Perspective - Interventional Cardiology (2009) Volume 1, Issue 1
Patient-specific treatment allocation for carotid artery disease
- Corresponding Author:
- Marc Bosiers
Department of Vascular Surgery
AZ St-Blasius, Kroonveldlaan 50
9200 Dendermonde, Belgium
Tel: +32 52 252 822
Fax: +32 52 252 289
E-mail: marc.bosiers@telenet.be
Abstract
Keywords
carotid, carotid artery stenting, carotid endarterectomy, endarterectomy, patient selection, stent
The optimal treatment of patients presenting with either asymptomatic or symptomatic carotid artery disease (CAD) has been the topic of many debates during the last few decades. Depending on their risk profile, patients with carotid artery disease can be treated with either carotid endarterectomy (CEA), carotid artery stenting (CAS) and/or optimal medical therapy. Based on a systematic review of the available literature, Hobson et al. recently published on behalf of the Society for Vascular Surgery (SVS), evidence-based guidelines for the management of carotid artery stenosis [1]. Although the presented guidelines contain profound valuable and valid information and considerations, it can be questioned whether the guidelines will resist time.
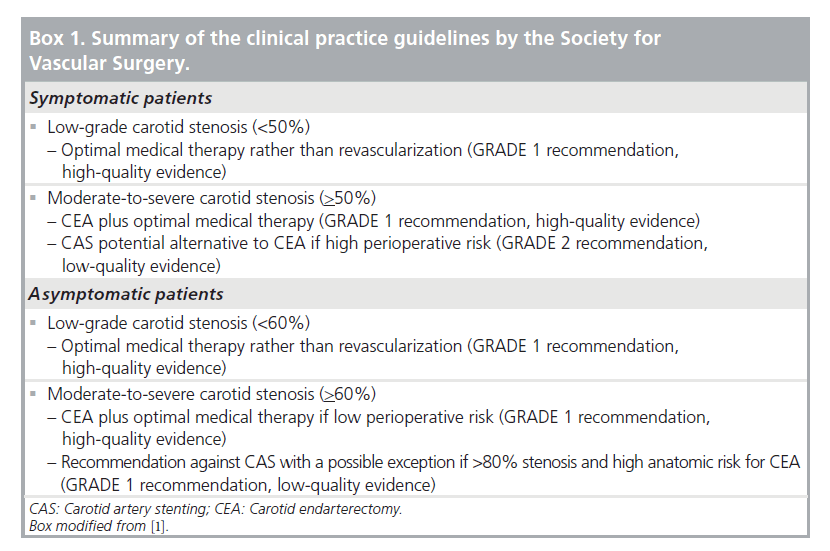
Summary of the clinical practice guidelines of the SVS
The expert committee of the SVS made the recommendations for treating CAD based on the best available evidence [1]. As depicted in Box 1, the quality of evidence is rated as high, moderate and low or very low. High-quality evidence is typically derived from well-conducted, large and consistent randomized controlled trials; moderate quality from less rigorous or inconsistent randomized controlled trials or some observational studies. Low or very low quality evidence is typically based on observational studies, case series and unsystematic clinical observations. Aside from the quality of evidence, the GRADE system has been applied to the proposed recommendations in order to indicate the strength of the supporting data and the strength of their convictions in offering the guidelines [2]. The GRADE system depicts recommendations as GRADE 1 being strong recommendations, or GRADE 2 being weak recommendations or mere suggestions. The GRADE system separates the quality of evidence from the strength of recommendations. This separation allows guideline users (clinicians, patients and policymakers) to recognize factors other than evidence, such interventionalists’ experience and preferences, and patients’ values and preferences that were considered by the expert committee while making the recommendations. Taking into consideration subjective factors such as patient preferences, guidelines supported by lesser degrees of clinical evidence might receive ‘strong recommendations’.
Indications for revascularization
As stipulated by the SVS recommendations, optimal medical therapy rather than revascularization is indicated for both asymptomatic and symptomatic patients with low-grade stenosis [1]. As shown in the European Carotid Surgery Trial (ECST), the minimal cut-off degree of internal carotid artery stenosis indicating the necessity to revascularize lesions in asymptomatic patients is 60% [3] and in the North American Symptomatic Carotid Endarterectomy Trial (NASCET), revascularization is indicated for all symptomatic patients presenting with at least 50% stenosis [4].
Carotid treatment assignment
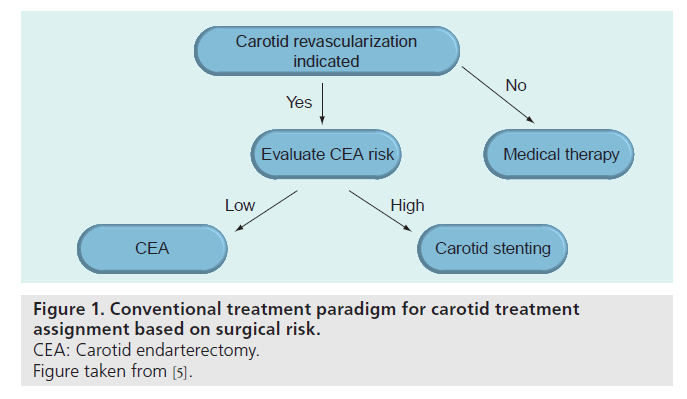
▪ Conventional paradigm based on the surgical risk
The recommendations proposed by the SVS are well reflected in the conventional paradigm for carotid treatment assignment published by Roubin et al. (Figure 1) [5]. Traditionally, candidates for carotid revascularization have been selected for either CEA or CAS on the basis of the presumed surgical risk. This means that all patients presenting with low risk for surgery are referred for CEA, and that CAS is only an option for the group of patients considered at high risk for open surgery. This conventional approach means first that CAS is only made available to a very limited population and that second, potential risk factors for CAS that can be easily identified by readily available clinical and angiographic features are neglected. This may result, particularly in asymptomatic patients, in procedural risks for CAS that outweigh the long-term risk of ipsilateral stroke with best medical therapy.
▪ CAS & CEA are not competitive but complementary treatment options
Both the standing recommendations from the SVS and the conventional treatment paradigm described by Roubin described are supported in the initial publications of the Endarterectomy versus Stenting in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) [6] and Stent- Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trials [7]. Based on the published 30-day outcome of these two randomized controlled trials, it was claimed there was no place for the widespread use of CAS. Although the 30‑day findings of these studies created strong skepticism against CAS, more recent publications on the same patient populations describing the long-term outcomes of the intervention are again more optimistic towards the endovascular approach. Both manuscripts demonstrate that if CAS can be safely achieved and the 30‑day complication rate can be kept low, it is as durable as CEA. This requires that CAS should be performed by experienced investigators in carefully selected patients [8]. The EVA-3S investigators published that up to 4 years after the index interventions there was no difference in the risk of ipsilateral stroke among CAS and CEA patients who did not have a stroke within the first 30 days [8], and in the SPACE trial, the ipsilateral ischemic strokes up to 2 years after the procedure and any periprocedural stroke or death did not differ between CAS and CEA patients [9]. In addition, the long-term results (5 years) of the Belgian–Italian Carotid (BIC) study [10] indicate that it is clear that CAS has durable benefit in terms of stroke-free survival. The annual rate of 1.36% (95% CI: 1.08–1.69) for any type of ipsilateral stroke (excluding the perioperative period), as reported in our total population, seemed to be in the same range as those of post-CEA complications reported by the historical randomized controlled trials [3,4,10–12]. In particular, the any type of stroke or perioperative death rate of 6.42% at 5 years reported for asymptomatic patients by the Asymptomatic Carotid Surgery Trial (ACST) [11] or of 5.1% reported by the Asymptomatic Carotid Atherosclerosis Study (ACAS) [12] are very close to the 6.1% rate in our asymptomatic population. In the symptomatic population the any stroke and death rates at 3 and 5 years were 5.6 and 6.9%, respectively, which are clearly comparable with the 8.5% rate of ECST at 3 years [3], and the 13% rate in NASCET [4].
The different publications of the above-mentioned trials were the source of many heroic debates between the protagonists and antagonists of the endovascular approach and they strongly impacted patient selection, treatment evolution and reimbursement policy in many countries. Now, with the most recent long-term results of CAS at hand, we might conclude that CAS and CEA do not have to be seen as competitive treatment strategies but that both are complementary. Approximately 80% of all carotid patients are eligible for both techniques, while only in the remaining 20% must careful attention be paid to predisposing factors putting them at increased risk for complications with one of the two techniques.
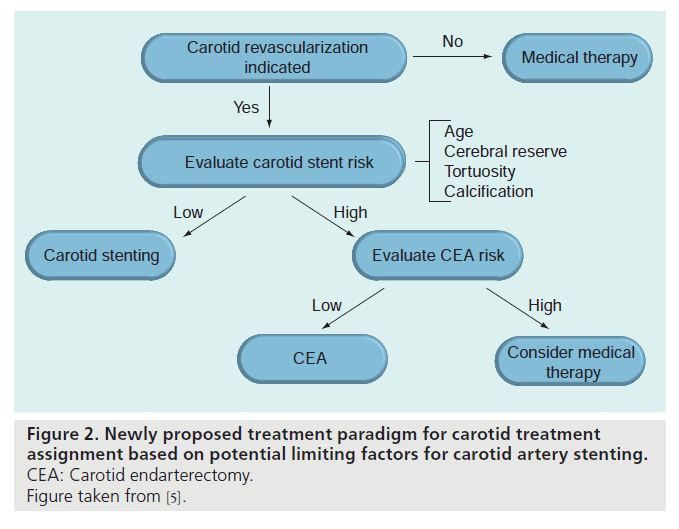
▪ An alternative proposed paradigm based on limiting factors for stenting
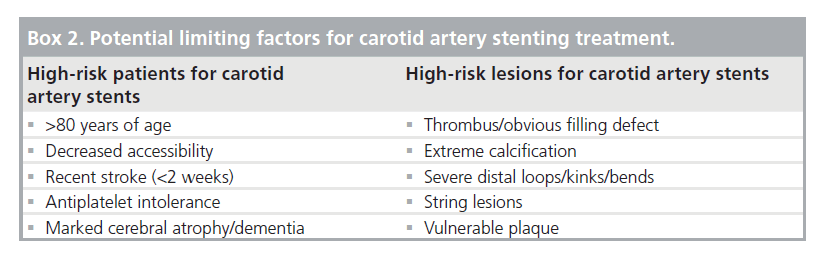
Taking into account the complementarity of CEA and CAS, an alternative treatment decision algorithm has been proposed by Roubin et al. [5]. In contrast to the conventional flow chart, the decision to allocate patients with CAD to either CEA or CAS in the newly proposed paradigm is based on potential limiting factors for CAS (Figure 2). Practically, this means that every patient with CAD might be considered as a potential candidate for CAS, but that CEA is preferred in patients presenting with any factor that potentially influences the outcome of CAS. In different publications with varying levels of evidence, both high-risk patient profiles and lesion characteristics have been identified and were used as potential CAS exclusion criteria (Box 2).
High-risk patient profile for CAS
The most important factor that has been associated with increased procedural complications is advanced age. In both the Carotid Revascularization Endarterectomy versus Stent Trial (CREST) and SPACE trial, increasing age has been directly related to a higher number of neurological complications at 30 days after CAS [13,14]. The SPACE investigators found that for the CAS population, the risk of ipsilateral stroke or death was significantly associated with age in the CAS group, whereas for the CEA group there was no difference in complication rates within the defined age groups. For patients treated with CAS, the estimated relative risk increase was estimated to be 7.2% (95% CI: 2.8–11.7%; p = 0·001) per year of age [14]. In their recent review based mostly on observational data, CEA was judged by Narins et al. to be the procedure of choice for patients aged 80 years or older [15].
Over 40% of all intraprocedural CAS events occur during the catheterization phase [16]. As any contact of the guidewire or catheter during catheterization can cause distal embolization and during this stage of the intervention no embolic protection device (EPD) is yet installed to protect the brain, all extra or unforeseen wire and catheter manipulations have to be avoided. Therefore, in order to define the best treatment strategy, preprocedural mapping of the anatomy of the access vessels is mandatory and can help exclude CAS for patients presenting with decreased accessibility due to tortuous iliac vessels and/or diseased or elongated aortic arches. Schneider et al. demonstrated that in CAS patients presenting with a proximal tortuosity index, being the sum of angles from the aortic arch to the carotid stenosis, of over 150, both neurologic complication and technical failure was significantly increased [17].
Antiplatelet intolerance might be considered as an exclusion criterion for CAS [18]. Acute stent thrombosis is a rare (incidence: 0.5–2%), but potentially fatal complication after CAS [19]. As the occurrence of acute stent thrombosis appears to be related to the lack of adequate antiplatelet therapy, most centers currently administer dual antiplatelet therapy for at least 4 weeks after CAS [20].
As shown in different studies, CAS is often associated with some degree of cerebral embolization [21]. Although the detected cerebral embolization generally does not lead to symptoms and is well tolerated in patients with good cerebral reserve, it might cause further deterioration in those with a history of decreased cerebral reserve. Therefore both marked cerebral atrophy and dementia might be important factors when one considers the risk of CAS [5].
High-risk lesion characteristics for CAS
Successful endovascular management for intraluminal carotid thrombus has been reported anecdotally [22,23]. But as any manipulation of the thrombotic area during catheterization carries a significant risk for thromboembolism and related neurological complications, it is suggested not to consider CAS as a primary treatment strategy in patients with obvious filling defects and intraluminal thrombus. We prefer CEA in those patients, but CAS remains a valid alternative if a high surgical risk patient presents with intraluminal thrombus. If so, the authors suggest administering statins for at least 4 weeks prior to the intervention to stabilize the plaque and decrease its emboligenic risk [24]. If CAS is the intervention of choice, it might be better to use, based on its theoretical working principle, a proximal occlusion device rather than a distal EPD in order to guarantee cerebral protection before lesion manipulation.
Heavy circumferential calcification is an important predictor of CAS-related complications. As the presence of highly calcified concentric plaques cause difficulties in the tracking and positioning of the stents and since, to date, no carotid stents are available that offer enough radial force to adequately achieve optimal stent expansion, CEA is probably the best option in this category of patients [25].
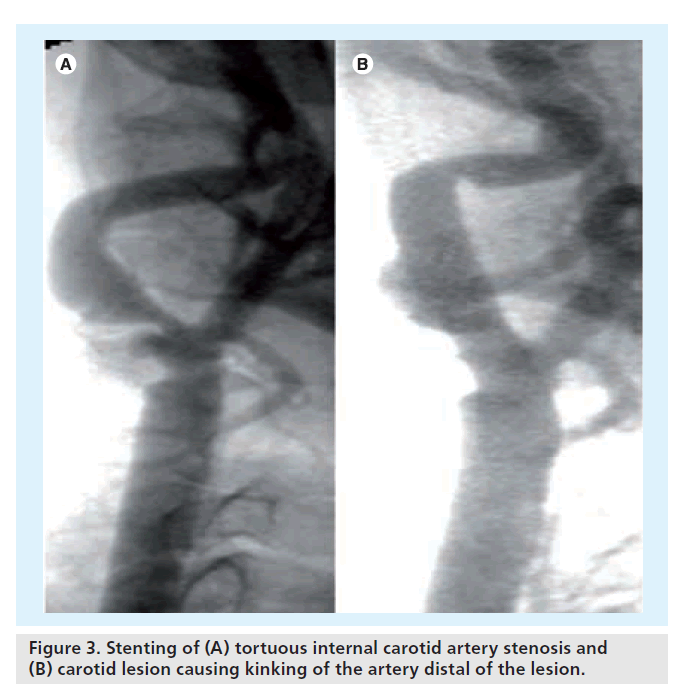
Excessive distal tortuosity might limit the successful outcome after CAS. First, it interferes with the optimal positioning of distal filtration devices. The potential suboptimal deployment of these filters exposes the patient to the risk of cerebral embolization and should be avoided. Second, stent implantation in the presence of loops, bends and/or kinking of the internal carotid artery alters the vessel’s original curve. If not placed accurately, the insertion of a carotid stent can result in even more pronounced kinking of the carotid vessel just distal of the implanted stent (Figure 3) [25].
Figure 3. Stenting of (A) tortuous internal carotid artery stenosis and (B) carotid lesion causing kinking of the artery distal of the lesion.
Patients presenting with vulnerable plaque are at significantly increased risk for complication after CAS. Biasi et al., performed the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) trial and concluded that carotid plaques with a grayscale median less than or equal to 25 are defined as echogenic and that in these patients the risk of stroke in CAS is significantly increased (p = 0.005) [26]. Therefore, the first treatment option in these patients should also be CEA. Nevertheless, if it is opted to treat these patients with CAS, it is suggested to prescribe statins in order to ‘stabilize’ the plaque before performing CAS. It was found by Gröschel et al., that preoperative statin intake for at least a week prior to CAS effectively reduced (p < 0.05) the 30-day incidence of stroke, myocardial infarction and death after CAS in symptomatic patients [24]. In the spirit of the findings of the BIC Registry [27], any patient presenting with a vulnerable lesion should receive a stent with as low a free cell area as possible [25,28]. It has to be stated that to date, no stent platform has been proven superior. Furthermore, although the findings on the free cell area of the BIC trial have been discussed and debated by the group of Schillinger et al. [29], these were recently confirmed by Jansen et al. based on a subanalysis of the SPACE trial data [30]. If the selection of a stent with such high scaffolding capacities would potentially compromise the maintenance of the vessel’s initial anatomy (e.g., potential distal kink or significant mismatch in proximal and distal diameter), the authors can only suggest performing CEA in these cases. Selecting a more flexible stent with less scaffolding potential in order to achieve optimal angiography after CAS, could place patients at a potential increased risk for postprocedural cerebral embolization [25].
▪ Incorporating the patient selection strategy into daily practice
The newly proposed algorithm has been successfully integrated into daily practice at the Department of Vascular Surgery at AZ St Blasius and the Department of Cardiovascular Surgery of the Imelda Hospital in Bonheiden and it did positively impact the outcome of both CAS and CEA.
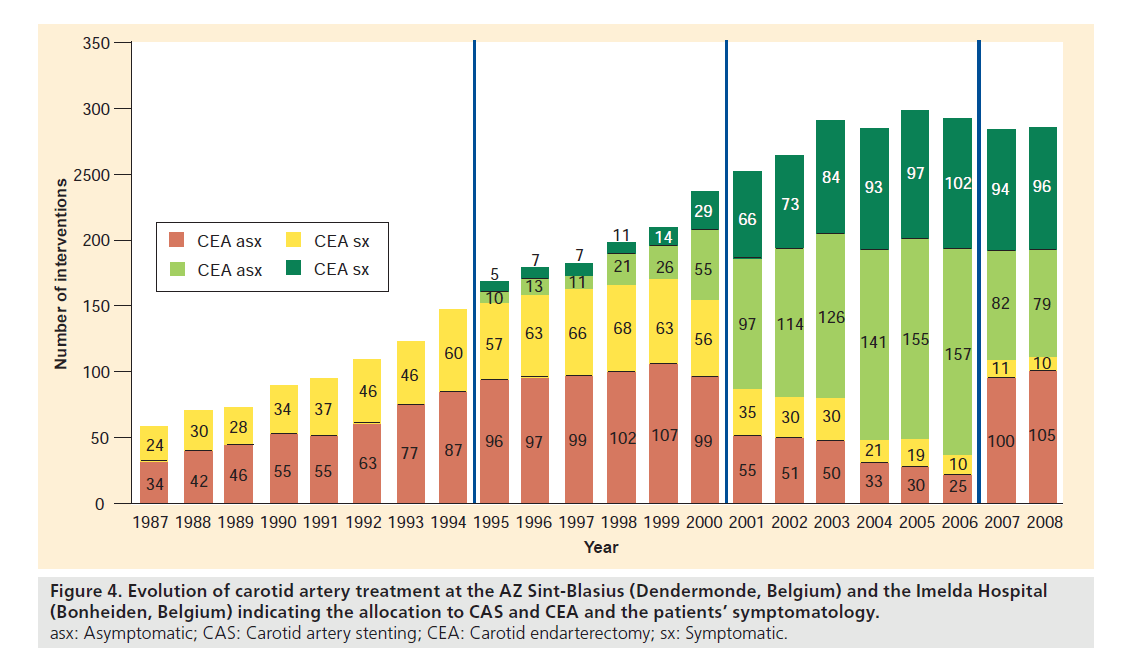
Since the start of patient data collection on carotid revascularization in 1987 until the end of 2008, a total of 4219 carotid artery revascularizations (CEA and CAS) were performed in the two centers (Figures 4 & 5). The global evolution in number of procedures is characterized by a gradual increase until the year 2003, after which a steady state of approximately 300 procedures per year was reached.
Figure 4. Evolution of carotid artery treatment at the AZ Sint-Blasius (Dendermonde, Belgium) and the Imelda Hospital (Bonheiden, Belgium) indicating the allocation to CAS and CEA and the patients’ symptomatology. asx: Asymptomatic; CAS: Carotid artery stenting; CEA: Carotid endarterectomy; sx: Symptomatic.
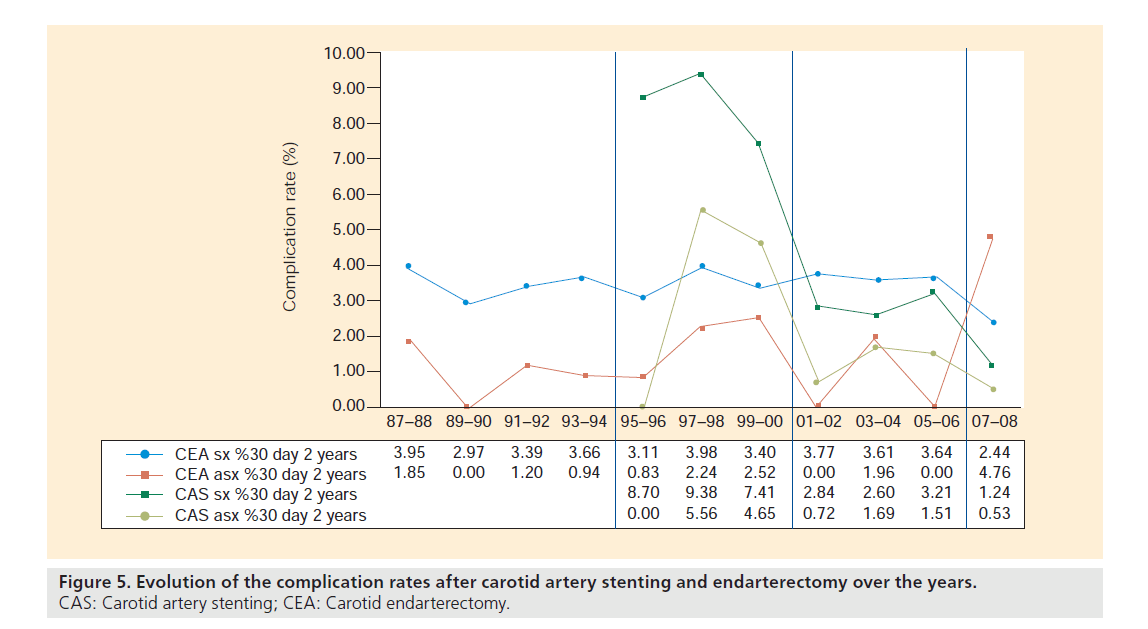
Figure 5. Evolution of the complication rates after carotid artery stenting and endarterectomy over the years. CAS: Carotid artery stenting; CEA: Carotid endarterectomy.
Historically, the evolution chart can be divided into four successive time periods (1987–1994; 1995–2000; 2001–2006; and 2007–2008) with specific characteristics in the revascularization patterns, available intervention techniques and complication rates.
The first period that can be identified goes from 1987 to 1994, and covers the first years of dedicated patient data collection in the aforementioned services. During this early time frame, CEA was the only treatment option for all carotid patients that were candidates for revascularization. There was a gradual increase in the yearly number of patients treated, going from 58 patients in 1987 up to 147 in 1994, with a constant distribution between symptomatic (avg.: 60.1%; range: 57.8–626%) versus asymptomatic patients (avg.: 39.9%; range: 42.2–37.4%). The average complication rate, defined as the combined transient ischemic attack, stroke and death rate at 30 days after the index intervention, was 2.5%.
The years 1995–2000 are considered as the second referral period, which was initiated with the performance of the first CAS procedure at the services. During these years, no EPD devices nor specific carotid stents were available and the use of CAS was limited to high surgical risk patients (e.g., recent myocardial infarction or ejection fraction <30%). Owing to the lack of dedicated materials, and probably even more importantly, experience, the average reported 30‑day stroke/death rate in our service was high (9.26%), but comparable to the outcome in the survey by Wholey et al. in the same era [31]. Although CAS had a high complication rate, the proportion of CAS interventions gradually increased until in the year 2000, approximately a third (35.15%) of all CAD patients that needed revascularization were treated endovascularly. The proportion of symptomatic patients treated with CEA and CAS between 1995 and 2000, were 61.7 and 65.1%, respectively.
The third referral period starts in the year 2001, with the development and introduction of EPD and specific low-profile carotid devices. This, in combination with growing experience, has led to a clear drop in the stroke and death rate after CAS to as low as 2.84 and 0.72% (2001–2002) for symptomatic and asymptomatic patients, respectively. With the improved results, it was decided to no longer reserve CAS for high surgical risk patients and to widen its indications. This resulted in a steep increase in the number of CAS procedures and a clear shift away from the classical towards the endovascular approach. In the years 2005–2006, approximately 85% of all carotid revascularization candidates were treated with CAS, and only 15% with CEA. Also in this period, on average a little more than six out of ten interventions (CEA: 62.7%; CAS: 60.6%) were performed in symptomatic carotid patients. Looking at the 30‑day complication rates, we observed a trend towards a lower complication rate in the symptomatic patients treated with CAS versus those treated with CEA. In the last 2 years of this period, 2005–2006, a new slight increase in the complication rate after CAS in our symptomatic patient population (3.21%) was noted.
As the latter observation might be potentially explained by the too liberal selection criteria for CAS used at the time, this necessitated a re-evaluation of the treatment selection algorithm for CAS in our services. Simultaneously with this evaluation, the publication of the 30‑day results of the EVA-3S [6] and SPACE [7] trials brought to light that the results of CAS might have been worse than generally accepted at the time. Both facts led to the introduction of the above-described paradigm based on potential limiting factors for CAS [5], which indicated the start of the fourth and last referral period from 2007 to date. With the newly introduced algorithm, more symptomatic patients were excluded from CAS and it was opted to perform CEA in those patients. This meant that again a higher number of patients were treated with CEA in 2007–2008. While in the three earlier periods the vast majority of symptomatic patients were treated with CAS (up to 86.3% in 2006 and 2007), this drastically changed in the last period. Following the algorithm, only approximately 40% of symptomatic patients are referred to stenting and now 60% receive endarterectomy. For asymptomatic patients, no shift in treatment allocation was observed, with as many as 90% being treated with CAS and less than 10% with CEA. This led to the observation that in 2007–2008, 90.7% of all CEA patients and only 45.9% of those treated with CAS were symptomatic. More importantly, the change in treatment allocation specifically for the symptomatic population meant that the 30‑day complication rate after CAS and CEA again lowered to 1.24 and 2.44%, respectively, as was the aim of introducing the new allocation algorithm.
Conclusion
Interventionalist groups who can offer both CEA and CAS in the same service are best placed to treat CAD patients. It should be understood that both strategies are complementary and not competitive and that a well thought out treatment allocation is crucial for good results in both CAS and CEA.
The SVS-supported allocation algorithm for patients with CAD is based on high-level evidence and limits CAS to a small number of high surgical risk patients. The treatment algorithm as currently used in our services and as initially proposed by Roubin et al. [5] allocates patients to CEA or CAS based on both patient profiles and lesion characteristics. Different publications, with varying levels of evidence were used to identify potential risk factors for suboptimal CAS outcome. The algorithm was introduced in our service at the end of 2006, and it was observed that again more CEA procedures were carried out in symptomatic patients and that there was a new decrease in the number of complications in both the CEA and CAS populations following this approach.
Future perspective
Carotid artery stenting will undoubtedly prevail as a complementary alternative to CEA. If performed safely, the long-term results are equivalent to those of CEA [8–10]. Concerning the 30‑day outcome, it became clear since the publication of the EVA-3S [6] and SPACE [7] results, that this needs to be improved. Therefore, in the future, interventionalists need to gain more experience and need better devices, especially stents. As it is the stent’s scaffolding capacity that influences clinical events [25,28,30], and as current generation stents with good scaffolding capacities tend to have insufficient flexibility to be optimally accommodated with the vessel’s original anatomy, future stent design improvements should focus primarily on optimally combining excellent scaffolding and flexibility.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Writing assistance was utilized in the production of this manuscript. The authors take great pleasure in thanking Koen De Meester (www.komedical.be) for performing the systematic review of the literature and providing substantial support to the writing of the article.
Executive summary
▪ Carotid artery stenting (CAS) and carotid endarterectomy (CEA) can be considered as being complementary and not competitive: well thought out treatment allocation is crucial to optimize the results for both strategies.
▪ The clinical practice guidelines for carotid revascularization published by the Society for Vascular Surgery are based on the available evidence and support an allocation algorithm for CAS and CEA based on the patients’ surgical risk.
▪ With the recently published evidence from both large, randomized controlled (level 1) and different case cohort trials (level 4), and the constant evolution in interventional techniques, the current value of the conventional algorithm might be questioned. The alternatively proposed paradigm allocates patients to CEA or CAS based on high patient and lesion profiles for suboptimal CAS outcome.
▪ The introduction of the algorithm allocating patients based on potential risk factors for CAS successfully lowered the 30‑day complication rates for both carotid revascularization strategies.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- Hobson R, Mackey W, Ascher E et al.: Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J. Vasc. Surg. Aug. 48(2), 480–486 (2008).
- Atkins D, Best D, Briss P et al.: Grading quality of evidence and strength of recommendations. BMJ 328(7454), 1490 (2004).
- ECST collaborators: Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial. Lancet 351(9113), 1379–1387 (1998).
- NASCET collaborators: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 325, 445–453 (1991).
- Roubin G, Iyer S, Halkin A, Vitek J, Brennan C: Realizing the potential of carotid artery stenting: proposed paradigms for patient selection and procedural technique. Circulation 113(16), 2021–2030 (2006).
- Mas J, Chatellier G, Beyssen B et al.: Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N. Engl. J. Med. 355(16), 1660–1671 (2006).
- SPACE Collaborative group, Ringleb P, Allenberg J et al.: 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet 368(9543), 1239–1247 (2006).
- Mas J, Trinquart L, Leys D et al.: Endarterectomy versus angioplasty in patients with symptomatic severe carotid stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 7(10), 885–892 (2008).
- Eckstein H, Ringleb P, Allenberg J et al.: Results of the Stent-Protected Angioplasty Versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 7(10), 890–902 (2008).
- de Donato G, Setacci C, Deloose K et al.: Long-term results of carotid artery stenting. J. Vasc. Surg. 48(6), 1431–1440 (2008).
- Halliday A, Mansfield A, Marro J et al.: Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 363(9420), 1491–1502 (2004).
- ACAS collaborators: Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 273(18), 1421–1428 (1995).
- Hobson R, Howard V, Roubin G et al.: Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J. Vasc. Surg. 40(6), 1106–1111 (2004).
- Stingele R, Berger J, Alfke K et al.: Clinical and angiographic risk factors for stroke and death within 30 days after carotid endarterectomy and stent-protected angioplasty: a subanalysis of the SPACE study. Lancet Neurol. 7(3), 216–222 (2008).
- Narins C, Illig K: Patient selection for carotid stenting versus endarterectomy: a systematic review. J. Vasc. Surg. 44, 661–672 (2006).
- Verzini F, Cao G, De Rango P et al.: Appropriateness of learning curve for carotid artery stenting: an analysis of periprocedural complications. J. Vasc. Surg. 44(6), 1205–1211 (2006).
- Schneider P: Carotid arteriography. In: Carotid Interventions. Schneider P, Bohannen W, Silva M (Eds). Boca Raton, FL, USA, Marcel Dekker, 33–68 (2005).
- Goodney P, Powell R: Carotid artery stenting: what have we learned from the clinical trials and registries and where do we go from here? Ann. Vasc. Surg. 22(1), 148–158 (2008).
- Tong F, Cloft H, Joseph G, Samuels O, Dion J: Abciximab rescue in acute carotid stent thrombosis. Am. J. Neuroradiol. 21(9), 1750–1752 (2000).
- Chatuverdi S, Yadav J: The role of antiplatelet therapy in carotid stenting for ischemic stroke prevention. Stroke 37(6), 1572–1577 (2006).
- Tummala R, Jahromi B, Yamamoto J et al.: Carotid artery stenting under flow arrest for the management of intraluminal thrombus: technical case report. Neurosurgery 63(1 Suppl. 1), ONSE87– ONSE88 (2008).
- Ecker R, Tummala R, Levy E: ‘Internal cross-clamping’ for symptomatic internal carotid artery thrombus. Report of two cases. J. Neurosurg. 107(6), 1223–1227 (2007).
- Gröschel K, Ernemann U, Schulz J et al.: Statin therapy at carotid angioplasty and stent placement: effect on procedure-related stroke, myocardial infarction, and death. Radiology 240(1), 145–151 (2006).
- Bosiers M, Deloose K, Verbist J, Peeters P: What practical factors guide the choice of stent and protection device during carotid angioplasty? Eur. J. Vasc. Endovasc. Surg. 35(6), 637–643 (2008).
- Wyers M, Powell R, Fillinger M, Nolan B, Cronenwett J: The value of 3D-CT angiographic assessment prior to carotid stenting. J. Vasc. Surg. 49(3), 614–622 (2009).
- Biasi G, Froio A, Diethrich E et al.: Carotid plaque echolucency increases the risk of stroke in carotid stenting: the Imaging in Carotid Angioplasty and Risk of Stroke (ICAROS) study. Circulation 10(110), 756–762 (2004).
- Topakian R, Strasak A, Sonnberger M et al.: Timing of stenting of symptomatic carotid stenosis is predictive of 30-day outcome. Eur. J. Neurol. 14(6), 672–678 (2007).
- Bosiers M, de Donato G, Deloose K et al.: Does free cell area influence the outcome in carotid artery stenting? Eur. J. Vasc. Endovasc. Surg. 33(2), 135–141 (2007).
- Schillinger M, Gschwendtner M, Reimers B et al.: Does carotid stent cell design matter? Stroke 39(3), 905–909 (2008).
- Jansen O, Fiehler J, Brückmann H: Protection or nonprotection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE Trial. Stroke 40(3), 841–846 (2009).
- Wholey M: Current global status of carotid artery stent placement. Cathet. Cardiovasc. Diagn. 44, 1–6 (1998).
▪▪ Basic clinical practice guidelines from the Society for Vascular Surgery to allocate carotid artery disease patients to either carotid artery stenting or carotid endarterectomy.
▪▪ Original description of an alternative paradigm for carotid treatment allocation.
▪ Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: 30-day outcome.
▪ Stent-Supported Percutaneous Angioplasty of the Carotid Artery versus Endarterectomy (SPACE) trial: 30-day outcome.
▪ EVA-3S trial: long-term follow-up.
▪ SPACE trial: long-term follow-up.
▪ Belgian–Italian Carotid (BIC) study: long-term follow-up.
▪▪ Review article on optimal device selection for carotid artery stenting.