Review Article - Interventional Cardiology (2023)
Mitral annular flutter: Review of mechanisms, challenges of ablation, and assessment of block
- Corresponding Author:
- Mekala Komandoor Srivathsan
Division of Cardiovascular Diseases, Mayo Clinic, Phoenix, Arizona, United States,
E-mail: srivathsan.komandoor@mayo.edu
Received date: 09-Mar-2023, Manuscript No. FMIC-23-91193; Editor assigned: 13-Mar-2023, PreQC No. FMIC-23-91193 (PQ); Reviewed date: 27-Mar-2023, QC No. FMIC-23-91193;Revised date: 03- Apr-2023, Manuscript No. FMIC-23-91193 (R);Published date: 10-Apr-2023, DOI: 10.37532/1755-5310.2023.15(S16).413
Abstract
Mitral Annular Flutter (MAF) occurs with increased frequency after procedures such as atrial fibrillation ablation and mitral valve surgeries. Catheter ablation of the mitral annulus is challenging due to its complex anatomy. We wish to highlight the anatomical considerations, mechanisms of MAF, challenges during ablation, and assessment of bidirectional block. The mechanism of MAF mostly revolves around previous extensive ablation in the left atrium for AF or mitral valve surgeries such as the Maze procedure. The commonly used lines for MAF ablation are the Lateral Mitral Isthmus line (LMI) and Left Atrial Anterior wall (LAAW) line. LMI line often necessitates ablation within the Coronary Sinus, which can lead to additional complications. There is also variable myocardial thickness in this region, increasing the difficulty. LAAW line ablation can lead to complications like left circumflex artery injury and strokes, and TIAs in rare cases. There is no significant difference in bidirectional block and ablation time rates in the different approaches. Although the success rates are high, assessment of bidirectional block is of utmost importance to reduce recurrence. Evaluation of the block involves the presence of widely split fixed double potentials, electro anatomic mapping, and differential pacing.
Keywords
Mitral annular flutter; Radiofrequency ablation; Atypical atrial flutter; Bidirectional block
Abbreviations
MAF: Mitral Annular Flutter; RF: Radiofrequency; AF: Atrial Flutter; MA: Mitral Annulus; PV: Pulmonary Vein; AFL: Atrial Flutter; CS: Coronary Sinus; LAT: Local Activation Time; LA: Left Atrium; MI: Mitral Isthmus; LMI: Lateral Mitral Isthmus; LAAW: Left Atrial Anterior Wall; VOM: Vein of Marshall; LAA: Left Atrial Appendage; LSPV: Left Superior Pulmonary Vein; RSPV: Right Superior Pulmonary Vein; EIVOM: Ethanol infusion into Vein of Marshall
Introduction
Mitral Annular Flutter (MAF) is the most common left atrial macro-re-entrant atrial arrhythmia occurring after catheter ablation of Atrial Fibrillation (AF) [1]. As with other atypical atrial flutters, MAF’s pathogenesis depends on the presence of abnormal electrical substrate resulting in areas of slow conduction [2]. These arrhythmias are mostly resistant to anti-arrhythmic medications and rate-controlling therapy and require ablation procedures [3]. The probability of MAF occurring in a patient is higher if there is a prior history of mitral valve surgery [4]. While there is success in eliminating recurrent tachyarrhythmia in most patients, achieving bidirectional block with catheter ablation is challenging due to complex anatomic relationships. We wish to highlight the anatomical considerations, mechanisms, challenges of MAF ablation, and methods to assess bidirectional block.
Literature Review
Mitral Annular Anatomy (MAF)
The Mitral Annulus (MA) is a thin, nonconductive, fibro fatty membrane that separates the left atrium from the left ventricle. It is divided into anterior and posterior portions. The anterior annulus spans between the left and right fibrous trigones and are anatomically coupled to the aortic annulus. The posterior annulus is externally related to the musculature of the left ventricular inflow region and internally to the left atrium, where it merges with the base of the posterior mitral leaflet [5].
In anatomic studies, MA has been described as having a ‘D-Shaped’ aspect. The straight component is conventionally named anterior MA. It accommodates the aortic valve allowing the latter to be wedged between the ventricular septum and the mitral valve, while the curved component is the posterior MA. This non-planar shape has been shown to lessen stress exerted on the mitral valve leaflets during systole [6].
Although the term annulus implies a solid ring-like fibrous cord to which the leaflets are attached, this is far from the case. In the area of aortic-mitral fibrous continuity, the distal margin of the atrial myocardium over the leaflet defines the hinge line. However, the hinge line is indistinct when viewed from the ventricular aspect since the fibrous continuity is an extensive sheet.
Mechanism of development of Mitral Annular Flutter (MAF)
Most cases of MAF arise after procedures such as atrial fibrillation ablation or Maze procedure. The current approach of AF ablation involves isolating the pulmonary veins by ablating on the left atrial side of the left atrial/Pulmonary Vein (PV) junction, either circumferentially or segmentally, to isolate the arrhythmic PVs electrically. This results in a much lower incidence of PV stenosis and prevents other triggers within the PV from initiating AF. A pro-arrhythmic side effect of extensive left atrial ablation can be the development of organized left atrial tachycardia, of which mitral annular flutter is one of the most common.
Mountantonakis, et al. conducted a study examining 21 patients with a history of MV surgery who developed MAF and required ablation. It was found that MAF was present in 31% of patients referred for catheter ablation of atrial arrhythmias occurring after MV surgery. This high incidence may be explained by a perimetrical scar (either due to MV surgery or prior ablation) that facilitates slow conduction around the mitral annulus [7].
Electroanatomic mapping
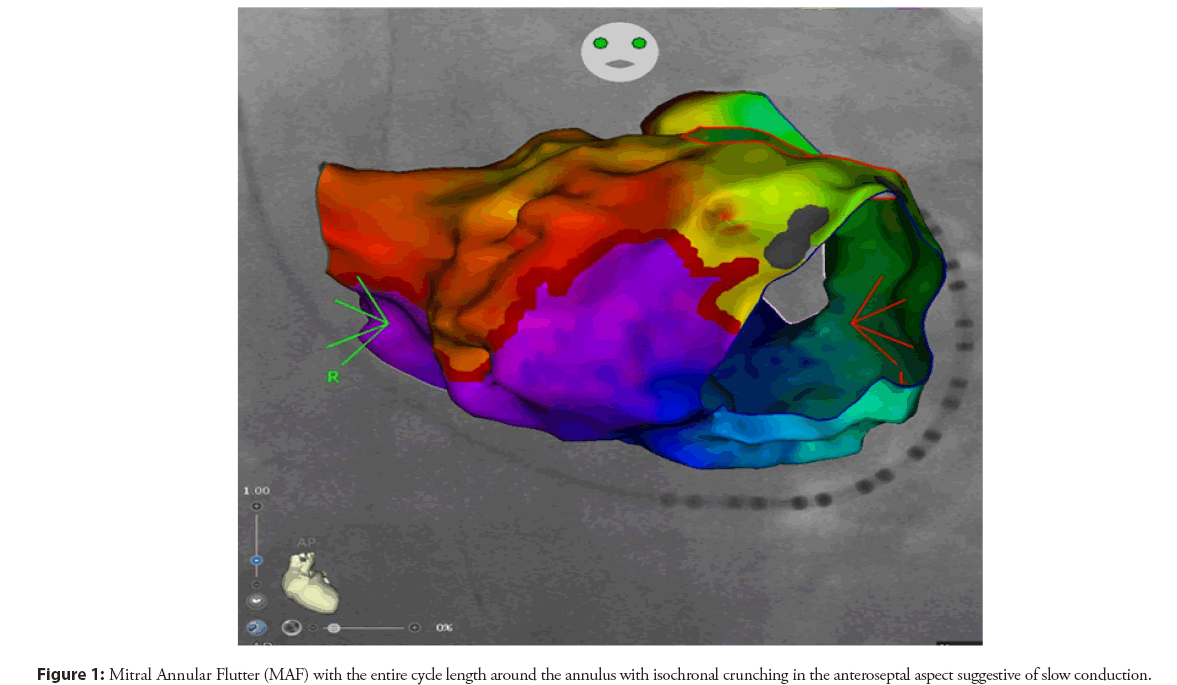
To accurately create electroanatomical maps for ablation of atypical atrial flutter, it is important to identify certain characteristics. These include the presence of electrical activation and two limbs with different conduction velocities, one fast and one relatively slow. These limbs have different refractory periods and create a functional core with unidirectional block. Critical regions and the dimensions and geometry of the circuit must also be identified in order to effectively carry out the ablation procedure to effectively carry out the ablation procedure (Figure 1) [4].
There are many reasons which could hinder our understanding of Macro-reentrant tachycardias. Therefore, it is critical to have a preconceived suspicion of the possible mechanism and location of Atrial Flutter (AFL) to avoid more exhaustive and confusing mapping and optimally strategize the entrainment maneuvers (Table 1).
| Recommendation for electroanatomic mapping |
|
|
|
|
|
|
|
|
Table 1: Recommendations for electroanatomic mapping.
Catheter ablation of Mitral Annular Flutter (MAF)
The mitral annulus acts as an anatomical barrier, and complex connections between the Left Atrium (LA) and Coronary Sinus (CS) play an intricate role in initiating and perpetuating these arrhythmias. Mitral Isthmus (MI) ablation is a time-consuming procedure, with the mean pure ablation time being almost 11 minutes, something to consider during the procedural planning [4]. The two most common approaches for ablating peri-mitral flutter include a Left Atrial Anterior Wall (LAAW) line and a Lateral Mitral Isthmus (LMI) line. Table 2 lists some recommendations for catheter ablation of MAF.
| Recommendation for percutaneous catheter ablation of mitral annular flutter |
|
|
|
|
|
|
|
|
|
Table 2: Recommendation for percutaneous catheter ablation of Mitral Annular Flutter (MAF).
When a standard mitral isthmus line is needed, a stratified approach can begin with endocardial ablation with preferably a balloon occlusion of CS, followed by epicardial ablation within the CS. If needed, a Vein of Marshall (VOM) ethanol infusion until the bidirectional block is achieved may be performed [8].
Challenges in mitral annular ablation
Although there are multiple lines in which ablative therapy can be applied, each has its anatomical challenges. The two most common approaches for ablating peri-mitral flutter include a LMI line and LAAW line.
Lateral Mitral Isthmus (LMI) line
This connects the left inferior pulmonary vein and the mitral valve annulus at the 4 o’clock position lateral to the left atrial appendage [2]. It can be difficult to achieve endocardial ablation only, and studies conducted through autopsies have revealed a significant range of thickness in the myocardium within this region [9]. The operator is usually unaware of the variability in tissue thickness and generally adopts fixed output ablation along the mitral isthmus.
Secondly, achieving complete bidirectional may be challenging due to blood flow in the CS that acts as a heat sink, removing heat from the ablation site and reducing the efficacy of radiofrequency ablation [10]. As a result, epicardial ablation within the CS may be required to achieve mitral annular block up to 70% of the time. Also, the CS has a diameter myocardial sleeve extending a variable distance from the CS ostium. This muscle cuff can act as an epicardial bridge, bypassing the endocardial mitral isthmus at the site adjacent to endocardial ablation [11].
Another factor that imposes a challenge during ablation involves the bundle of Marshall, a fibromuscular tissue that surrounds the Vein of Marshall. Traversing epicardially along the ridge between the Left Atrial Appendage (LAA) and left pulmonary veins, it may have connections to the CS musculature and the left atrium, thereby providing another source of epicardial connection that can prevent bidirectional block in peri-mitral flutters [12].
The left circumflex artery may lie in close proximity to the CS in some patients and may be susceptible to injury during ablation. Other potential complications of the LMI line include coronary spasm or occlusion, perforation of the CS, and pericardial tamponade due to the necessity to ablate the CS [8].
Left Atrial Anterior Wall (LAAW) line
This line connects the superior aspect of the mitral annulus and the Left Superior Pulmonary Vein (LSPV) to the LAA (“anterolateral line”), roof line (“true anterior line”), or right superior pulmonary vein (RSPV; “anteromedial line”) [2]. This approach avoids the epicardial connections of the CS muscle coat and the bundle of Marshall. But this approach has its own set of anatomical challenges.
The anterior mitral line is significantly longer than the lateral mitral isthmus line [1]. Furthermore, thick epicardial muscle bundles within Bachmann’s bundle that extend anteriorly and invaginate the base of the LAA can make transmural ablation difficult. Despite adequate oral anticoagulation, some case reports have described transient ischemic attacks and strokes in patients following anterior linear ablation, resulting in a greater activation delay of the left atrial appendage than lateral mitral isthmus lines [13]. If performed with a lateral mitral isthmus line or in the presence of a posterolateral scar, the LAA may become electrically isolated with a consequent increased risk of thromboembolic events.
With the anterolateral line, there is potential for injuring the sinus node artery branch of the left circumflex artery. In the anteromedial line, it is often difficult to get adequate contact without a steerable catheter; therefore, this line is reserved for refractory cases [9]. LAAW ablation results in significant conduction delay to the left atrial appendage and may result in intra-atrial or atrioventricular dyssynchrony [14].
Superolateral mitral isthmus line
This line connects the posterior base of the LAA orifice adjacent to the LSPV and the mitral annulus. It has been associated with high rates of successful transmural block without CS ablation. It also interrupts the epicardial connections within the ligament of Marshall. However, the thin atrial myocardium over the superolateral aspect of the LAA increases the risk of cardiac tamponade [15].
Mountantonakis, et al. described the challenges of MAF ablation in patients with previous mitral valve surgery and prosthetic mitral valves [7]. Catheter ablation presents several challenges in these patients. One significant hurdle is creating a block across the mitral isthmus, which typically requires ablation on both the mitral annulus and, in some cases, the ventricular aspect of the annulus. As a result, physicians may avoid attempting to create a mitral isthmus line in patients who have undergone mitral valve replacement, as there is a risk of the catheter becoming trapped in the prosthetic valve. Additionally, creating deep, contiguous lesions can be difficult in patients with advanced atrial myopathy and scar around the mitral annulus. Despite these challenges, catheter ablation is often necessary for patients with symptomatic MAF, as medical management may prove ineffective.
A meta-analysis conducted by Aldaas, et al. compared the procedural and peri-procedural outcomes of two ablation approaches for MAF ablation, namely the left atrial anterior line and lateral mitral isthmus line [1]. The study findings indicated no significant differences between the two approaches in bidirectional block rates, ablation time, and pericardial effusion risk. However, the left atrial anterior line method required a longer ablation line length, caused delayed left atrial appendage activation, improved sinus rhythm maintenance during follow-up, and eliminated the need for coronary sinus ablation. Conversely, the lateral mitral isthmus line method necessitated coronary sinus ablation, which could result in complications such as coronary spasm or occlusion, coronary sinus perforation, and pericardial tamponade.
Assessment of bidirectional block
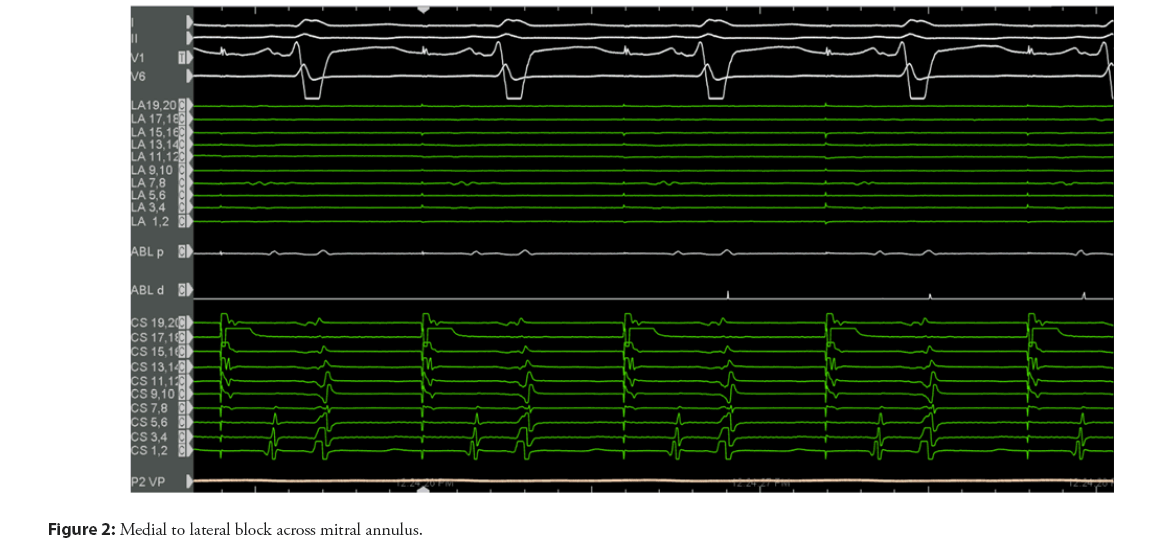
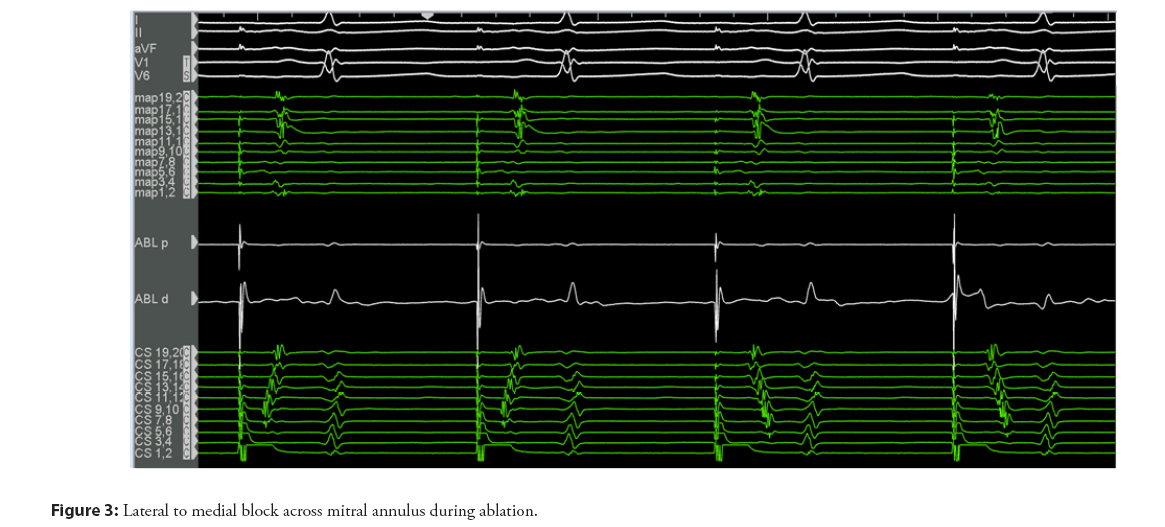
Irrespective of the approach, careful mapping and pacing maneuvers need to confirm transmural bidirectional conduction block as a mandatory prerequisite for successful long-term outcomes (Figures 2 and 3).
This can be confirmed by:
• Pacing medial and lateral to ablation lines both endocardial and epicardially.
• Spatial differential pacing.
A lateral mitral isthmus line block is suspected in the presence of widely split double potentials of fixed separation recorded along the length of the ablation line during pacing from the distal CS electrode. Electroanatomic mapping is utilized to identify the activation detour when pacing is performed from either side of the line. Pacing on the septal side of the line using the CS should reveal activation in both the septal and lateral directions [16]. Differential pacing is then conducted by placing the ablation or multipolar catheter in the LAA. When lateral mitral isthmus block is present, pacing from the LAA will result in the atrial electrogram being recorded earlier on the His electrode than the CS proximal electrode. This will be followed by counter-clockwise activation around the mitral annulus with proximal to distal CS activation. The activation time should be the same when pacing in the reverse direction from the CS distal electrode to the catheter in the LAA. An activation map may be completed immediately superior to the line to confirm the latest activation immediately adjacent to the line. Finally, the stimulus-to-electrogram interval in the catheter placed in the LAA should be longer with pacing from the distal CS electrode than with pacing from a more proximal CS electrode [2].
If Radiofrequency (RF) ablation fails to achieve conduction block within the Coronary Sinus (CS), Ethanol Infusion into the Vein of Marshall (EIVOM) may be performed. A study conducted by Takigawa, et al. found that EIVOM can potentially shorten the RF duration needed for peri-mitral flutter termination and result in improved outcomes at one year when used as a treatment for peri-mitral flutter [17].
Physicians may use differential pacing maneuvers to verify the left atrial anterior wall line block. However, the line length can be challenging to distinguish a conduction delay from the complete block. When a multipolar mapping catheter is placed lateral to the line, the ablation catheter should detect widely split local double potentials of fixed separation along the length of the ablation line during pacing. Afterward, the ablation catheter is placed septal to the line for testing. The LAA should have a long activation time when pacing from the ablation catheter. This activation time should be the same when pacing in the opposite direction from the LAA and recording from the ablation catheter septal to the line.
Conclusion
MAF is highly resistant to anti-arrhythmic therapy and cardio version and often requires ablation. Achieving a successful bidirectional block in mitral annular flutter can be challenging due to the complex anatomy involved. The most commonly used lines for ablation are the left atrial anterior wall line and lateral isthmus line, each having its own set of difficulties. Although MAF ablation has high success rates, it is important to consider these challenges faced during it and understand how to assess the block.
Financial Support
This Research did not receive any specific grant from funding agencies in public, commercial or not-for-profit sectors.
Compliance with Ethical Standards
N/A
Conflict of Interest
Drs Iyengar, Mekala, and Srivathsan declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the authors.
References
- Aldaas OM, Lupercio F, Lin AY, et al. Ablation of mitral annular flutter ablation utilizing a left atrial anterior line versus a lateral mitral isthmus line: A systematic review and meta-analysis. J Interv Card Electrophysiol. 63: 87-95 (2002).
- Lim MW, Kistler PM. Managing peri-mitral flutter. J Cardiovasc Electrophysiol. 1-7 (2023).
- Maheshwari A, Shirai Y, Hyman MC, et al. Septal versus lateral mitral isthmus ablation for treatment of mitral annular flutter. JACC: Clinical Electrophysiology. 5(11): 1292-1299 (2019).
- Sriramoju A, Elbanna M, Cheema KP, et al. Risk factors and management of mitral annular atrial flutter after mitral valve surgery. Curr Cardiovasc Risk Rep. 16: 87-96 (2022).
- Silbiger JJ. Anatomy, mechanics, and pathophysiology of the mitral annulus. Am Heart J. 164(2): 163-176 (2012).
- Faletra FF, Leo LA, Paiocchi VL, et al. Anatomy of mitral annulus insights from non-invasive imaging techniques. Eur Heart J Cardiovasc Imaging. 20(8): 843-857 (2019).
- Mountantonakis S, Frankel DS, Hutchinson MD, et al. Feasibility of catheter ablation of mitral annular flutter in patients with prior mitral valve surgery. Heart Rhythm. 8(6): 809-814 (2011).
- Báez-Escudero JL, Morales PF, Dave AS, et al. Ethanol infusion in the vein of marshall facilitates mitral isthmus ablation. Heart Rhythm. 9(8): 1207-1215 (2012).
- Becker AE. Left atrial isthmus: Anatomic aspects relevant for linear catheter ablation procedures in humans. J Cardiovasc Electrophysiol. 15(7): 809-812 (2004).
- Hamoud NS, Abrich VA, Shen W, et al. Achieving durable mitral isthmus block: Challenges, pitfalls, and methods of assessment. J Cardiovasc Electrophysiol 30(9): 1679-1687 (2019).
- Chauvin M, Shah DC, Haissaguerre M, et al. The anatomic basis of connections between the coronary sinus musculature and the left atrium in humans. Circulation. 101(6): 647-652 (2000).
- Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: A structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 36(4): 1324-1327 (2000).
- Yeo KK, Davenport J, Raff G, et al. Life-threatening coronary sinus thrombosis following catheter ablation: Case report and review of literature. Cardiovasc Revasc Med. 11: e1-e5 (2010).
- Pak HN, Oh YS, Lim HE, et al. Comparison of voltage map-guided left atrial anterior wall ablation versus left lateral mitral isthmus ablation in patients with persistent atrial fibrillation. Heart Rhythm. 8: 199-206 (2011).
- Maurer T, Metzner A, Ho SY, et al. Catheter ablation of the superolateral mitral isthmus line: A novel approach to reduce the need for epicardial ablation. Circ Arrhythm Electrophysiol. 10: e005191 (2017).
- Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation. 110(19): 2996-3002 (2004).
- Takigawa M, Vlachos K, Martin CA, et al. Acute and mid-term outcome of ethanol infusion of vein of Marshall for the treatment of perimitral flutter. Europace. 22(8): 1252-1260 (2020).