Case Report - International Journal of Clinical Rheumatology (2023) Volume 18, Issue 5
Lymphadenopathy as the initial presentation in systemic lupus erythematosus: A disease manifestation or co-existing association and review of the literature
Tamer A Gheita11* Shams M Reda1, Anan H El Aini1, Aya El-Hindawy1, Heba H El-Hadidi2 , Lobna Thabet1, Lobna A Maged1, Nora Y Elsaid1, Nahla N Eesa1, Samar M Fawzy1
1 Rheumatology Department, Faculty of Medicine, Cairo University, Egypt
2Dermatology Department, Faculty of Medicine, Cairo University, Egypt
Rheumatology Department, Faculty of Medicine, Cairo University, Egypt
E-mail: gheitamer@hotmail.com
Received: 02-May-2023, Manuscript No. fmijcr-23-98113; Editor assigned: 04- May-2023, Pre-QC No. fmijcr-23-98113 (PQ); Reviewed: 18-May-2023, QC No. fmijcr-23-98113; Revised: 23-May- 2023, Manuscript No. fmijcr-23-98113 (R); Published: 28-May-2023, DOI: 10.37532/1758-4272.2023.18 (5).84-92
Abstract
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that exhibits a variety of clinical symptoms in many organs.Lymphadenopathyis a frequent and usually nonspecific feature of SLE. Some patients have lymphadenopathy at diagnosis or at follow-up. Objective: to present a rare case of lymphadenopathy presenting as the initial presentation of SLE. Case presentation: A 34 years old female patient presented with cervical and axillary LN enlargement for 2 month duration after which she developed arthritis affecting mainly small joints of both hands initial labs workup revealed microcytic hypochromicanemia, leukopenia and elevated ESR hematological work up revealed antinuclear antibodies and anti-smith antibodies were positive rheumatoid factor and anti CCP were negative. As that time the diagnosis of SLE was made but suspicious LNS lead us to extensive work up for infection and malignancy which revealed generalized lymphadenopathy suspicious for lymphoma, malignancy concern lead us to excisional biopsy from cervical LN and revealed at first a picture highly suggestive for Hodgkin lymphoma but repeated biopsy showed a picture of reactive hyperplasia with positive CD staining PET scan also wasn’t conclusive and revealed metabolically active supra and infra diaphragmatic LNS with lymphoma among possibilities for histopathological correlation. After ruling out infections and not being able to confirm malignancy, patient was started on steroids 20mg with a significant improvement of joint condition and she is under regular follow-up. Conclusion: Diffuse lymphadenopathy may be a presenting feature of SLE and should be considered in the differential diagnosis of patient presenting with diffuse lymphadenopathy
Keywords
Lymphadenopathy • Systemic lupus erythematosus • Initial presentation
Introduction
In Egypt, SLE has a wide variety of clinical and immunological manifestations, with some similarities with that in other nations and differences within the same country. The clinical characteristics, autoantibodies and comorbidities are comparable between Ao- SLE and Jo-SLE. The frequency of various clinical and immunological manifestations varied between genders [1].
Localized or generalized lymphadenopathy, which may be associated with systemic symptoms such as fever, is frequently found in patients with systemic lupus erythematosus (SLE) [2]. Lymphadenopathy is a benign finding in SLE, commonly seen in young patients with cutaneous involvement and constitutional symptoms, with good response to corticosteroids. Reactive follicular hyperplasia is the most frequent finding in biopsies [3].
Generalized diffuse lymphadenopathy has been associated with SLE but is much less frequent now than in the past [4]. Lymphadenopathy commonly occurs in association with active SLE and is characterized by the presence of enlarged, soft, nontender lymph nodes. Lymphadenopathy can be focal or generalized; the cervical, axillary and inguinal regions are typically involved. Lymph node histopathology demonstrates reactive hyperplasia and varying degrees of coagulative necrosis. The presence of hematoxylin bodies is specific for SLE. Histologic features of Castleman’s disease have been reported. The differential diagnosis of lymphadenopathy in a patient with SLE includes infection and/or a lymphoproliferative process; lymph node biopsy is sometimes required for diagnosis. Splenomegaly can be observed in patients with SLE and may be associated with hepatomegaly. Histopathologic studies demonstrate periarterial fibrosis (onion-skin lesions). Splenic atrophy and asplenism has been reported [5, 6].
Lymphadenopathy could represent a vast spectrum of etiologies including infectious or non-infectious diseases. Some of the infectious causes include HIV, Castleman’s disease, tuberculosis, brucellosis, and syphilis. Common causes of non-infectious etiologies of diffuse lymphadenopathy include lymphoma, sarcoidosis, connective tissue disease, and Kikuchi-Fujimoto disease [7]. Identified lymphadenopathy and splenomegaly in SLE mostly denote high disease activity. However, other causes must be taken into account to adopt a more complete therapeutic approach [8].
Although generalized lymphadenopathy is a rare initial presentation of SLE, it should be included in the differential diagnosis of the disease [9]. After extensive workup for malignant and infectious diseases, if unrevealing, rheumatologic workup is required as generalized diffuse lymphadenopathy may be a presenting feature of SLE [7]. Generalized lymphadenopathy as the first and only manifestation makes the diagnosis of SLE challenging [4].
The aim of the current study is to present a rare case of lymphadenopathy presenting as the initial presentation of SLE and to review the literature on the likely causes of SLE associated lymphadenopathy.
Search methodology
The database Scopus and MEDLINE were searched and limited to the keywords ‘lymphadenopathy’ and 'systemic lupus erythematosus' with no date restriction on the 1st of November, 2022. The article titles, abstracts, keywords and full text (when possible) were revised for the search terms. Advanced search was considered for articles including in their titles both keywords and to those with lymphadenopathy as the initial presentation of the disease. Out of 32250 titles for SLE, there were 228 results (0.7%) for the keywords ‘lymphadenopathy’ and 'systemic lupus erythematosus' on PubMed and for those titles that include the 2 keywords, there were only 35 (0.11%). For those cases presenting with lymphadenopathy as the initial presentation, to the best of our knowledge, 9 reports were recorded.
Case presentation
A 34 years old female patient from Cairo, married with 2 offspring’s presented to the rheumatology outpatient clinic, Cairo university hospitals with pain and swelling of the hand joints and morning stiffness for more than 2 hours. She reported neck swelling for a 2 months duration and dry mouth. No history of weight loss, fever, recent infection, dysphagia , Raynaud's or skin tightness, malar rash, photosensitivity or oral ulcer, dry eye or ocular manifestations, inflammatory back pain or psoriasis, manifestations suggestive chest, cardiac or gastrointestinal manifestations or recent dr
ug intake.
On examination the patient was fully conscious and oriented. The pulse, blood pressure, temperature and respiratory rate were within normal.
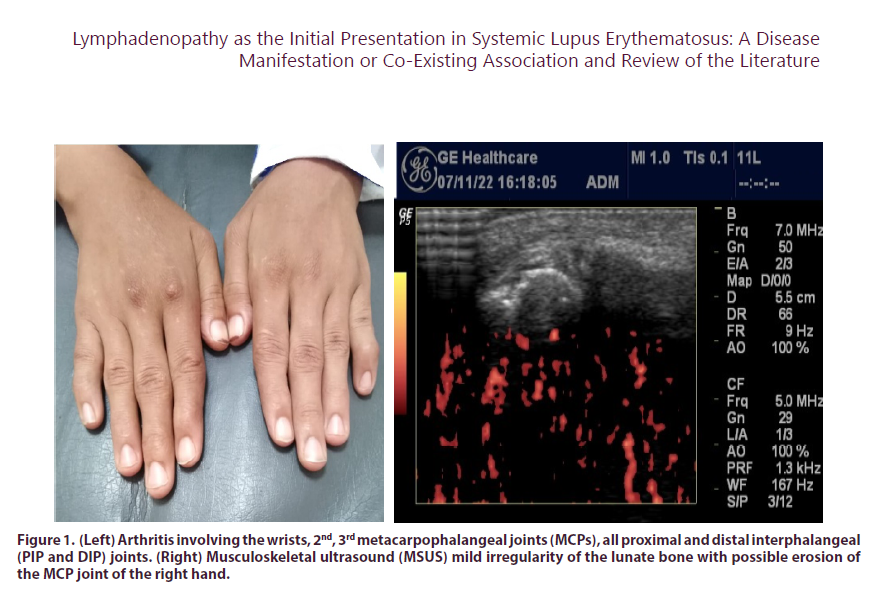
There was arthritis involving the wrists, 2nd, 3rd metacarpophalangeal joints (MCPs), all proximal and distal interphalangeal (PIP and DIP) joints (Figure 1a). The range of motion (ROM) was limited in both shoulders. The clinical disease activity index (CDAI) was calculated (43) denoting high activity. There was an enlarged posterior cervical LN on the right side, firm, 2 cm in size and bilateral enlarged axillary LNs. No other LNs were clinically detected. Cardiovascular, respiratory, and neurological systems examination were unremarkable.
Figure 1: (Left) Arthritis involving the wrists, 2nd, 3rd metacarpophalangeal joints (MCPs), all proximal and distal interphalangeal (PIP and DIP) joints. (Right) Musculoskeletal ultrasound (MSUS) mild irregularity of the lunate bone with possible erosion of the MCP joint of the right hand.
Plain x-ray of the hands and wrists, plain x-ray chest and computerized tomography (CT) chest were normal. Laboratory investigations were requested including infection, autoimmune and malignancy work up (Table 1). There was microcytic hypochromic anemia, leucopenia and a highly elevated ESR. Tests for infection were negative and the autoimmune profile was remarkably positive. There was no history suggestive of coronavirus disease 2019 (COVID-19) infection and the patient did not receive the COVID-19 vaccine.
| Investigations | |
|---|---|
| Hemoglobin (g/dl) | 8.8 |
| TLC (x103/mm3) | 3.27 |
| staff: segmented | 02:52 |
| Platelets (x103/mm3) | 210 |
| ESR (mm/1st hour) | 120 |
| CRP (mg/dl) | Negative |
| Creatinine (mg/dl) | 0.68 |
| Urea (mg/dl) | 40.7 |
| LDH (U/L) | 264 |
| CPK (ug/L) | 37 |
| SUA (mg/dl) | 4.4 |
| T3 (nmol/L) | 2.9 |
| T4 (ng/dl) | 1.1 |
| TSH (mU/L) | 3.06 |
| PTH (pg/ml) | 29.4 |
| Urine | normal |
| Infection work-up | |
| Tuberculin skin test | negative |
| HBV sAg | negative |
| HCV Ab | negative |
| HIV Ab | negative |
| CMV IgM | negative |
| EBV IgM | negative |
| Autoimmune profile | |
| ANA | positive 1/640 |
| pattern | speckled |
| Anti-dsDNA | positive |
| ENA: | positive |
| Anti-RNP | positive |
| Anti-histone | positive |
| Anti-Smith | positive |
| Anti-La | positive |
| Anti-Ro | negative |
| Anti-Scl70 | positive |
| Anti-Rib-P | positive |
| Anti-Jo | negative |
| RF | negative |
| Anti-CCP | negative |
TLC: total leucocytic count, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein, LDH: lactate dehydrogenase, CPK: creatine phosphokinase, SUA: serum uric acid, T3/T4: Thyroid hormone, TSH: thyroid stimulating hormone, PTH: parathormone, HBV: hepatitis B virus, HCV: hepatitis C virus, HIV: human immunodeficiency virus, CMV: cytomegalovirus, EBV: Epstein Barr virus, ANA: antinuclear antibody, dsDNA: ant double stranded deoxyribonucleic acid, ENA: extractable nuclear antigen, anti-RNP: anti-ribonuclear protein, Scl-70: anti-scleroderma 70, anti-Rib-P: anti-ribosomal P protein, RF: rheumatoid factor, anti-CCP: anti-cyclic citrullinated peptide.
Table 1. Laboratory investigations of the young female with lymphadenopathy and systemic lupus erythematosus.
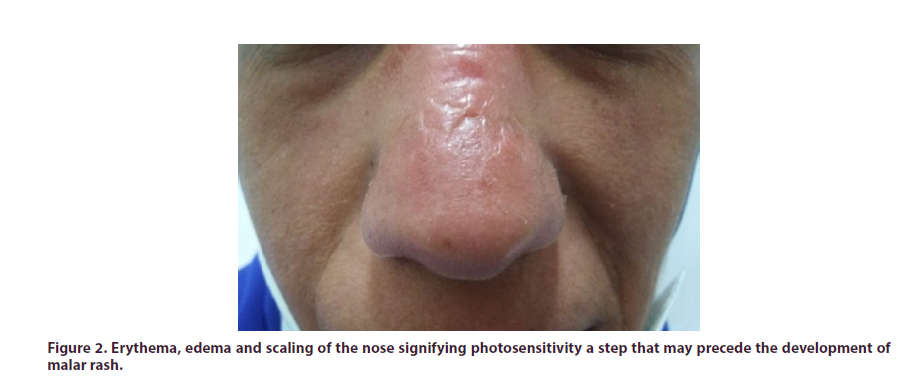
During investigation the patient started to develop malar rash and photosensitivity. Dermatology consultation revealed erythema, edema and scaling of the nose signifying photosensitivity a step that may, or may not, precede the development of malar rash (Figure 2). At this point the diagnosis of SLE was made as the patient fulfilled the 2019 European league against rheumatism/ American College of rheumatology (EULAR/ACR) criteria for the classification of SLE [10]. The SLE disease activity index (SLEDAI) [11] was calculated to be 7 (mild/moderate activity). The case was presented in the Rheumatology and Clinical Immunology "Case of the Week" meeting among Professors in the Rheumatology Department, Cairo University and further consultations were suggested to complete the work up of the case. Ophthalmologic examination revealed bilateral dry eye with meibomian gland dysfunction and was prescribed topical treatment. Musculoskeletal ultrasound was performed and revealed mild irregularity of the lunate bone with possible erosion of the MCP joint of the right hand (Figure 1b). However, the suspicious LNs lead us to further investigate and extra malignancy-work up was considered. Tumor markers, Ca125, CA 19 9, AFP, CEA, CA15 were normal. Cervical ultrasound revealed multiple enlarged lymph nodes 18 mm (right) and 12 mm (left), matted with preserved oval shape and fatty hilum; CT neck revealed bilateral upper and lower deep cervical multiple small discrete lymph node with no matrix calcification or breaking down. Pelviabdominal ultrasonography revealed hepatospenomegaly and CT with contrast reported hepatosplenomegaly, enlarged discrete abdominal and inguinal lymphadenopathy, possibly neoplastic (lymphoma) for histopathological correlation.
Accordingly an excisional biopsy was performed of the cervical LN and was found to be a single nodule 2x 1.5 cm rubbery grayish and microscopically showed partially effaced nodule with infiltration by mononuclear cells (lacunar cells) in a background of inflammatory cells with fibrotic bands. A diagnosis of highly suspicious for Hodgkin lymphoma (mixed celluarity) was still held and immunohistochemical staining suggested. There was positive staining for CD20 and CD3 while negative for CD15 and CD30 and a picture suggestive of reactive lymphoid hyperplasia was held. However, the oncologist requested a revision of the immunohistochemistry staining and positive staining for CD15 and CD30 was found for further immunophenotyping.
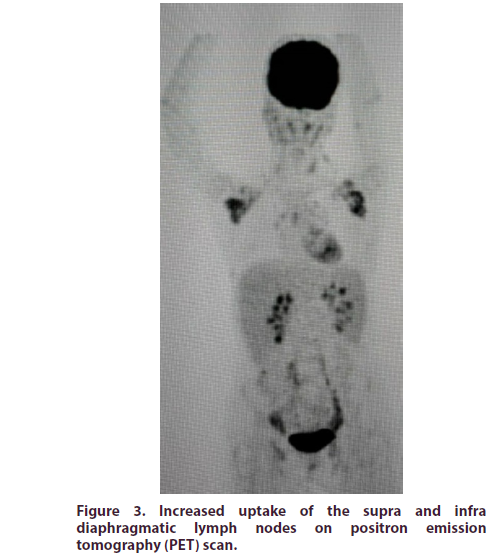
Furthermore, positron emission tomography (PET) scans also reported metabolically active supra and infra diaphragmatic lymphadenopathy with lymphoma a possibility for histopathological correlation and oncology consultation revealed that the possibility of lymphoma cannot be excluded as it showed increase uptake by PET scan (Figure 3) and re-examination of the histopathology could not confirm malignancy.
It was decided to postpone steroids until the biopsy was done so as not to alter the findings. Patient was only prescribed symptomatic treatment. After ruling out infections and not being able to confirm malignancy, patient was started on oral steroids 20 mg/day and hydroxychloroquine 300 mg/day with a significant improvement of arthritis and she is under regular follow-up.
Discussion
Lymphadenopathy in SLE is often such a challenging presentation for rheumatologists. There are many examples for a differential diagnosis of this condition that add to the dilemma of any SLE case. Table 2 presents the reported causes associating lymphadenopathy with SLE in published articles.
| Causes of lymphadenopathy in SLE | |
| As part of the disease manifestation | |
| Disease activity is more commonly associated | |
| Ageing (late-onset SLE) | |
| Juvenile SLE (higher tendency) | |
| Co-existence with SLE | |
| HS / MAS | |
| Sarcoidosis | |
| Amyloidosis | |
| IgG4-related disease (IgG4-RD) | |
| Rosai-Dorfman Disease: (SHML) | |
| Necrotizing histiocytic lymphadenopathy (NHL) | |
| Autoimmune lymphoproliferative syndrome (ALPS) | |
| Mimics/Associations with SLE | |
| Castleman's disease (CD) | |
| TAFRO syndrome (CD variant) | |
| Kikuchi-Fujimoto disease (KFD) | |
| Infections | |
| COVID-19 | TB |
| Syphilis | Brucellosis |
| EBV HIV CMV | |
| Medications | |
| Rituximab | |
| Vaccines: COVID-19 / meningeococcal | |
| Malignancies | |
| ALPIBP | |
| Angioimmunoblastic T-cell lymphoma | |
| Burkitt lymphoma Burkitt lymphoma | |
| (predispose to) Thyroid cancer | |
| non-Hodgkins lymphoma | |
HS: Hemophagocytic syndromes, MAS: Macrophage activating syndrome, SHML: Sinus histiocytosis with massive lymphadenopathy TAFRO: thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly, COVID-19: coronavirus disease 2019, EBV: Epstein Barr virus, HIV: human immunodeficiency virus, CMV: cytomegalovirus, TB: tuberculosis, ALPIBP: Atypical lymphoplasmacytic and immunoblastic proliferation.
Table 2. Reported causes of lymphadenopathy in systemic lupus erythematosus (SLE) from the search on PubMed from 228 articles.
Lymphadenopathy is a frequent and usually nonspecific feature of SLE and in spite of the fact that it usually carries no risk to the patient, disease activity levels are high. Besides the disease itself, other causes of lymphadenopathy such as infections, immunological or malignant diseases must be considered in the differential diagnosis [12]. In the present case, in addition to the cervical lymphadenopathy, the patient initially presented by arthritis of the wrists and hands as well as prolonged morning stiffness. In SLE, arthralgia or arthritis, are usually transient but can mimic rheumatoid arthritis (RA), with persistent pain, swelling, stiffness, and disability. Lupus arthritis usually involves the metacarpophalangeal and interphalangeal, wrist, and knee joints, it can develop at onset and is associated with morning stiffness of at least 30 min. [13]. Lack of erosions and negative RF and anti-CCP make the diagnosis of rhupus unlikely. Lymphadenopathy is not uncommon with SLE disease and activity. The association has been reported to be present in 4% of adult Egyptian SLE patients [14] and in 14.2% of Iranian cases [15], yet it is unusual to be the initial presentation [16, 17].
The precise prevalence of lymphadenopathy in SLE is unknown but has been reported in 26%. SLE patients presenting with lymphadenopathy are more likely to manifest constitutional symptoms such as fever, fatigue, weight loss, cutaneous manifestations, hepatomegaly and splenomegaly, anti-dsDNA antibodies and consumed complement indicative of active disease [18]. The current case had mild/moderate activity. Lymphadenopathy is common in active SLE and exhibits marked histological diversity and reactive LN hyperplasia is possible [17] The patient was 34 years old. However, lymphadenopathy was part of the initial presentation of a very late-onset SLE Octogenarian woman [19]. Moreover, in the recent study of El-Garf et al. on a large number of Egyptian SLE cases there was a tendency to a higher frequency of lymphadenopathy in juvenile patients (7%) [14]. Lymphadenopathy is occasionally the presenting feature in children with SLE and granulomatous lupus lymphadenitis though rare has been reported [20].
The present case was not feverish and the possibility of an associated infection was ruled out and there was no history suggestive of a recent COVID-19 infection or vaccination. While an obvious association was found between the age and steroid escalation in SLE patients, the development of COVID-19 was also associated with lymphadenopathy [21]. In young individuals, Epstein-Barr virus (EBV) infection is a major differential diagnosis of SLE and age-related EBV-lymphoproliferative disorder may masquerade as SLE [22]. Infectious mononucleosis is the acute clinical manifestation of EBV. It is characterized by low-grade fever, malaise, lymphadenopathy, splenomegaly, and occasionally symmetrical arthralgias. EBV may trigger new-onset SLE [23]. Newly developed SLE has been reported in a patient with human immunodeficiency virus (HIV) presenting with lymphadenopathy [24]. Cytomegalovirus (CMV) infections have been associated with exacerbations of SLE and may present with persistent fever, cervical lymphadenopathy, elevated liver function tests, and leucopenia [25]. Overlapping features of syphilis and SLE may include lymphadenopathy [26].
Persistent suspicion of malignancy in the current case, made its exclusion challenging. Burkitt lymphoma is a highly aggressive non-Hodgkin B-cell lymphoma and even though clinically detected lymphadenopathy may be absent, PET scan may show extensive hypermetabolic lymphadenopathy in multiple areas in a known case of SLE [27]. In SLE patients, the presence of lymphadenopathy and splenomegaly may predispose to thyroid cancer [28]. A symmetrically increased (18) F-FDG uptake in small lymph nodes with multiple serous cavity effusion helps in differentiating between SLE related autoimmune haemolytic anaemia (AIHA) with lymphadenopathy and lymphoma [29]. The simultaneous occurrence of angioimmunoblastic T-cell lymphoma (AITL) and SLE has been reported usually with multiple swollen intra-abdominal and intra-pelvic LNs [30]. Furthermore, atypical lymphoplasmacytic and immunoblastic proliferation (ALPIBP) may be associated with SLE presenting with lymphadenopathy [17]. The increased risk of lymphoproliferative diseases in SLE with considerable overlap in features adds to the difficulty in diagnosing lymphoma in lupus patients. An increased awareness is recommended in dealing with SLE patients with lymphadenopathy and careful clinical, laboratory and pathological evaluation are often needed in order to establish an accurate diagnosis [12].
SLE flare and infection could trigger macrophage activation syndrome (MAS), one of the hemophagocytic syndromes (HS) [31]. Although lymphadenopathy and splenomegaly are common features, however, absence of fever and the mild increase in SLEDAI with a low probability of having HS (0.4%; H-score 80) make the diagnosis of MAS unlikely in our case. HS are not frequent manifestations of SLE yet the high suspicion of its possible association must be maintained for timely treatment [32]. It may be difficult to distinguish HS from SLE flare-up or non-Hodgkins lymphoma and on treatment; opportunistic infections remain a difficult issue [33]. There is a potential concurrence of sarcoidosis and SLE although uncommon [34]. The lack of bilateral hilar lymphadenopathy and histological evidence of non-caseating granuloma make this possibility implausible. SLE and IgG4- related disease can co-exist with overlapping features making accurate diagnosis challenging. Undetected in the present case with lymphadenopathy, increased IgG4 level with eosinophilia raise suspicion of an IgG4-related systemic disease [35]. Histopathologic examination of the LNs shows IgG4+ plasma-cells infiltrates and fibrosis in most cases, regardless of the stage of the disease [36]. Lymphadenopathy due to amyloidosis secondary to Sjögren syndrome and SLE is rare and amyloid lymphadenopathy is detected by PET [37]. Other reported conditions associated with SLE and causing lymphadenopathy include autoimmune lymphoproliferative syndrome (ALPS) [38], necrotizing histiocytic lymphadenopathy (NHL) [39] and Rosai- Dorfman disease; Sinus histiocytosis with massive lymphadenopathy (SHML) [40].
Kikuchi-Fujimoto disease (KFD) (histiocytic necrotizing lymphadenitis) is a rare and benign disease characterized by fever and lymphadenopathy that can mimic SLE with abnormal serologic testing or malignancies with unwanted invasive diagnostic testing and even treatments. It is mostly an immune response of T cells and histiocytes to infections [12, 41]. KFD is a self-limiting lymphadenopathy that typically affects young females and is usually cervical, accompanied by leukopenia. Although the association of KFD with SLE is rare, the number of reports is increasing [42]. Furthermore, SLE may eventually develop in KFD patients [6]. As the prognosis and treatment of KFD and SLE are different, it is important to differentiate these two entities [12].
Castleman's Disease (CD) is a rare, systemic disease with histopathological features of angiofollicular lymph node hyperplasia. There are case reports that mimic or coexist with SLE. TAFRO "thrombocytopenia, anasarca, fever, reticulin fibrosis and organomegaly" syndrome is a newly recognized variant of idiopathic multicentric CD. Confusing presentation may affect the treatment decision [43]. SLE with intraabdominal lymphadenopathy compatible with CD on histopathological examination of the LN biopsy has been reported even though such association is rare [44].
The present case was not receiving any disease modifying antirheumatic drugs (DMARDs) or steroids prior to the development of lymphadenopathy. It has been reported that lymphoid follicular hyperplasia can develop in patients with refractory SLE who received multiple cycles of rituximab with CD20 depletion. The compensatory overexpression of B lymphocyte stimulator (BLyS), biomarkers of apoptosis, in LNs added to the potential value of switching to belimumab [45]. Interestingly, Baricitinib, a selective inhibitor for Janus kinase (JAK) is recently regarded as a potential option in SLE as it was found to remarkably suppress splenomegaly, lymphadenopathy, proteinuria, circulating autoantibodies and pro-inflammatory cytokines [46].
Immune hyperactivation has been linked to various vaccines with a recently potential association of new-onset SLE post-COVID-19 immunization. Lymphadenopathy is among the many manifestations reported following receiving doses of the vaccine [47]. Meningococcal vaccine was reported to newly precipitate SLE with positive ANA, anti-ds DNA and cervical lymphadenopathy.
The current patient developed lymphadenopathy as an initial presentation of SLE and this has been rarely reported in the literature. There are several case reports of generalized lymphadenopathy as the first manifestation of SLE [4, 7, and 20] and the present case is considered another leading report (Table 3). Although not included in the American College of Rheumatology (ACR) classification criteria for SLE, generalized lymphadenopathy is frequently observed in children and may be the presenting feature in the absence of other clinical manifestations. This may pose a diagnostic dilemma, and therefore a lymph node biopsy is warranted in this subset of patients [20].
| Case Report | Year | Country | Age onset | Sex | Lymph nodes | SM | Other | Time till SLE Dx | Treatment /outcome |
|---|---|---|---|---|---|---|---|---|---|
| Kitsanou | 2000 | Greece | 25 | female | Cervical, Axillary, | yes | Pericardial effusion | 6 mo | Steroids |
| et al. [49] | Supraclav., Inguinal. | (remission) | |||||||
| Smith | 2013 | USA | 27 | male | preAuric, supraClav, | yes | Pleural effusion | 7 mo | Steroids, HCQ,MMF |
| et al.[4] | Afro | Occiputal, Inguinal. | (remission) | ||||||
| Shrestha | 2013 | Nepal | 12 | female | Cervical, Axillary, | no | Lupus nephritis | 3 years | Steroids, HCQ |
| et al. [20] | Abdominal | (remission) | |||||||
| Gillmore | 2014 | UK | 30 | female | Cervical, Axillary | - | - | 3 mo | HCQ, MMF |
| and Sin [50] | Mediastinal, Pelvic | Antibiotics | |||||||
| Afzal | 2016 | USA | 23 | female | Cervical, Axillary, Inguinal | yes | Seizures | 8 mo | Steroids |
| et al. [7] | Afro | Lost on FU | |||||||
| Tamaki | 2017 | Japan | 52 | male | Generalized lymphadenopathy | yes | - | 9 mo | Steroids |
| et al. [51] | |||||||||
| Michailidou | 2018 | USA | 35 | male | Cervical | - | Confusion | 2 mo | Steroids |
| et al [52] | Afro | Submandibular | Seizures | HCQ, CYC | |||||
| Muhammad | 2021 | India | 23 | female | Cervical, Axillary, Inguinal, Intertroch. | - | Seizures | 2mo | Steroids, antiTB |
| et al. [53] | Pericardial effusion | Phenytoin | |||||||
| (died after 1 wk) | |||||||||
| Maeda | 2021 | Japan | 25 | female | Cervical | - | LIP | During hospital | Steroids |
| et al. [54] | LR | AZA | |||||||
| Magendiran | 2021 | India | 27 | female | Generalised lymphadenopathy | - | IVH | 1 yr | Steroids |
| et al. [55] | HCQ | ||||||||
| Current | 2023 | Egypt | 34 | female | Cervical, Axillary | yes | - | 2 mo | Steroids, HCQ |
| case | Supra and infra diaphragmatic | (remission) |
Supra Clav: supraclavicular, preAuric: preauricular, Intertroch: intertrochanteric, PE: pericardial effusion, Pl E: pleural effusion, LIP: Lymphoid interstitial pneumonia, LR; livido-reticularis, IVH: intravascular haemolysis, HCQ: hydroxychloroquine, MMF: mycophenolate mofetil, FU: follow up, CYC: cyclophosphamide, AZA: azathioprine.
Table 3. Case reports of systemic lupus erythematosus patients initially presenting with lymphadenopathy.
The patient was finally managed by steroids and hydroxychloroquine. An atypical presentation of a middle-aged SLE woman initially presenting with multiple lung nodules (lymphoid interstitial pneumonia) followed by cervical lymphadenopathy, arthritis and livedo reticular is was later diagnosed as having SLE (positive dsDNA and low complement). All symptoms responded to prednisolone and azathioprine. In conclusion, the initial atypical presentation of SLE with lymphadenopathy should be managed in due time seriously to rule out any masquerade of an underlying malignancy or paraneoplastic syndrome. Such rare presentation of SLE should not be forgotten.
Conflict of Interest
The authors declare no conflict of interest
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Gheita TA, Noor RA, Abualfadl E et al. On behalf of the Egyptian College of Rheumatology (ECR) SLE Study Group. Adult systemic lupus erythematosus in Egypt The nation-wide spectrum of 3661 patients and world-wide standpoint. Lupus. 30(9), 1526-1535 (2021).
- Kojima M, Motoori T, Asano S et al. Histological diversity of reactive and atypical proliferative lymph node lesions in systemic lupus erythematosus patients. Pathol Res Pract. 203(6), 423-431 (2007).
- Neto NS, Bonfiglioli KR, Milanez FM et al. Lymphadenopathy and systemic lupus erythematosus. Rev Bras Reumatol. 50(1), 96-101 (2010).
- Smith LW, Gelber AC, Petri M et al. Diffuse lymphadenopathy as the presenting manifestation of systemic lupus erythematosus. J Clin Rheumatol. 19(7), 397–399 (2013).
- Firestein GS, Dall'Era M, Wofsy D et al. Clinical features of systemic lupus erythematosus. In Kelley and Firestein's Textbook of Rheumatology. Budd RC, Gabriel SE, McInnes IB, O; Dell JR (Eds.). Tenth edition, Elsevier. 1345-1367 (2017).
- Afzal W, Arab T, Ullah T et al. Generalized Lymphadenopathy as presenting feature of systemic lupus erythematosus Case report and review of the literature. J Clin Med Res. 8(11), 819-823 (2016).
- Santacruz JC, Mantilla MJ, Rueda et al. A practical perspective of the hematologic manifestations of systemic lupus erythematosus. Cureus. 14(3), e22938 (2022).
- Muhammad O, Jindal H, Sharath M et al. Systemic lupus erythematosus with multi-organ involvement in a young female Lymphadenopathy, lupus cerebritis, lupus nephritis, and cardiac manifestations. Cureus.13 (6), e15517 (2021).
- Aringer M, Costenbader K, Daikh D. et al. European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. 78(9), 1151-1159 (2019).
- Bombardier C, Gladman DD, Urowitz MB et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 35, 630–640 (1992).
- Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Reumatol Port. 33(4), 402-406 (2008).
- Ceccarelli F, Govoni M, Piga M et al. Arthritis in Systemic Lupus Erythematosus From 2022 International GISEA/OEG Symposium. J Clin Med. 11(20), 6016 (2022).
- El-Garf K, El-Garf A, Gheith R et al. A comparative study between the disease characteristics in adult-onset and childhood-onset systemic lupus erythematosus in Egyptian patients attending a large university hospital. Lupus. 30(2), 211-218 (2021).
- Nazarinia MA, Ghaffarpasand F, Shamsdin A et al. Systemic lupus erythematosus in the Fars Province of Iran. 17(3), 221-227 (2008).
- Girard A, Ohnona J, Bernaudin JF et al. Generalized lymph node FDG uptake as the first manifestation of systemic lupus erythematosus. Clin Nucl Med. 42(10), 787-789 (2017).
- Kojima M, Matsuda H, Iijima M et al. Reactive hyperplasia with giant follicles in lymph node lesions from systemic lupus erythematosus patients. Report of three cases. APMIS. 113(7-8)558-63 (2005).
- Shapira Y, Weinberger A, Wysenbeek AJ et al. Lymphadenopathy in systemic lupus erythematosus. Prevalence and relation to disease manifestations. Clin Rheumatol. 15(4), 335-338 (1996).
- de Montjoye S, Boland B, Van Raemdonck J et al. Very late-onset systemic lupus erythematosus as unusual cause of reversible functional and cognitive impairments in an octogenarian patient. Eur J Case Rep Intern Med. 7(8), 001570 (2020).
- Shrestha D, Dhakal AK, Shiva RK et al. Systemic lupus erythematosus and granulomatous lymphadenopathy. BMC Pediatr. 13,179 (2013).
- Ramirez GA, Argolini LM, Bellocchi C et al. Impact of the COVID-19 pandemic in patients with systemic lupus erythematosus throughout one year. Clin Immunol. 231, 108845 (2021).
- Kadoba K, Nishimura K, Uchino K et al. Age-related Epstein-Barr Virus-associated Lymphoproliferative Disorder Masquerading as Systemic Lupus Erythematosus. Intern Med. 60(15), 2495-2497 (2021).
- Wang S, Wang S, Singh S. Development of systemic lupus erythematosus after infectious mononucleosis in a 64-year-old woman. J Investig Med High Impact Case Rep. 8, 2324709620961613 (2020).
- O'Kelly B, McNally C, McConkey S et al. HIV and systemic lupus erythematosus where immunodeficiency meets autoimmunity. Lupus. 29(9),1130-1132 (2020).
- Pérez-Mercado AE, Vilá-Pérez S. Cytomegalovirus as a trigger for systemic lupus erythematosus. J Clin Rheumatol. 16(7), 335-337 (2010).
- Shatley MJ, Walker BL, McMurray RW et al. Lues and lupus syphilis mimicking systemic lupus erythematosus (SLE). Lupus. 10(4), 299-303 (2001).
- Irshad Y, Tariq EF, Asif H et al. Renal infiltration as a primary presentation of Burkitt lymphoma secondary to systemic lupus erythematosus A rarity unto a rarity. Cureus. 12(9), e10512 (2020).
- Kawano Y, Nambu M, Uejima Y et al. Risk factors for thyroid cancer in systemic lupus erythematosus. Glob Pediatr Health. 4, 2333794X17736700 (2017).
- Zhang X, Xu C, Wang X et al. (18) F-FDG PET/CT imaging in systemic lupus erythematosus related autoimmune haemolytic anaemia and lymphadenopathy. Hell J Nucl Med. 19(1), 42-45 (2016).
- Suzuki A, Shoji N, Aoki N et al. Systemic lupus erythematosus as the concomitant manifestation of angioimmunoblastic T-cell lymphoma. Mod Rheumatol. 27(2), 360-363 (2017).
- Wafa A, Hicham H, Naoufal R et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome a study of 20 Moroccan adult patients. Clin Rheumatol. 41(7), 2021-33 (2022).
- Castillo JM, MÁrquez AMB, Cabada IAB et al. Systemic lupus erythematosus and its association with hemophagocytic syndrome as an initial manifestation. Maedica (Bucur). 15(4), 556-60 (2020).
- Jeremić I, Dorđević-Kontić S, Nikolić M et al. Systemic lupus erythematosus progressing to non-Hodgkin's lymphoma complicated by fatal hemophagocytic syndrome case report. Acta Dermatovenerol Croat. 20(1)21-26 (2012).
- Prieto-Peña D, Ferrer-Pargada D, Atienza-Mateo B et al. Coexisting sarcoidosis and systemic lupus erythematosus a case report and literature review. Acta Reumatol Port. 46(2), 177-185 (2021).
- Naramala S, Biswas S, Adapa S et al. An overlapping case of IgG4-related disease and systemic lupus erythematosus. J Investig Med High Impact Case Rep. 7, 2324709619862297 (2019).
- Horger M, Lamprecht HG, Bares R et al. Systemic IgG4-related sclerosing disease spectrum of imaging findings and differential diagnosis. AJR Am J Roentgenol. 199(3), W276-82 (2012).
- Serizawa I, Inubushi M, Kanegae K et al. Lymphadenopathy due to amyloidosis secondary to Sjögren syndrome and systemic lupus erythematosus detected by F-18 FDG PET. Clin Nucl Med. 32(11), 881-882 (2007).
- Tamaki K, Morishima S, Nakachi S et al. An atypical case of late-onset systemic lupus erythematosus with systemic lymphadenopathy and severe autoimmune thrombocytopenia/neutropenia mimicking malignant lymphoma. Int J Hematol. 105(4), 526-31 (2017).
- Komócsi A, Tóvari E, Pajor L et al. Histiocytic necrotizing lymphadenitis preceding systemic lupus erythematosus. J Eur Acad Dermatol Venereol.15 (5), 476-480 (2001).
- Kaur PP, Birbe RC, De Horatius RJ. Rosai-Dorfman disease in a patient with systemic lupus erythematosus. J Rheumatol. 32(5), 951-953 (2005).
- Kazmi TR, Greear EL, Lavallee CA et al. Kikuchi-Fujimoto disease A differential for when it is not systemic lupus erythematosus. Case Rep Rheumatol.20227709246 (2022).
- Kim SK, Kang MS, Yoon BY et al. Histiocytic necrotizing lymphadenitis in the context of systemic lupus erythematosus (SLE) Is histiocytic necrotizing lymphadenitis in SLE associated with skin lesions? Lupus. 20(8), 809-819 (2011).
- Okyar B, Torun B, Öktem ES et al. Mimic or coincidentally? TAFRO syndrome and systemic lupus erythematosus A case-based review. Mod Rheumatol Case Rep. 2022rxac045. Epub ahead of print (2022).
- Demirkan FG, Doğan S, Kalyoncu Uçar A et al. Systemic lupus erythematosus complicated with Castleman disease a case-based review. Rheumatol Int. 41(2), 475-479 (2021).
- Ruiz-Ordóñez I, Santos VA, Bonilla-Abadía F et al. Lymphoid Follicular Hyperplasia in Patients with Systemic Lupus Erythematosus after Multiple Cycles of Rituximab. Mod Rheumatol Case Rep. 2022rxac066. epub ahead of print (2022).
- Lee J, Park Y, Jang SG et al. Baricitinib Attenuates Autoimmune Phenotype and Podocyte Injury in a Murine Model of Systemic Lupus Erythematosus. Front Immunol. 2021, 12704526 (2021).
- Maeda A, Kinjo M, Kinjo K et al. Systemic lupus erythematosus presenting with lymphoid interstitial pneumonia as an initial manifestation. BMJ Case Rep. 14(12), e243539 (2021).
- Magendiran B, Jose A, Kolar Vishwanath V et al. Immune-mediated Coombs negative intravascular haemolysis in systemic lupus erythematosus (SLE). BMJ Case Rep.14 (8), e244459 (2021).
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at , Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref