities, and social factors. Baseline data were extracted from the 12 months preceding the index event, and patients were followed for 5 years. The primary outcomes were the proportions of patients prescribed traditional DMARDs, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, biologic therapies, and interleukin inhibitors, as determined by RxNorm codes. Group differences were evaluated using Z-tests and Kaplanââ¬âMeier survival analyses. Results: After matching, each cohort comprised 99,063 patients with comparable demographics (mean age 58.1±15.2 years; ~81% female). While DMARD utilization was similar between white (33.35%) and Black patients (34.45%), notable differences emerged in other treatment modalities. Black patients had higher rates of NSAID (22.70% vs. 16.89%) and corticosteroid (5.56% vs. 4.29%) use, whereas white patients were more likely to receive biologic therapies (13.62% vs. 11.29%) and interleukin inhibitors (2.78% vs. 2.00%). Conclusion: These findings reveal significant racial disparities in RA treatment patterns despite balanced baseline characteristics. The observed differences in therapeutic approaches may contribute to divergent long-term outcomes, underscoring the need for targeted interventions to ensure equitable care across racial groups.
Keywords
Rheumatoid arthritis • Treatment disparities • Racial disparities • DMARD • NSAIDs • Biologic therapies • Interleukin inhibitors • Healthcare inequity • Disease burden
Introduction
Rheumatoid Arthritis is a well-known and well-studied inflammatory polyarthritis involving erosive joint destruction. Early disease detection and prompt treatment with disease-modifying drugs (DMARDs) is crucial to prevent further progression of joint damage and decreased functionality [1]. Untreated and uncontrolled inflammation can also contribute to increased risk of cardiovascular disease, osteoporosis, and certain types of cancer such as lymphoma. Rheumatoid arthritis has various distinctive signs and symptoms, including rheumatoid nodules, morning stiffness, symmetric joint pain and swelling, systemic symptoms, and other extra- articular manifestations. These signs and symptoms are worsened in patients with uncontrolled, untreated, and long-standing disease [2]. There is evidence that suggests that treatment disparities exist between people of color and Caucasian patients with rheumatoid arthritis. A recent study found that at two rheumatology clinics, African American and Hispanic patients were more likely to be in a public rather than private clinic (83% vs 18%) and wait significantly longer before being started on DMARD therapy. The median wait time was 7 years for African American patients and 1 year for Caucasian patients. Of the patients with early disease of less than 5 years, Caucasian patients were more likely to have previously tried DMARD compared to their counterparts of color (64% vs 32%) [3]. Strait A et al. conducted a systematic review of 240 rheumatoid arthritis randomized clinical trials that demonstrated Caucasian patients with rheumatoid arthritis are over-represented in research. Caucasian patients with rheumatoid arthritis comprised 74.6% of research participants in 2010 and 97% in 2013 [4]. An additional study by Schmajuk et al examined 93,143 patients with rheumatoid arthritis enrolled in Medicare and found significant correlations of DMARD use with socioeconomic factors. Living in an area considered low SES, possessing low personal income (defined by needing state assistance for their Medicare Part B), male gender, and African American race were all factors that were associated with a lower likelihood of being prescribed a DMARD [5]. Various research suggests that patients with RA living in under-resourced areas and those of lower socioeconomic status had higher disease activity, worse function, as well as increased pain and disability [6-8]. Additionally, a study by Del Rincon et al demonstrated higher Erythrocyte Sedimentation Rate (ESR) levels in Hispanic and African American patients with rheumatoid arthritis compared to non-Hispanic whites from various clinical sites in Texas [9]. Although advancements in genetic epidemiology reveal ethnic differences in patients with RA of various racial backgrounds, there are certainly many factors contributing to the vast differences in outcome and prognosis [10]. This suggests a significant racial and economic disparity among patients with rheumatoid arthritis. Intrinsic biases could be preventing minorities from receiving equal access to quality care. As early detection and treatment is imperative to prevent further joint damage and other manifestations of disease progression, minorities without equitable care will experience increased rates of uncontrolled disease, disease-related disability, and poorer prognosis. The objective of this study is to conduct a retrospective cohort study using the TriNetX Global Network to investigate the patterns of commonly prescribed medications among white and African American patients with rheumatoid arthritis. By examining these prescription trends, the study seeks to uncover potential racial disparities contributing to inequitable treatment practices. The analysis will explore whether biases in healthcare are leading to differences in early detection and subsequent adequate treatment. Ultimately, this research aims to add valuable insights to the limited existing literature on racial disparities in rheumatoid arthritis treatment and highlight the urgent need for strategies that ensure equitable access to quality care for all patient populations.
Methods
We conducted a retrospective cohort study using the TriNetX Global network, a de-identified electronic health record-based database of ~160 million patients from 143 healthcare organizations. We used International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM), and RxNorm codes to identify patients with a rheumatoid arthritis diagnosis (ICD-10-CM). Meeting the above criteria defined the index event. We identified two study groups based on racial identification from the above RA cohort: White and African American. Using 1:1 propensity score matching (PSM), study cohorts were matched for demographics, co-morbidities and social factors that may influence the prescription of therapies or disease severity (Table 1). Baseline variables were captured using ICD-10-CM codes 12 months before the index event. Patients were followed for 5 years from the index event. The study outcome was the proportion of patients who were prescribed DMA, NSAID, Biologics, and Interleukin inhibitors (defined as the appearance of the corresponding RxNorm code within 5 years post-index event). The significance of the difference in proportion between the study groups was assessed using the Z-test. Analyses were performed in real-time on Feb 22, 2025, using the TriNetX live platform.
Results
Table 1 presents demographic data comparing Caucasian and African American patients diagnosed with rheumatoid arthritis, both before and after propensity score matching. The variables include mean age at index with standard deviation, total number of patients, and gender distribution. Prior to matching, Caucasian patients (n = 508,802) had a higher mean age at index (61.3 ± 15.7) compared to African American patients (n = 99,703; 58.1 ± 15.2). After matching, the mean age was equalized at 58.1 ± 15.2 for both groups (n = 99,603 each). Female patients were more prevalent in all groups, with the highest proportion observed among African American patients. Matching maintained consistent gender distributions between groups.
| Category | Age at Index (Mean ± SD) |
Total Patients | Female Patients (%) | Male Patients (%) | |
|---|---|---|---|---|---|
| 1 | Caucasian (All Index) | 61.3 ± 15.7 | 508,802 | 373,151 (73.34%) | 135,434 (26.62%) |
| 2 | African American (All Index) |
58.1 ± 15.2 | 99,703 | 80,368 (81.12%) | 18,662 (18.84%) |
| 3 | Caucasian (Matched) |
58.1 ± 15.2 | 99,603 | 80,436 (81.20%) | 18,599 (18.78%) |
| 4 | African American (Matched) |
58.1 ± 15.2 | 99,603 | 80,358 (81.12%) | 18,662 (18.84%) |
Table 1: Demographic Characteristics of Rheumatoid Arthritis Patients by Race and Matching Status
Table 2 summarizes the prevalence of common comorbid conditions among Caucasian and African American patients with rheumatoid arthritis, both before and after propensity score matching. Diagnoses are listed by ICD-10-C codes and include circulatory system diseases, hypertensive diseases, diabetes mellitus, obesity, and social determinants of health such as housing, employment, and education-related issues. African American patients, in both the unmatched and matched cohorts, show a higher prevalence of hypertensive disease, diabetes mellitus, and social risk factors (Z-codes), while circulatory system diseases remain the most common diagnosis across all groups. Propensity score matching preserved the distribution of comorbidities between racial cohorts, facilitating equitable comparisons. The analysis examined treatment utilization disparities between white and Black patients with rheumatoid arthritis, with each cohort consisting of 99,063 patients after matching on baseline characteristics such as age, gender, and comorbidities (Table 1). The average age of patients was 58.1 ± 15.2 years, with a nearly equal gender distribution (approximately 81% female and 19% male) in both cohorts and a total study population of 198,126 patients (Table 1). In terms of treatment utilization, no significant difference was observed in the use of traditional disease- modifying antirheumatic drugs (DMARDs) between the two groups, with slightly higher utilization among Black patients (34.45%) compared to white patients (33.35%) (risk difference: –1.10%, p < 0.0001; risk ratio: 0.968; odds ratio: 0.952). However, a notable disparity was found in the use of nonsteroidal anti-inflammatory drugs (NSAIDs). Black patients had a higher prevalence of NSAID use (22.70% versus 16.89% in white patients) (risk difference: –5.82%, p< 0.0001; risk ratio: 0.744; odds ratio: 0.692). This was supported by Kaplan–Meier analyses demonstrating lower survival probabilities without NSAID initiation (77.12% for white vs. 69.26% for Black; hazard ratio: 0.715, p=0.0173). Corticosteroid use was also varied among patient populations (5.56% in Black patients versus 4.29% in white patients). The differences yielded a risk difference of –1.27%, p <0.0001; risk ratio: 0.772; odds ratio: 0.762, although the hazard ratio from the Kaplan–Meier analysis (0.768) did not reach statistical significance (p = 0.1344). Moreover, white patients were more likely to receive biologic treatment (13.62% versus 11.29% in Black patients) and interleukin inhibitors (2.78% versus 2.00%). The biologic therapy disparity yielded a risk difference of 2.33% (p <0.0001; risk ratio: 1.206; odds ratio: 1.239) and a corresponding hazard ratio of 1.232 (p <0.0001) on survival analysis, while the interleukin inhibitor disparity yielded a risk difference of 0.78%, p <0.0001; risk ratio: 1.39; odds ratio: 1.401 with a hazard ratio of 1.403 (p=0.0028). Kaplan–Meier survival curves and corresponding hazard ratios indicated that white patients had an earlier initiation of biologic therapies and interleukin inhibitors.
| Treatment | White Incidence (%) |
Black Incidence (%) |
Risk Ratio | Odds Ratio | p-value | |
|---|---|---|---|---|---|---|
| 1 | DMARDs | 33.3 | 34.4 | 0.698 | 0.952 | 0.0 |
| 2 | NSAIDs | 16.9 | 22.7 | 0.744 | 0.692 | 0.0 |
| 3 | Biologics | 13.6 | 11.3 | 1.206 | 1.239 | 0.0 |
| 4 | Interleukin Inhibitors | 2.8 | 2.0 | 1.39 | 1.401 | 0.0 |
Table 2: Prevalence of Select Comorbid Diagnoses in Rheumatoid Arthritis Patients by Race and Matching Status
Table 3 compares the incidence of four classes of rheumatoid arthritis treatments—DMARDs, NSAIDs, biologics, and interleukin inhibitors—between white and Black patients. It includes corresponding risk ratios, odds ratios, and p-values for each treatment. Black patients had a higher incidence of NSAID and DMARD use, while white patients were more likely to receive biologics and interleukin inhibitors. Risk and odds ratios below 1 indicate lower relative use by Black patients, whereas values above 1 indicate greater use by white patients. All differences were statistically significant (p=0.0), highlighting racial disparities in treatment allocation.
| DIAGNOSIS (ICD-10-C) |
CAUCASIAN (ALL INDEX) | AFRICAN AMERICAN (ALL INDEX) |
CAUCASIAN (MATCHED) |
AFRICAN AMERICAN (MATCHED) | |
|---|---|---|---|---|---|
| 1 | Diseases of the circulatory system (100-199) | 1,95,243 (38.37%) | 48,054 (48.50%) | 48,011 (48.47%) | 48,044 (48.50%) |
| 2 | Hypertensive diseases (IlO-IlA) | 1, 41,959 (27.90%) | 41,027 (41.41%) | 41,008 (41.40%) | 41,017 (41.41%) |
| 3 | Diabetes mellitus (E08-E13) | 56,195 (11.05%) | 18,872 (19.05%) | 18,866 (19.04%) | 18,864 (19.04%) |
| 4 | Obesity, unspecified (E66.9) | 41,357 (8.13%) | 13,640 (13.77%) | 13,605 (13.73%) | 13,631 (13.76%) |
| 5 | Problems relate to housing and economic circumstances (Z59) |
1,585 (0.31%) | 806 (0.81%) | 728 (0.73%) | 796 (0.80%) |
| 6 | Problems related to employment and unemployment | 621 (0.12%) | 294 (0.30%) | 241 (0.24%) | 288 (0.29%) |
| 7 | Problems related to education and literacy (Z55) | 382 (0.08%) | 90 (0.09%) | 62 (0.06%) | 90 (0.09%) |
Table 3: Differences in Rheumatoid Arthritis Treatment Utilization by Race
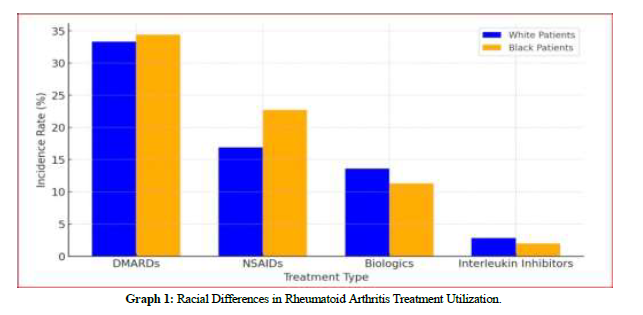
Graph 1 illustrates the incidence rates of four treatment types for rheumatoid arthritis— DMARDs, NSAIDs, biologics, and interleukin inhibitors—by race. Black patients had a slightly higher incidence of DMARD use (34.45%) compared to white patients (33.35%) (risk difference: –1.10%, p < 0.0001; risk ratio: 0.968; odds ratio: 0.952). NSAID use was more prevalent among Black patients (22.70%) than white patients (16.89%), indicating potential differences in symptom management (risk difference: –5.82%, p < 0.0001; risk ratio: 0.744; odds ratio: 0.692). Conversely, white patients had higher rates of biologic therapies (13.62% vs. 11.29%) and interleukin inhibitors (2.78% vs. 2.00%). The biologic disparity yielded a risk difference of 2.33% (p < 0.0001; risk ratio: 1.206; odds ratio: 1.239; hazard ratio: 1.232, p < 0.0001), while the interleukin inhibitor disparity showed a risk difference of 0.78% (p <0.0001; risk ratio: 1.39; odds ratio: 1.401; hazard ratio: 1.403, p=0.0028). These findings underscore significant racial variations in the prescribing patterns of rheumatoid arthritis treatments.
Graph 1: Racial Differences in Rheumatoid Arthritis Treatment Utilization.
Interpretation of Findings
- DMARDs were prescribed at similar rates between White and Black patients, but White patients were slightly less likely to continue long-term treatment, as indicated by the hazard ratio.
- NSAIDs were significantly more commonly used in Black patients (22.7% vs. 16.9%), which may reflect differences in prescribing practices, pain management approaches, or healthcare access.
- Biologic therapies and interleukin inhibitors were more frequently used by White patients, suggesting racial disparities in access to advanced RA treatments. White patients were 20.6% more likely to receive biologics and nearly 40% more likely to receive interleukin inhibitors.
- Black patients had higher survival probabilities (less exposure) for biologics and interleukin inhibitors, which may suggest barriers to accessing these advanced therapies or differences in physician prescribing patterns.
- The significant p-values across all analyses indicate strong evidence of racial disparities in RA treatment, highlighting the need for further investigation into access, affordability, and physician decision-making.
Discussion
The results shed light on various discrepancies between white and African American patients. However, there was no significant difference between the groups regarding the prescription of traditional disease-modifying antirheumatic drugs (DMARDs). There was a noteworthy difference in the prevalence of NSAIDs and corticosteroids compared to biologics. African American patients were significantly more likely to be prescribed NSAIDs and corticosteroids, while their white counterparts had an increased likelihood of receiving biologic and interleukin inhibitor treatment. This suggests that providers tend to prioritize symptomatic management in African American patients while priority is placed on slowing disease progression in white patients. This study had findings similar to those of the study by Suarez-Almazor et al. which found that African American and Hispanic patients had significantly longer wait times before being started on DMARD therapy compared to their white counterparts, with the median wait time for African Americans being 7 years and 1 year for white patients [11]. Although the findings of our study did not demonstrate significant differences in DMARD therapy, it did show that white patients had an earlier initiation of biological therapies and interleukin inhibitors. The time from diagnosis to initiation of treatment is very important in managing rheumatoid arthritis. The longer patients must wait to be treated contributes to the worsening severity of the disease and the poorer outcomes that accompany that. This study suggests that African American patients are disproportionately experiencing significantly increased time from diagnosis to treatment, which is likely contributing to their overall disease severity and progression. In addition, the results of this study align with those of Kerr et al, who demonstrated that in the private healthcare setting, African American patients with RA were less likely to receive biologics than their white counterparts [12]. The results of this study confirm the findings of various prior research, which demonstrated patients with rheumatoid arthritis of lower economic status had overall worse disease activity, disability, and function. These patients tend to be people of color, particularly African Americans, causing this population to be disproportionately negatively affected [6-8,13]. Additionally, further research has demonstrated that patients at financial risk and those paying out of pocket for medical care are more likely to have more severe disease and twice as likely to be a racial minority [14,15]. These findings contribute to the poorer prognosis, worsened outcomes, and decreased quality of life evident in African American patients with rheumatoid arthritis. Intrinsic biases and systematic injustice may be contributing to these disparities and preventing African Americans and other minorities from receiving equal access to quality healthcare. Patients of lower socioeconomic status may have only received care from primary care providers rather than rheumatology specialists. Studies have shown there are underlying racial biases specific to pain management and assessment among both medical providers and laypeople, showing persistent misconceptions regarding biological differences between races [16]. The implications of these results demonstrate the need for targeted interventions to address these disparities and ensure equitable care for all rheumatoid arthritis patients, regardless of race. Strengths of this study include a large sample size of 99,603 patients, which promotes increased statistical power and improves generalizability of the study results in its application to a broader population. We also include a meaningful subgroup analysis that can highlight inequities and treatment practices within and between racial groups, with the potential to inform future policy and practice. Possible limitations of this study include the reliance on de-identified electronic health records from the TriNetX Global Network, which may not accurately capture all relevant clinical or social factors influencing treatment decisions. Additionally, although matching was used to balance demographic, comorbidity, and social factors, unmeasured confounders may still exist. The retrospective design of the study prevents the establishment of causal relationships between racial disparities in the treatment of rheumatoid arthritis. Further research is needed to more concretely identify the factors directly influencing these disparities. In addition, the study does not account for patient preferences, physician prescribing behaviors, or potential structural barriers within the healthcare system that may influence treatment choices. Lastly, although the study population is large, the generalizability of findings may be limited, as the study population primarily consists of patients within the TriNetX database, which may not be fully representative of all individuals with rheumatoid arthritis across different healthcare settings.
Conclusion
This study highlighted the significant difference in the patterns of commonly prescribed medications between white and African American patients with rheumatoid arthritis, suggesting that despite balanced baseline characteristics, disparities in therapeutic approaches persist between white and Black patients with rheumatoid arthritis. These differences in therapeutic strategies appear to contribute to a poorer prognosis, accelerated disease progression, and a diminished quality of life among African American patients. This disparity underscores the urgent need for further research to identify additional patterns and biases within the healthcare system that may be driving these inequities. By building a robust body of evidence, there will be increased support for the imperative need for systemic change aimed at ensuring quality care and the equitable allocation of resources regardless of race or background. Possible solutions to address the disparities in rheumatoid arthritis treatment can begin with implementing comprehensive programs and frequent meetings specifically designed to combat intrinsic bias within the healthcare system. Such initiatives would raise awareness among providers about the existing treatment disparities and encourage them to consider these differences when prescribing medications to patients with rheumatoid arthritis of various racial and socioeconomic backgrounds. Additionally, encouraging the development and implementation of culturally competent care can help providers better understand and meet the unique needs of diverse patient populations. Another imperative action is increasing investment in and support of healthcare providers from a variety of racial, ethnic, and socioeconomic backgrounds. Promoting diversity within the healthcare system will bring a broader range of perspectives and experiences, fostering more inclusive and equitable care. These combined efforts can contribute to reducing racial disparities in the treatment of rheumatoid arthritis, ultimately improving patient outcomes across all demographics and assist in achieving more equitable care.
References
- Nell VP, Machold KP, Eberl G et al. Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford, England). 43(7), 906–914 (2004).
- Baker, Joshua F. Diagnosis and Differential Diagnosis of Rheumatoid Arthritis. UpTo Date, Mar. 2024.
- Yip K, Navarro-Millán I. Racial, ethnic, and healthcare disparities in rheumatoid arthritis. Curr Opin Rheumatol. 33(2), 117-121 (2021).
- Strait A, Castillo F, Choden S et al. Demographic Characteristics of Participants in Rheumatoid Arthritis Randomized Clinical Trials: A Systematic Review. JAMA Network Open. 2(11), 1914745 (2019).
- Schmajuk G, Trivedi AN, Solomon DH et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 305(5), 480-486 (2011).
- Barton JL, Trupin L, Schillinger D et al. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis Care Res (Hoboken). 63(9), 1238-1246 (2011).
- Yang G, Bykerk VP, Boire G et al. CATCH Investigators. Does socioeconomic status affect outcomes in early inflammatory arthritis? Data from a Canadian multisite suspected rheumatoid arthritis inception cohort. J Rheumatol. 42(1), 46-54 (2015).
- Parks CG, D'Aloisio AA, DeRoo LA et al. Childhood socioeconomic factors and perinatal characteristics influence development of rheumatoid arthritis in adulthood. Ann Rheum Dis. 72(3), 350-356 (2013).
- Del Rincon I, Battafarano DF, Arroyo RA et al. Ethnic variation in the clinical manifes-tations of rheumatoid arthritis: role of HLA– DRB1 alleles. Arthritis Rheum 49, 200 – 208 (2003).
- Kochi Y, Suzuki A, Yamada R et al. Genetics of rheumatoid arthritis: underlying evidence of ethnic differ-ences. J Autoimmun 32, 158 – 162 (2009).
- Suarez-Almazor ME, Berrios-Rivera JP, Cox V et al. Initiation of disease-modifying antirheumatic drug therapy in minority and disadvantaged patients with rheumatoid arthritis (in eng). The Journal of rheumatology 34(12) 2400–2407 (2007).
- Kerr GS, Swearingen C, Mikuls TR et al. Use of Biologic Therapy in Racial Minorities With Rheumatoid Arthritis From 2 US Health Care Systems (in eng). Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases 23(1), 12–18 (2017).
- Constantinescu F, Goucher S, Weinstein A et al. Understanding why rheumatoid arthritis patient treat-ment preferences differ by race. Arthritis Rheum 61, 413– 418 (2009).
- Heidari P, Cross W, and Crawford K. Do out-of-pocket costs affect medication adherence in adults with rheumatoid arthritis? A systematic review. Seminars in arthritis and rheumatism 48(1), 12–21 (2018).
- Wolfe F and Michaud K. Out-of-pocket expenses and their burden in patients with rheumatoid arthritis. Arthritis and rheumatism 61(11), 1563–1570 (2009).
- Hoffman, Kelly M et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences of the United States of America 113(16), 4296-4301 (2016).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref