Research Article - International Journal of Clinical Rheumatology (2017) Volume 12, Issue 3
Mycophenolate mofetil is an effective therapy for connective tissue disease-associated interstitial lung disease
- *Corresponding Author:
- Mohammed A Omair
Department of Medicine
Rheumatology Division
College of Medicine
King Saud University
Riyadh, Saudi Arabia
E-mail: momair@ksu.edu.sa
Abstract
Introduction: Mycophenolate mofetil (MMF) is increasingly used in the management of connective tissue disease-related interstitial lung disease (CTD-ILD). The aim of this study was to evaluate the safety and efficacy of MMF in patients with CTD-ILD based on the ILD subtype regardless of the underlying CTD. Method: This was a retrospective single-center study. Patients with ILD-CTD who had either non-specific interstitial pneumonia (NSIP) or usual interstitial pneumonia (UIP) who received MMF therapy were included. Patients who received cyclophosphamide (CYC) or rituximab (RTX) were excluded. A 5% change in the forced vital capacity (FVC) and diffusion capacity of carbon monoxide (DLco) and a 30-meter change in the 6-min walk test (6MWT) were considered significant. Results: Forty patients were included. There was a female predominance (67.5%), with a mean (± standard deviation) age and disease duration of 54.1 ± 15.8 years and 61.4 ± 75.5 months, respectively. The mean ILD duration was 43.2 ± 27.7 months. The mean FVC and DLco were 54.6 ± 16.0% and 59.4 ± 14.2%, respectively. There were 22 (55%) and 18 (45%) patients in the CTD-NSIP and CTD-UIP groups, respectively. All patients achieved a target dose of 2-3 g/day. Treatment with MMF resulted in stabilization and/or improvement of FVC in 28 (70%) patient, of DLco in 24/33 (72.7%) patients, and of 6MWT in 23/37 (62.1%) patients. The response to MMF was identical in both ILD subtypes, with all measures achieving a statistical significance. No safety signals were detected. Conclusion: MMF is safe and effective in the management of CTD-ILD regardless of the underlying ILD subtype.
Keywords
non-specific interstitial pneumonia, usual interstitial pneumonia, mycophenolate mofetil, connective tissue disease, interstitial lung disease
Introduction
Interstitial lung disease (ILD) is a serious and common complication of different connective tissue diseases (CTD) [1,2] and can lead to significant morbidity and mortality [3]. In Saudi Arabia, CTD-related ILD (CTD-ILD) is the most frequently encountered type of ILD [4]. Treatment aims at improving symptoms and functional status in addition to halting physiological and radiological deterioration [5]. Mycophenolate mofetil (MMF), an inhibitor of inosine monophosphate dehydrogenase, influences T and B lymphocyte proliferation by altering the synthesis of guanosine nucleotides and inhibits transforming growth factor β (TGF-β), leading to immunosuppressive and anti-fibrotic effects, respectively [6]. MMF has shown promising results in the management of different CTD-ILD [7]. MMF has been used as induction and maintenance therapy in many uncontrolled trials, predominantly in patients with systemic sclerosis (SSc). The majority of these studies included patients from European and North American origin [8]. Currently, there are no published data looking at the effectiveness and safety of MMF in Saudi patients with CTD-ILD. This study reports the results of treatment with MMF in a Saudi population managed with a multidisciplinary approach.
Methods
This is a single center retrospective review study which included patients from the ILD database. Patients with CTD-ILD who presented between March 2010 and June 2015 were included. The study duration was 69 months. Patients had regular follow-up in the ILD Center every 3-6 months and were also seen during times when they had worsening of symptoms.
CTD was diagnosed if the patients fulfilled the established classification criteria for SSc [9], rheumatoid arthritis (RA) [10], systemic lupus erythematosus (SLE) [11], Sjogren’s syndrome (SS) [12], polymyositis (PM) and dermatomyositis (DM) [13,14], mixed connective tissue disease (MCTD) [15] and undifferentiated connective tissue disease (UCTD) [16]. The target MMF dose was 2-3 g/day starting at 1 g/day and was increased as tolerated over 2-4 weeks to the maximum tolerable dose. All patients received bone protection with vitamin D ≥800 international unit/day and calcium supplement ≥600 mg/ day, anti-reflux therapy with a proton pump inhibitor and Pneumocystis jiroveci pneumonia (PJP) prophylaxis with sulfamethoxazole and trimethoprim. Patients were classified according to their underlying ILD pattern either as nonspecific interstitial pneumonia (NSIP) or usual interstitial pneumonia (UIP) based on radiological and/or histological findings. Inclusion criteria included the following: Age 18 years or more, clinical diagnosis of a pre-specified type, availability of clinical/physiological data at 3 months of follow-up or more and having received a minimum of 3 months of uninterrupted therapy with MMF. Exclusion criteria were as follows: ILD pattern other than UIP and NSIP, a diagnosis of drug-induced or unclassified pulmonary fibrosis, pregnancy, and past/current use of cyclophosphamide (CYC) or rituximab (RTX). This study was approved by the Ethic Institutional Review Board. Written informed consent was waived because of the retrospective nature of the study.
The multidisciplinary team consisted of a pulmonologist, radiologist, pathologist and rheumatologist who met on a bimonthly basis to discuss clinical, serological, radiological and pathological findings of patients with CTD-ILD to reach a consensus on the final diagnosis and management plan.
Physiological measurements
Pulmonary function tests (PFT Masterscreen; Jaeger, Hoechberg, Germany) were performed using standard methods, including spirometry, plethysmography and measurement of the diffusion capacity of the lung for carbon monoxide (DLco) (10–12). Arterial blood gas (ABG) values (Rapid Lab 865; Bayer, Plymouth, UK) were obtained for the partial pressure of oxygen (PaO2), the partial pressure of carbon dioxide (PaCO2) and oxygen saturation. Following the PFTs and ABG sampling, the patients were asked to perform the 6-min walk test (6MWT) in accordance with American Thoracic Society guidelines [17]. Oxygen saturation was recorded at the beginning and end of the 6-min walk. At the end of the test, the total distance walked in meters was recorded.
Chest high-resolution computed tomography (HRCT)
All patients underwent computed tomography scanning (Light Speed 16 or VCT XT; GE Medical Systems, Milwaukee, WI, USA). Full-volume scans reconstructed every 2.5 mm were obtained through the entire thorax. Scans were performed during suspended inspiration with patients in the supine position. Additional limited scans using 1.25 mm thin collimation at 10 mm intervals from the level of the aortic arch to the lung bases, with high spatial resolution reconstruction, were obtained at end-expiration with patients in the prone position.
Statistical analysis
Descriptive statistics are presented as the mean ± standard deviation or number (percentage). The unpaired Student’s t-test, Mann-Whitney rank sum test, chi-square test and Fisher’s exact test were used as appropriate to compare the studied variables. A 5% change in the forced vital capacity (FVC) and DLco values and a change of 30 m in the 6MWT were considered significant. Odds ratios and 95% confidence intervals for relative risks were calculated. A two-sided p value <0.05 was considered statistically significant. SPSS version 18 software (SPSS Inc., Chicago, IL, USA) was used for all analyses.
Results
Forty patients were included in the final analysis. Baseline characteristics and underlying disease are shown in Table 1. There was a female predominance (67.5%), with a mean (± standard deviation) age 54.1 ± 15.8 years. The mean duration of the CTD and ILD were 61.4 ± 75.5 months and 43.2 ± 27.7 months respectively. The mean body mass index was 28.4 ± 5.9, with 15 (37.5%) obese patients and 15 (37.5%) overweight patients. The mean FVC and DLco of the study group were 54.6 ± 16.0% and 59.4 ± 14.2%, respectively (Table 2). There were 22 patients in the CTD-NSIP (55%) group and 18 patients in the CTD-UIP group (45%). Patients in the CTD-NSIP group were younger (mean 48.2 ± 15.6 vs. 61.3 ± 13 years; p=0.007) and had a lower PaCO2 at baseline (38.6 ± 5.3 vs. 42.5 ± 6.1 mmHg; p=0.034).
| Characteristic | Overall (n=40) |
|---|---|
| Age, years | 54.1 ± 15.8 |
| Female gender, n (%) | 27 (67.5) |
| Ever smoker, n (%) | 5 (12.5) |
| Disease duration, months | 61.4 ± 75.5 |
| ILD duration, months | 43.2 ± 27.7 |
| Duration of therapy, months† | 18.2 ± 12.8 |
| BMI | 28.4 ± 5.9 |
| Overweight, n (%) | 15 (37.5) |
| Obese, n (%) | 15 (37.5) |
| Co-morbidities | |
| Ischemic heart disease | 4 (10) |
| Hypertension | 11 (27.5) |
| Diabetes mellitus | 6 (15) |
| Treatment | |
| Mycophenolate | 15 (37.5) |
| Prednisolone + Mycophenolate | 20 (50) |
| Prednisolone+Mycophenolate+Tacrolimus | 4 (10) |
| Pirfenidone+Mycophenolate | 1 (2.5) |
| CTD type | |
| Rheumatoid arthritis | 1 (2.5) |
| Systemic lupus erythematosus | 1 (2.5) |
| Scleroderma | 13 (32.5) |
| Sjogren’s syndrome | 11(27.5) |
| Dermatomyositis | 1 (2.5) |
| Undifferentiated connective tissue disease | 10 (25) |
| Mixed connective tissue disease | 3 (7.5) |
The data are presented as the mean ± standard deviation or number (percentage). BMI: Body Mass Index; CTD: Connective Tissue Disease. †Range of treatment 3-67 months.
Table 1. Baseline characteristics of the study group.
| Overall (n=40) | |
|---|---|
| Pulmonary function test | |
| FVC, % predicted | 54.6 ± 16.0 |
| TLC, % predicted | 59.4 ± 14.2 |
| DLCO, % predicted | 38.8 ± 20.5 |
| 6-minute walk test | |
| Initial SpO2, % | 95.6 ± 2.7 |
| Final SpO2, % | 85.8 ± 6.4 |
| Distance, meters | 367.4 ± 68.9 |
| Arterial blood gas | |
| PaO2, mmHg | 75.0 ± 17.2 |
| PaCO2, mmHg | 40.4 ± 5.9 |
| SaO2, % | 92.9 ± 11.5 |
The data are presented as the mean ± standard deviation. FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in One Second; TLC: Total Lung Capacity; DLco: Diffusion Capacity of the Lung for Carbon Monoxide; SpO2: Oxygen Saturation by Pulse Oximetry; PaO2: Partial Pressure of Oxygen; PaCO2: Partial Pressure of Carbon Dioxide; SaO2: Oxygen Saturation.
Table 2. Baseline physiologic parameters of the study group.
The majority of patients received MMF with prednisolone (60%), and 37.5% of patients received MMF monotherapy. A minority of patients received adjunctive tacrolimus (10%) and one patient received MMF combined with pirfenidone (2.5%). There was no significant difference between the groups regarding underlying disease or therapy received.
Physiological measures
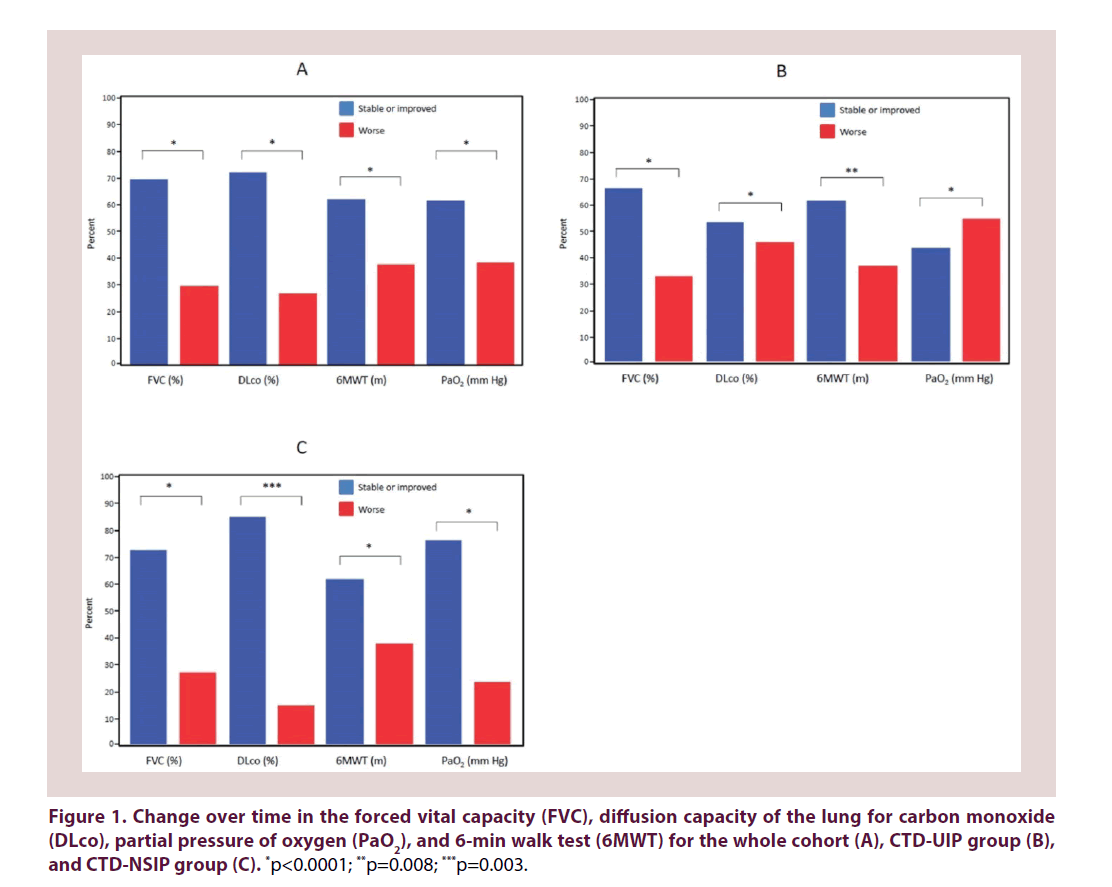
At the time of the last follow-up, treatment with MMF resulted in stabilization or improvement of FVC in 28 (70%) patients, of DLco in 24/33 (72.7%) patients, of 6MWT in 23/37 (62.1%) patients and of PaO2 in 24/39 (61.5%) patients. The response to MMF was similar for both ILD subtypes. However, the PaO2 value was more likely to be stable or improved in the CTD-NSIP group than in the CTD-UIP group (76% vs. 44%, p=0.042) (Figure 1 and Table 3). Twelve patients (6 from each group) showed a decrease of five percentage points or more of the predicted FVC, including 3, 1 and 2 patients with underlying SSc, SS and UCTD, respectively, in the UIP-CTD group, compared to 2, 3 and 1 patients suffering from SSc, UCTD and mixed CTD, respectively.
| Variable | Overall (n=40) | CTD-UIP (n=18) |
CTD-NSIP (n=22) |
OR | 95% CI | p-value |
|---|---|---|---|---|---|---|
| FVC, % | (n=40) | (n=18) | (n=22) | |||
| ≥ 5% decrease | 12 (30) | 6 (33.3) | 6 (27.3) | 0.750 | 0.193–2.913 | 0.677 |
| Stable or improved | 25 (70) | 12 (66.7) | 16 (72.7) | 1.333 | 0.343–5.178 | 0.677 |
| DLco, % | (n=33) | (n=13) | (n=20) | |||
| ≥ 5% decrease | 9 (27.3) | 6 (46.2) | 3 (15) | 0.206 | 0.040–1.063 | 0.05 |
| Stable or improved | 24 (72.7) | 7 (53.8) | 17 (85) | 4.857 | 0.941–25.084 | 0.05 |
| Walking distance, m | (n=37) | (n=16) | (n=21) | |||
| ≥ 30 meters decrease | 14 (37.8) | 6 (37.5) | 8 (38.1) | 1.026 | 0.268–3.923 | 0.97 |
| Stable or improved | 23 (62.2) | 10 (62.5) | 13 (61.9) | 0.975 | 0.255–3.730 | 0.97 |
| PaO2, mmHg | (n=39) | (n=18) | (n=21) | |||
| ≥ 5 mmHg decrease | 15 (38.5) | 10 (55.6) | 5 (23.8) | 0.250 | 0.064–0.982 | 0.042 |
| Stable or improved | 24 (61.5) | 8 (44.4) | 16 (76.2) | 4.000 | 1.018–15.717 | 0.042 |
The data are presented as the number (percentage). CTD: Connective Tissue Disease; UIP: Usual Interstitial Pneumonia; NSIP: Nonspecific Interstitial Pneumonia; OR: Odds Ratios; ci: Confidence Intervals; FVC: Forced Vital Capacity; DLco: Diffusion Capacity Of The Lung For Carbon Monoxide; PaO2: Partial Pressure of Oxygen.
Table 3. Comparison between histological groups with reference to changes in physiological measures.
Radiological measures
Follow-up HRCT was available for 10 and 13 patients with CTD-UIP and CTD-NSIP, respectively. Both ILD subtypes showed similar radiological outcomes (Table 4).
| Variable | CTD-UIP | CTD-NSIP | p-value |
|---|---|---|---|
| HRCT interval, months* | 25.1 ± 15.2 (7–47) | 17.6 ± 6.4 (5–26) | 0.122 |
| HRCT available | (n=10) | (n=13) | - |
| Worse | 0 | 3 (23.1) | 0.229 |
| Stable or improved | 10 (100) | 10 (76.9) | 0.229 |
The data are presented as the mean ± standard deviation or number (percentage). CTD: Connective Tissue Disease; UIP: Usual Interstitial Pneumonia; NSIP: Nonspecific Interstitial Pneumonia; HRCT: High-resolution Computed Tomography.
Table 4. Comparison of changes in high-resolution CT scan (HRCT) results over time.
Tolerability and adverse events
All patients achieved the target dose of MMF between 2-3 g/day with 29 (72.5%) achieving 2 g/day and 11 (27.5%) achieving 3 g/day with recorded discontinuation events due to intolerability. During follow-up, there was no documented incidence of pancytopenia, malignancy, or bacterial or opportunistic infection.
Discussion
Treatment of CTD-ILD can be challenging, and it is essential that the chosen immunosuppressive agent targets the lung and other involved organs. MMF has been shown to be an effective therapeutic option in inflammatory myositis [18,19] and skin disease of SSc [8], and its effectiveness in treating CTD-ILD has been demonstrated in uncontrolled trials [20,21]. However, all previous studies have included patients who failed or developed intolerance to a prior immunosuppressive agent. Swigris et al. retrospectively evaluated the safety and efficacy of MMF in 25 patients with a predominant diagnosis of SSc (n=9). The most common reason for initiating MMF was intolerance to a previous immunosuppressive agent. Adverse events occurred in 6 patients, all of which resolved after a dose reduction. MMF resulted in stabilization of physiological parameters and a reduction in the daily steroid dose [20]. The same researchers reported their findings in a larger group of patients (n=125) followed over a 3-year period [7]. The cohort consisted mainly of patients with SSc (35.2%) but also included patients with lung-dominant CTD (15.2%) based on a proposed definition [22]. MMF was the first prescribed agent in 50% of patients with a discontinuation rate of 10%. Patients without the UIP pattern experienced a statistical improvement in lung parameters, whereas patients with the UIP pattern showed stabilization of their parameters. In 2012, Tzouvelekis et al. reported the results of a metaanalysis evaluating the efficacy of MMF in SSc lung disease [23]. Their analysis included 6 studies encompassing 69 patients, 10 of which were from unpublished data based on the authors’ experience, and the results showed stabilization of lung function after initiation of MMF.
Our study included only patients who received MMF as induction or maintenance treatment. We found that MMF effectively improved and/ or stabilized physiological pulmonary parameters in patients with CTD-ILD regardless of the ILD type.
The minimally important difference in physiological parameters used in the present work was derived from studies of idiopathic pulmonary fibrosis patients (22-23). Recently, the Outcome Measures in Rheumatology (OMERACT) group proposed using less stringent criteria to define progression in CTD-ILD [24]. These criteria use the term “clinically meaningful progression” and relate to the following 3 cut-off values: ≥10% relative decline in FVC predicted, ≥5 to <10% relative decline in FVC predicted and ≥15% relative decline in DLco predicted.
Compared to previously published reports, one important finding in our cohort was the absence of mortality [25]. Most of the patients who deteriorated had underlying UCTD, which may indicate that this group of patients represents an entity with a different prognosis from patients with established CTD. To standardize the approach to this group of patients, the term interstitial pneumonia with autoimmune features (IPAF) has been recently proposed by the European Respiratory Society and American Thoracic Society to include patients who do not fulfill the criteria established for a certain CTD [26]. To be classified as IPAF, the patient needs to have interstitial pneumonia and at least 1 feature of 2 of the 3 domains (clinical, serological and morphological). Ferri et al. evaluated patients with IPAF and compared them to patients with stable UCTD and unclassifiable ILD (U-ILD) who did not have extrathoracic or serological evidence of CTD [27]. These authors hypothesized that IPAF and U-ILD may represent 2 variants of the same disease spectrum and that selection bias plays a role when evaluating patients referred to pulmonology and rheumatology services based on lung involvement. In the same study, the researchers shared their interdisciplinary experience between the pulmonology and rheumatology groups. Because the management of patients with ILD in general requires a multidisciplinary team [28], the involvement of the rheumatologist is strongly encouraged when an underlying CTD is suspected or diagnosed [29]. Our studies underscore the importance of a multidisciplinary approach, which may explain the improved survival in the current study.
The present study had several limitations. The retrospective review of a database from a single tertiary center may have introduced both selection and recall biases. In addition, the number of CTD-associated ILD patients in each subgroup was relatively small. The amalgamation of patients with CTD-associated ILD caused by including various types of CTD is potentially an important problem, as evidenced by the influence of the UCTD subgroup in our study. Therefore, further studies are needed to explore each CTD subtype separately and improve our understanding of the natural history of specific disease patterns.
Conclusion
MMF is a safe and effective therapeutic agent for treating CTD-ILD regardless of the underlying ILD pattern. A multidisciplinary approach is desirable and may be associated with improved survival.
Funding Information
The authors received no financial support for the research, authorship and publication of this article.
Conflict of Interest
The authors disclose no conflict of interest.
References
- Afeltra A, Zennaro D, Garzia P et al. Prevalence of interstitial lung involvement in patients with connective tissue diseases assessed with high-resolution computed tomography. Scand. J. Rheumatol. 35(5), 388–394 (2006).
- Marigliano B, Soriano A, Margiotta D, Vadacca M, Afeltra A. Lung involvement in connective tissue diseases: a comprehensive review and a focus on rheumatoid arthritis. Autoimmun. Rev. 12(11), 1076–1084 (2013).
- Kocheril SV, Appleton BE, Somers EC et al. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 53(4), 549–557 (2005).
- Alhamad EH. Interstitial lung diseases in Saudi Arabia: A single-center study. Ann. Thorac. Med. 8(1), 33–37 (2013).
- Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 143(3), 814–824 (2013).
- Allison AC. Mechanisms of action of mycophenolate mofetil. Lupus. 14(Suppl 1), s2–s8 (2005).
- Fischer A, Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J. Rheumatol. 40(5), 640–646 (2013).
- Omair MA, Alahmadi A, Johnson SR. Safety and effectiveness of mycophenolate in systemic sclerosis. A systematic review. PLoS ONE. 10(5), e0124205 (2015).
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 23(5), 581–590 (1980).
- Aletaha D, Neogi T, Silman AJ et al. rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Annals of the rheumatic diseases 69(9), 1580–1588 (2010).
- Tan EM, Cohen AS, Fries JF et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25(11), 1271–1277 (1982).
- Vitali C, Bombardieri S, Jonsson R et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis.61(6), 554–558 (2002).
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N. Engl. J. Med. 292(7), 344–347 (1975).
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N. Engl. J. Med. 292(8), 403–407 (1975).
- Smolen JS, Steiner G. Mixed connective tissue disease: to be or not to be? Arthritis Rheum. 41(5), 768–777 (1998).
- Doria A, Mosca M, Gambari PF, Bombardieri S. Defining unclassifiable connective tissue diseases: incomplete, undifferentiated, or both? J. Rheumatol. 32(2), 213–215 (2005).
- ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 166(1), 111–117 (2002).
- Pisoni CN, Cuadrado MJ, Khamashta MA, Hughes GR, D'Cruz DP. Mycophenolate mofetil treatment in resistant myositis. Rheumatology (Oxford). 46(3), 516–518 (2007).
- Majithia V, Harisdangkul V. Mycophenolate mofetil (CellCept): an alternative therapy for autoimmune inflammatory myopathy. Rheumatology (Oxford). 44(3), 386–389 (2005).
- Swigris JJ, Olson AL, Fischer A et al. Mycophenolate mofetil is safe, well tolerated, and preserves lung function in patients with connective tissue disease-related interstitial lung disease. Chest 130(1), 30–36 (2006).
- Saketkoo LA, Espinoza LR. Experience of mycophenolate mofetil in 10 patients with autoimmune-related interstitial lung disease demonstrates promising effects. Am. J. Med. Sci. 337(5), 329–335 (2009).
- Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 138(2), 251–256 (2010).
- Tzouvelekis A, Galanopoulos N, Bouros E et al. (2012) Effect and safety of mycophenolate mofetil or sodium in systemic sclerosis-associated interstitial lung disease: a meta-analysis. Pulm. Med.
- Khanna D, Mittoo S, Aggarwal R et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD)-Report from OMERACT CTD-ILD Working Group. J. Rheumatol. 42(11), 2168–2171 (2015).
- Alhamad EH, Al-Kassimi FA, Alboukai AA et al. Comparison of three groups of patients with usual interstitial pneumonia. Resp. Med. 106(11), 1575–1585 (2012).
- Fischer A, Antoniou KM, Brown KK et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur. Resp. J. 46(4), 976–987 (2015).
- Ferri C, Manfredi A, Sebastiani M et al. Interstitial pneumonia with autoimmune features and undifferentiated connective tissue disease: Our interdisciplinary rheumatology-pneumology experience, and review of the literature. Autoimmun. Rev. 15(1), 61–70 (2016).
- Travis WD, Costabel U, Hansell DM et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care. Med. 188(6), 733–748 (2013).
- Omair MA. The role of serological testing in idiopathic interstitial pneumonia: a rheumatologist perspective. Curr. Pulmonol. Rep. 4(3), 130–134 (2015).