Case Report - Interventional Cardiology (2025) Volume 17, Issue 0
No Cut, Just Close: Trans catheter device closure of mitral Paravalvular leak
- Corresponding Author:
- Gaurav Kothari
Department of Cardiology, GGMC and Sir JJ Hospital, Mumbai, Maharashtra, India
E-mail: drgauravkothari@gmail.com
Received date: 0-Feb-2025, Manuscript No. FMIC-25-164780; Editor assigned: 05-Feb-2025, PreQC No. FMIC-25-164780 (PQ); Reviewed date: 19-Feb-2025, QC No. FMIC-25-164780; Revised date: 26-Feb-2025, Manuscript No. FMIC-25-164780 (R); Published date: 05-Mar-2025, DOI: 10.37532/1755-5310.2025.17(S27).699
Abstract
Paravalvular Leaks (PVLs) are increasingly recognized after valve replacement procedures, resulting from factors such as incomplete sealing, suture dehiscence, or annular calcification. Mitral PVLs are particularly common and can lead to symptoms ranging from mild dyspnea to severe heart failure. While surgical repair remains the traditional approach, transcatheter PVL closure has emerged as a less invasive alternative, offering comparable outcomes with reduced procedural risks. T his technique is guided by advanced imaging modalities, with three-dimensional Trans Esophageal Echocardiography (TEE) serving as the gold standard for assessing leak location, size, and severity. Recently, devices have been developed specifically for PVL closure, demonstrating high technical success rates and favorable post procedural outcomes. The procedure can be performed via antegrade or retrograde approaches, depending on the leak's anatomy, and is associated with a low incidence of complications such as device embolization or stroke. Overall, percutaneous PVL closure represents a promising strategy, particularly for patients with suitable anatomy and contraindications to surgery, leading to significant improvements in symptoms and quality of life.
keywords
Paravalvular leak . TEE . 3D TEE . Heart failure . Transcatheter closure . Minimally invasive procedure
Introduction
PVL is a condition where an abnormal passage forms between the prosthetic valve and the anatomical annulus, causing regurgitant flow between heart chambers [1]. Common presentations include congestive heart failure and/or hemolysis [2]. These symptoms require prompt intervention, emphasizing timely detection, effective management, and a thorough understanding of PVL pathophysiology. While redo valve replacement is traditionally needed, transcatheter PVL closure is gaining recognition as a safe and effective alternative [3]. This procedure is particularly complex, with success dependent on the defect's anatomical characteristics, especially in the mitral position [4]. A comprehensive baseline echocardiographic evaluation, including TTE and TEE with 2D and 3D imaging, is necessary to assess PVL's hemodynamic impact and suitability for percutaneous repair [5]. The effect of percutaneous PVL closure on quality of life remains underexplored, with the hypothesis that it improves quality of life and reduces NT-pro BNP levels [6,7].
Case Report
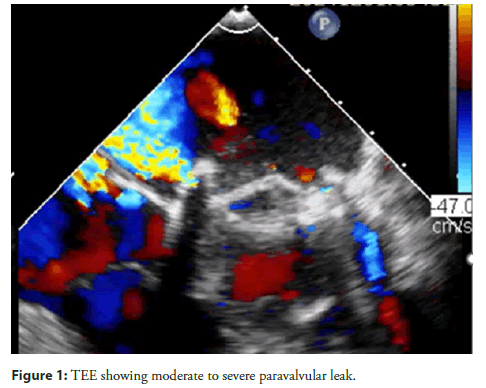
A 33-year-old male with a history of Rheumatic Heart Disease (RHD) and severe Mitral Stenosis (MS) underwent Mitral Valve Replacement (MVR) in 2023 with a TTK Chitra mechanical valve. Two months post-surgery, he presented with new onset Dyspnea on Exertion (DOE). Laboratory findings revealed elevated NT-pro BNP levels, suggestive of heart failure. 2D echocardiography and TEE was done which showed a moderate to severe paravalvular leak, a dilated Left Atrium (LA), a color jet flow area >50%, a Vena Contracta (VC) of 0.7 cm, a regurgitant fraction (RF) of 55%, and a Regurgitant Volume (R Vol) of 60 ml (Figure 1). Considering the patient's recent open-heart surgery and the feasibility of a transcatheter approach, he was planned for transcatheter device closure of the paravalvular leak.
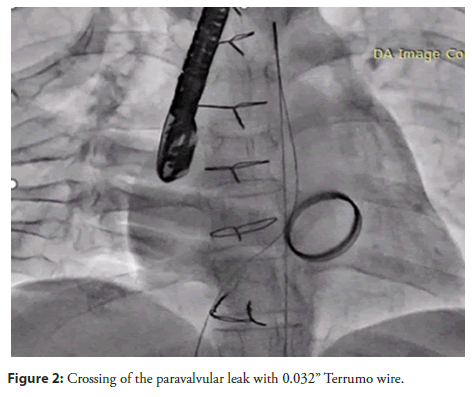
The procedure started by taking access through Right Femoral Artery (RFA), Right Femoral Vein (RFV), and Left Femoral Artery (LFA). A pigtail catheter was placed in the Left Ventricular (LV) cavity. A Transseptal (TSP) needle was used to puncture the septum, which was confirmed with a Left Atrial (LA) contrast injection. A JR catheter was advanced across the Paravalvular Leak (PVL) over a 0.032" Terumo wire into the LV cavity under 3D TEE guidance (Figure 2).
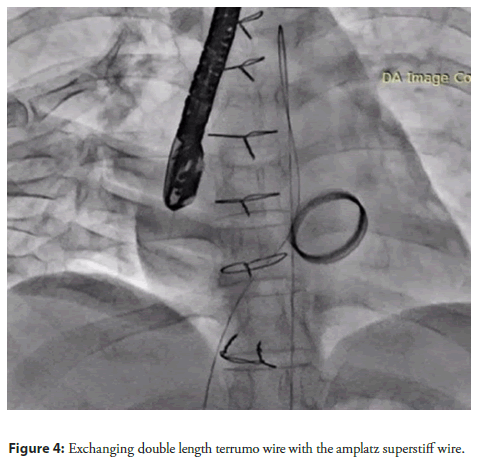
The 0.032" Terumo wire was then exchanged for a double-length Terumo wire, which was subsequently snared from the aorta using a 6F snare (Figure 3). The JR catheter was exchanged for an MPA catheter, which was positioned in the descending aorta. The Terumo wire was then exchanged for an Amplatz Super Stiff wire (Figure 4).
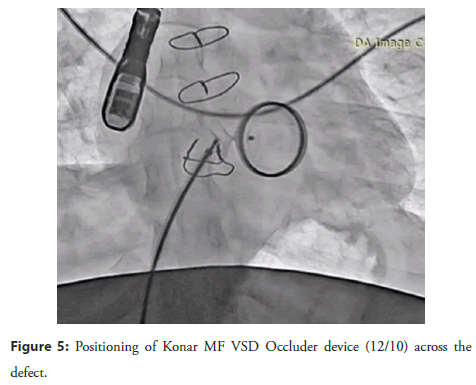
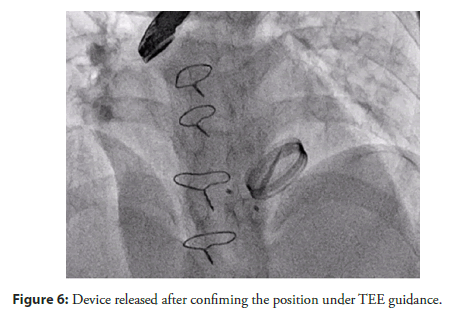
A Lifetech Konar MF VSD Occluder device (12/10) was deployed across the defect, and its position was confirmed by 2D echocardiography, TEE, and 3D TEE (Figure 5). After confirming the correct positioning of the device and the absence of any residual leak, the device was released (Figure 6).
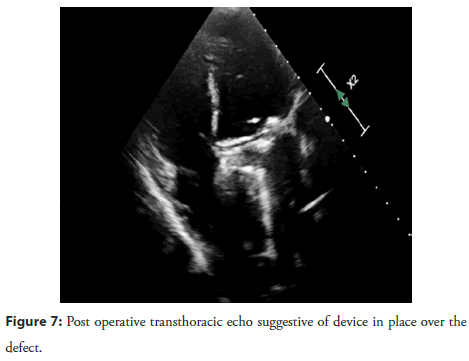
Post-procedure, the patient was monitored in the Intensive Coronary Care Unit (ICCU) and was started on Vitamin K Antagonist (VKA) anticoagulation after bridging with heparin. The patient was discharged after stabilization. At 1-month followup, the patient remained stable without any complaints. 2D echocardiography and 3D TEE confirmed that the device was well-positioned, with no residual paravalvular leak (Figures 7 and Figure 8).
Discussion
PVL has become relatively common nowadays following surgical and trans catheter valve replacement. It can result from incomplete sealing of the prosthetic valve, suture dehiscence, malposition, improper sizing, or a calcified or friable annulus. PVL is defined as a defect between the anatomical and prosthetic valve annulus, leading to regurgitation of blood through this defect. The incidence of PVL after MVR ranges from 7% to 17%, of which fewer than 5% of patients remain symptomatic and have significant regurgitation. Surgical repair remains the gold standard in these patients. However, because many patients have comorbidities, redo sternotomy for PVL carries a high procedural risk. Surgical intervention is also associated with higher 30-day mortality and a trend toward increased stroke risk and prolonged hospital stays. With the emergence of trans catheter techniques for PVL closure which are less invasive and more cost-efficient-it has become a promising alternative with lower operative risk and outcomes comparable to surgical repair. In some centers, it is now considered the first-line treatment option. PVL can cause symptoms ranging from mild dyspnea to pulmonary edema and heart failure. Hemolysis is more common in mitral PVL than in aortic PVL, due to the occurrence of PVL during systole. The appearance of a new murmur in a valve replacement patient may raise suspicion and should be evaluated using echocardiography. TTE may be limited in these patients due to acoustic shadowing from calcium and the prosthetic valve. TEE remains the gold standard for diagnosing PVL. It provides detailed information about the location, size, and severity of the leak. 3D TEE is superior to 2D TEE in assessing PVL. A semi-quantitative assessment can be performed using pulsed-wave Doppler to evaluate flow pattern distortion upstream (e.g., pulmonary veins in mitral PVL). An elevated trans prosthetic gradient, despite a normal appearance and mobility of the cusps or discs, should raise suspicion for significant PVL causing volume overload. Progressive LV enlargement may also suggest severe PVL. TEE is also useful during percutaneous procedures. Severe mitral PVL is indicated by significant flow convergence, a vena contracta =7 mm, regurgitant volume >60 mL, regurgitant fraction >50%, and an effective regurgitant orifice area (EROA) >40 mm². Cardiac MRI may be considered in patients with multiple PVLs. The location of mitral PVL can be described using a clock-face analogy: near the aortic valve at the 12 oâclock position, toward the Left Atrial Appendage (LAA) at 9 oâclock, and near the Interatrial Septum (IAS) at 3 oâclock. The most common locations for mitral PVL are the 10 and 11 oâclock positions. A meta-analysis by Busu et al., showed a 72.1% success rate for transcatheter PVL closure. The current ACC/AHA guidelines recommend device closure of PVL in patients with symptomatic heart failure or persistent hemolytic anemia, provided they have suitable anatomy for percutaneous intervention. PVL can be approached antegradely via trans-septal puncture or retrogradely by crossing the PVL from the left ventricle to the left atrium (trans-apical approach). A snaring technique may be used to establish an arteriovenous wire loop when advancing the delivery sheath is difficult. This method facilitates sheath passage through sharp angles and calcified lesions. Local anesthesia may be considered in patients undergoing trans-femoral access, whereas general anesthesia is preferred for the trans-apical route. The most common complications after PVL device closure includes Valve interference (3.5%-5.0%), Stroke, Endocarditis, Post-procedural hemolysis, Device erosion, Emergent cardiac surgery (0.7%-2.0%), and Death (1.4%-2.0%). Percutaneous PVL closure has been associated with improved quality of life in patients with successful procedures during 12 months of follow-up.
Conclusion
Percutaneous closure of PVLs has emerged as a viable and effective alternative to traditional surgical repair, particularly for patients at high surgical risk. This minimally invasive approach offers favorable outcomes, including significant reductions in leak severity and improvements in symptoms, with a low incidence of major adverse events. Advanced imaging techniques, such as threedimensional transesophageal echocardiography, play a crucial role in accurately diagnosing and guiding the closure of PVLs. While the procedure has shown promising short-term results, long-term prognosis remains influenced by underlying patient comorbidities. Therefore, percutaneous PVL closure should be considered a firstline treatment option in appropriately selected patients, offering a safe and effective means to manage this challenging complication.
References
- Cruz-Gonzalez I, Antunez-Muiños P, Lopez-Tejero S, et al. Mitral paravalvular leak: Clinical implications, diagnosis and management. J Clin Med. 11(5):1245 (2022).
- Amar K, Kapadia Samir R, Murat TE, et al. Percutaneous paravalvular leak closure. Circ J. 77(1):19-27 (2013).
- Kalogeras K, Ntalekou K, Aggeli K, et al. Transcatheter closure of paravalvular leak: Multicenter experience and follow-up. Hellenic J Cardiol. 62(6):416-422 (2021).
- Liu Y, Xu C, Ding P, et al. Transcatheter closure of mitral paravalvular leak via multiple approaches. J Interv Cardiol. 2021(1):6630774 (2021).
- Pysz P, Wojakowski W, Smolka G, et al. Paravalvular leak echo imaging before and during the percutaneous procedure. J Clin Med. 11(11):3155 (2022).
- Hayashi Y, Russell JK, Dvorak CJ, et al. Echocardiographic characteristics of paravalvular leak following surgical aortic valve replacement: A retrospective cohort study. J Thorac Dis. 17(3):1249 (2025).
- Kozlowski M, Malczewska M, Pysz P, et al. Quality of life after percutaneous paravalvular leak closure-a prospective registry. Postepy Kardiol Interwencyjnej. 18(3):261-268 (2022).