Research Article - International Journal of Clinical Rheumatology (2021) Volume 16, Issue 3
Long-term results of joint damage in patients with rheumatoid arthritis treated with abatacept: 5-year results of a clinical observational study
- *Corresponding Author:
- Takeshi Mochizuki
Department of Orthopedic Surgery, Kamagaya General Hospital, Chiba, Japan
E-mail: twmutamo@gmail.com
Abstract
Background: In abatacept treatment for RA, there are no studies investigating the long-term results of joint damage in daily clinical practice. We aimed to investigate the long-term efficacy of abatacept in Japanese patients with rheumatoid arthritis.
Methods: We examined 120 patients who received abatacept for 5 years. Joint damage was radiographically analyzed using the van der Heijde-modified total Sharp score. Disease activity score was assessed using the disease activity score in 28 joints-erythrocyte sedimentation rate (DAS28-ESR). The data analyses were used by observed case analysis.
Results: Changes in the Sharp score was 0.60 ± 2.03, 0.93 ± 2.40, 1.23 ± 2.92, 1.53 ± 3.38, and 1.71 ± 3.84 at years 1, 2, 3, 4, and 5, respectively. Progression of joint damage did not differ significantly between the Bio-naïve and Bio-switch groups and methotrexate [MTX](+) and MTX(MTX(-)) groups. DAS28-ESR at baseline was associated with radiographic progression (p = 0.035). In all patients, the remission rates of DAS28-ESR were 44.6% and 50.0% at years 1 and 5, respectively. These rates were 45.2% and 50.8% in the biological disease-modifying anti-rheumatic drugs (Bio)-naïve¯ve group, and 42.9% and 47.1% in the Bio-switch group, respectively. Moreover, these rates were 45.2% and 52.6% in the MTX(+) group and 43.6% and 47.6% in the MTX(-) group, respectively. The remission rates were not significantly different between the groups at any of time points.
Conclusions: we have analyzed the efficacy of abatacept treatment in patient with RA for 5 years in daily clinical practice. The present study suggested that improvement of joint damage, disease activity, and physical function are maintained in the long-term.
Keywords
abatacept • joint damage • long-term • observational study • rheumatoid arthritis
Introduction
Rheumatoid Arthritis (RA) is associated with joint inflammation and destruction, which leads to pain, swelling, stiffness, and loss of function in joints throughout the body. Gradually, patients with RA experience a diminished quality of life, including disability, which impacts both the activities of daily living and work [1-3]. Although the pathogenic mechanisms of RA remain unknown, the involvement of inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-1, and interleukin-6), has been established [4]. Activated T cells promote and stimulate monocytes, macrophages, and synovial fibroblasts to produce inflammatory cytokines. Thus, the modulation of T cells could bean effective strategyfor preventing RA progression. T cells require both an antigen-specific and a co-stimulatory signal for complete activation. One of the best characterized pathways for T cell activation is the engagement of CD80/86 on antigen-presenting cells with the CD28 on T cells [5,6]. During normal immune responses, endogenous cytotoxic T-lymphocyte antigen-4 (CTLA-4) down regulates CD28-mediated T cell activation by binding to CD80/86 with higher affinity than CD28 [7,8]. Given theimportant roleof T cells in theimmune responseofpatients with RA, they may be a reasonable therapeutic target for the treatment of RA.
Abatacept is a fully human, soluble fusion protein comprising the extracellular domain of human CTLA-4 linked to the fragment crystallizable portion of human immunoglobulin G1. This protein functions as a selective modulator of T cell co-stimulation. A combination of abatacept with Methotrexate (MTX) improves signs, symptoms, physical function, quality of life, and disease activity, in patients with active RA despite ongoing therapy with MTX [9,10]. Similarly, in Japan, administration of 10 mg/kg abatacept with MTX significantly improved Disease Activity Score in 28 joints based on C-reactive protein (DAS28-CRP) compared with that reported in the placebo group at week 24 [11]. Moreover, abatacept improved DAS28-CRP and the Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index, and Health Assessment Questionnaire Disability Index (HAQ-DI) compared with those recorded in the placebo group. It has been shown that abatacept suppresses the progression of joint damage, as evaluated using the van der Heijde-modified Total Sharp Score(mTSS), compared with the placebo group at week 52 [12]. Both subcutaneously and intravenously of abatacept demonstrated comparable efficacy and safety [13,14]. In Post-Marketing Surveillance (PMS), the DAS28-CRP and DAS28-Erythrocyte Sedimentation Rate(ESR) at week 24 were significantly lower than those observed at baseline [15]. Several similar results have been reported in daily practice [16-20]. In contrast, there are few long-term results regarding the use of abatacept for the treatment of RA. In the Abatacept in Inadequate Responders to Methotrexate (AIM) trial, the rates of patients who achieved the American College of Rheumatology (ACR) criteria of 20, 50, and 70 at y 1 were maintained for 3 y [21]. Moreover, the results were maintained for 5 y [22]. However, there are no studies investigating the long-term results of joint damage in actual clinical practice. Therefore, we investigated the change in joint damage in Japanese patients with RA treated with abatacept in a clinical observational study over a period of 5 y.
Methods
Patients
In thisstudy, we investigated the clinical course and background characteristics of patients with RA who fulfilled theACRclassification criteria(1987) and/or the ACR/European League Against Rheumatism criteria[23, 24]. A total of 120 consecutive patients who received at least one dose of abatacept were enrolled from January 2011 to October 2014. Patients without baseline data were excluded from analyses. In all patients, RA was poorly controlled for ≥3 months during therapy with Disease-Modifying Antirheumatic Drugs (DMARDs), there by satisfying the existing relevant criteria for the management of RA in the Japanese guidelines for the administration of biologic agents(e.g., more than six tender joints, more than six swollen joints, CRP level of >2.0 mg/dL, and ESR >28 mm/h). Inpatients who did not meetthese criteria, the following additionalcriteriacould bemet: progressive bone erosion, DAS28-CRP >2.7, and DAS28-ESR>3.2.
Abatacept was intravenously administered in patients weighing <60, 60–100, or >100 kg received 500, 750, or 1000 mg every 4 weeks, respectively. The subcutaneous formulation of abatacept was administered 125 mg weekly.
This study was conducted according to the principles of the Declaration of Helsinki, and informed consent was provided by all patients. The Ethics Committee for Clinical Research of Kamagaya General Hospital approved this study (approval number: TGE00888- 064).
Assessment of efficacy against joint damage
The joint damage was examined using mTSS. Changes from baseline in joint damage categorized total score (ΔTSS), erosion score (ΔEN), and joint-space narrowing score (ΔJSN) were determined at years 1, 2, 3, 4, and 5. The scores were assessed by two investigators. Structural remission using the ΔTSS was defined as ≤ 0.5 point per year [17,20]. In this study, structural remission was defined as ΔTSS ≤ 2.5 points at year 5, while no radiographic progression was defined as ΔTSS ≤0 point at year 5.
Assessment of efficacy against disease activity
DAS28-ESR was determined at years 1, 2, 3, 4, and 5. DAS28-ESR are divided into four categories: remission, <2.6; low disease activity, 2.6–<3.2; moderate disease activity, 3.2–≦5.1; and high disease activity, >5.1 [25,26].
Statistical analysis
The data analyses were used by observed case analysis. Comparisons of the changes in ΔTSS between the Bio-naïve (i.e., patients who received abatacept as their first treatment with a biological DMARD) and Bio-switch (i.e., patients who received treatment with other biological DMARDs prior to abatacept) groups, and MTX(+) (i.e., patients treated with MTX) and MTX(−) (i.e., patients treated without MTX) groups were conducted using paired t-tests. Factors associated with joint damage were analyzed by comparing variables in patients with RA and with or without structural remission and radiographic progression. The variables in the univariate analysis included age, sex, disease duration, Rheumatoid Factor (RF) positivity, anti-cyclic Citrullinated Peptide Antibody(anti-CCP Ab) positivity, Bio-naïve, MTX use, glucocorticoid use, CRP level, matrix metalloproteinase-3 level, DAS28- ESR, TSS, and HAQ-DI at baseline. Stepwise multiple regression analysis was performed to identify factors associated with joint damage.A p-value of <0.05 denoted statistical significance. All statistical analyses were performed using the R Statistical Package software, version 3.3.2 (http://www.r-project.org/).
Results
Patient characteristics
A total of 120 patients were enrolled in this study. Table 1 shows the patient characteristics (age, sex, disease duration, RF positivity, anti-CCP positivity, Bio-naïve, MTX use, MTX dose, glucocorticoid use, glucocorticoid dose, CRP, MMP-3, DAS28-ESR, TSS, and HAQ-DI in all groups at baseline.
| Variables | All patients (N=120) | Bio-naïve | Bio-switch | MTX(+) | MTX(−) |
|---|---|---|---|---|---|
| (N=86) | (N=34) | (N=70) | (N=50) | ||
| Age, years | 66.2 ± 10.3 | 68.9 ± 8.5 | 59.2 ± 13.9 | 63.0 ± 12.2 | 70.5 ± 7.6 |
| Sex, female, n (%) | 102 (85.0) | 70 (81.4) | 32 (94.1) | 62 (88.6) | 40 (80.0) |
| Disease duration, years | 9.7 ± 10.3 | 10.2 ± 11.5 | 8.3 ± 6.5 | 7.3 ± 8.6 | 13.0 ± 11.7 |
| RF positivity, n (%) | 92 (76.7) | 70 (81.4) | 22 (64.7) | 51 (72.9) | 41 (82.0) |
| Anti-CCP Ab positivity, n (%) | 97 (80.8) | 71 (82.6) | 26 (76.4) | 56 (80.0) | 41 (82.0) |
| Bio-naïve, n (%) | 86 (71.7) | 86 (100) | 0 (0) | 51 (72.9) | 35 (70.0) |
| MTX use, n (%) | 70 (58.3) | 51 (59.3) | 19 (55.9) | 100 (0) | 0 (0) |
| MTX dose, mg/weeks | 7.4 ± 2.5 | 7.3 ± 2.5 | 7.6 ± 2.7 | 7.4 ± 2.5 | − |
| Glucocorticoid use, n (%) | 61 (50.8) | 43 (50.0) | 18 (52.9) | 25 (35.7) | 36 (72.0) |
| Glucocorticoid dose, mg/day | 4.4 ± 1.7 | 4.5 ± 1.8 | 4.3 ± 1.3 | 4.2 ± 1.9 | 4.6 ± 1.5 |
| CRP, mg/dL | 1.8 ± 2.0 | 1.7 ± 1.8 | 1.8 ± 2.3 | 1.7 ± 2.1 | 1.8 ± 1.8 |
| MMP-3, ng/mL | 216.6 ± 199.3 | 214.1 ± 194.4 | 223.0 ± 214.0 | 206.2 ± 194.1 | 213.3 ± 207.4 |
| DAS28-ESR | 4.61 ± 1.19 | 4.62 ± 1.16 | 4.59 ± 1.27 | 4.59 ± 1.35 | 4.63 ± 0.92 |
| TSS | 53.5 ± 60.4 | 53.1 ± 64.5 | 54.5 ± 49.5 | 47.5 ± 59.1 | 62.0 ± 61.8 |
| HAQ-DI | 0.97 ± 0.76 | 0.95 ± 0.74 | 1.03 ± 0.83 | 0.86 ± 0.68 | 1.14 ± 0.84 |
Values are presented as the mean ± standard deviation. Bio-naïve, abatacept of first biological Disease-Modifying Antirheumatic Drug (DMARD); Bio-switch, other biological DMARD treatments prior to abatacept treatment; RF: Rheumatoid Factor; anti-CCP Ab: Anti-Cyclic Citrullinated Peptide Antibody; MTX: Methotrexate; CRP:C-Reactive Protein; MMP-3: Matrix Metalloproteinase-3;DAS28:Disease Activity Score in 28 joints; ESR: Erythrocyte Sedimentation Rate; TSS: Total Sharp Score using van der Heijde-modified total Sharp score; HAQ-DI: Health Assessment Questionnaire Disability Index
Table 1. Patient characteristics at baseline.
The rates of MTX use were 58.3%, 61.4%, 58.2%, 51.2%, 48.8%, and 47.5% at baseline, years 1, 2, 3, 4, and 5. The MTX doses (mg/week) were 7.4 ± 2.5, 5.4 ± 2.8, 5.5 ± 2.7, 5.8 ± 2.4, 6.1 ± 2.3, and 6.8 ± 2.4 at baseline, years 1, 2, 3, 4, and 5. The rates of glucocorticoid use were 50.8%, 41.6%, 35.2%, 32.6%, 29.8%, and 33.8% at baseline, years 1, 2, 3, 4, and 5. The glucocorticoid doses (mg/day) were 4.4 ± 1.7, 3.9 ± 1.2, 3.5 ± 1.6, 3.4 ± 1.5, 3.2 ± 1.2, and 2.9 ± 1.1 at baseline, years 1, 2, 3, 4, and 5.
Retention rate
The retention rate was 84.2% at year 1, 75.8% at year 2, 71.7% at year 3, 70.0% at year 4, and 66.7% at year 5. The reasons for discontinuation during this 5 year period were lack of efficacy (40%), adverse events (20%), other diseases (10%), change of hospitals (22.5%), and patient hope (7.5%).
Radiographic joint damage
All patients: The ΔTSS was 0.60 ± 2.03, 0.93 ± 2.40, 1.23 ± 2.92, 1.53 ± 3.38, and 1.71 ± 3.84 at years 1, 2, 3, 4, and 5, respectively (Figure 1). The ΔEN was 0.14 ± 0.70, 0.19 ± 0.87, 0.31 ± 0.85, 0.36 ± 1.31, and 0.43 ± 1.48 at years 1, 2, 3, 4, and 5. The ΔJSN was 0.48 ± 1.52, 0.75 ± 1.78, 0.94 ± 2.16, 1.20 ± 2.51, and 1.29 ± 2.91 at years 1, 2, 3, 4, and 5. On the other hand, the ΔTSS using linear extrapolation based on last scores was 0.67 ± 1.97 at 0years to 1 year, 0.48 ± 1.20 at 1yearsto 2 years, 0.45 ± 1.21 at 2years to 3 years, 0.43 ± 1.18 at 3 years to 4 years, 0.42 ± 1.15 at 4 years to 5 years.
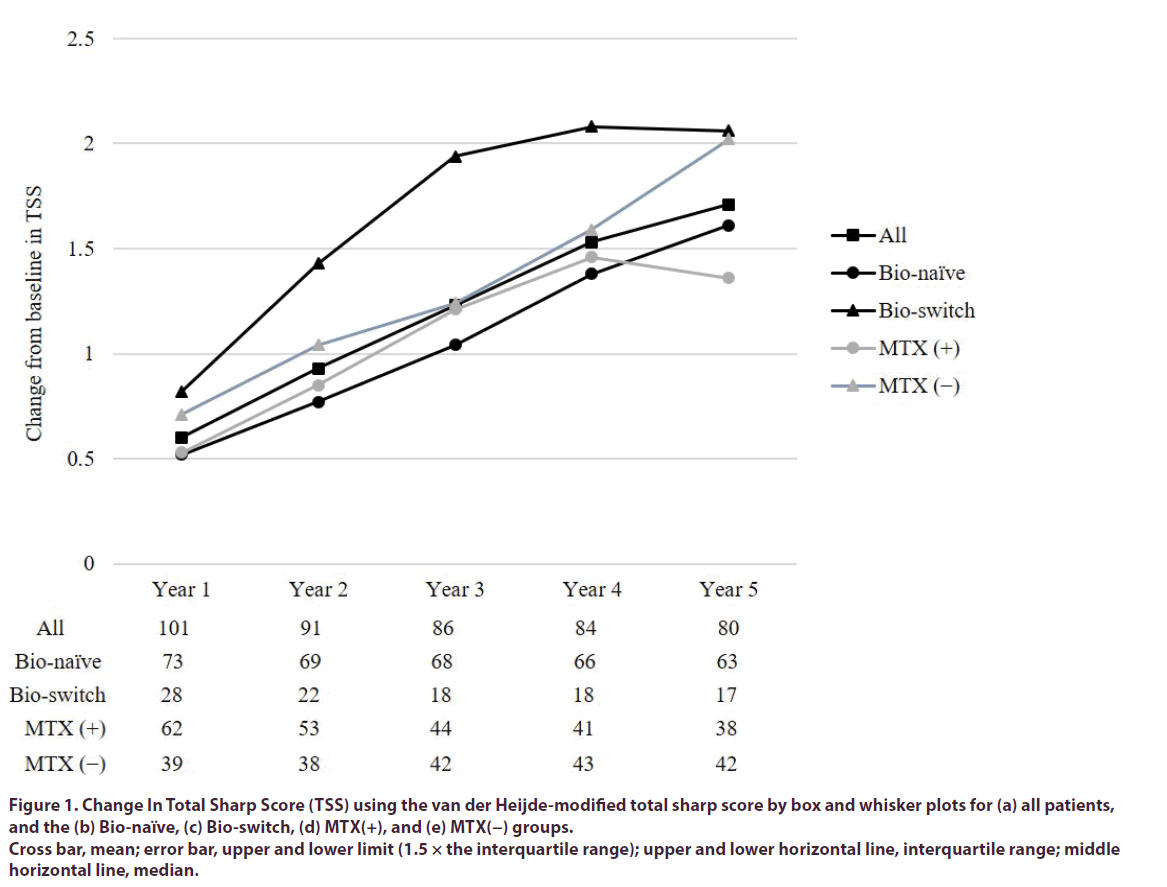
Figure 1. Change In Total Sharp Score (TSS) using the van der Heijde-modified total sharp score by box and whisker plots for (a) all patients, and the (b) Bio-naïve, (c) Bio-switch, (d) MTX(+), and (e) MTX(−) groups.
Cross bar, mean; error bar, upper and lower limit (1.5 × the interquartile range); upper and lower horizontal line, interquartile range; middle horizontal line, median.
Bio-naïve vs. Bio-switch
In Bio-naïve group, the ΔTSS was 0.52 ± 1.46, 0.77 ± 1.63, 1.04 ± 2.12, 1.38 ± 2.67, and 1.61 ± 3.09 at years 1, 2, 3, 4, and 5 (Figure 1). In Bio-switch group, the ΔTSS was 0.82 ± 3.09, 1.43 ± 3.97, 1.94 ± 4.92, 2.08 ± 5.29, and 2.06 ± 5.99 at years 1, 2, 3, 4, and 5 (Figure 1). There was no difference between these groups in ΔTSS each year (year 1: p = 0.625; year 2: p = 0.453; year 3: p = 0.454; year 4: p = 0.591, and year 5: p = 0.769).
MTX (+) vs. MTX (–)
In MTX (+) group, the ΔTSS was 0.53 ± 1.40, 0.85 ± 1.80, 1.21 ± 2.69, 1.46 ± 3.46, and 1.36 ± 3.63 at years 1, 2, 3, 4, and 5 (Figure 1). In MTX (−) group, the ΔTSS was 0.71 ± 2.78, 1.04 ± 3.07, 1.24 ± 3.18, 1.59 ± 3.34, and 2.02 ± 4.05 at years 1, 2, 3, 4, and 5 (Figure 1). There was no difference in these groups in ΔTSS each year (year 1: p = 0.700; year 2: p = 0.733; year 3: p = 0.972; year 4: p = 0.862, and year 5: p = 0.438).
Factors associated with structural remission and radiographic progression
The structural remission rate at year 5 (ΔTSS ≤ 2.5) was 78.8%. There were no significant factors associated with structural remission.
The rate of no radiographic progression at 5 years (ΔTSS ≤ 0) was 51.3%. The univariate analysis revealed that the following factor was significantly associated with no radiographic progression: DAS28-ESR (Table 2). Moreover, the multivariate analysis showed that DAS28-ESR (p = 0.035) was a significant factor.
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| All patients | 0.60 ± 2.03 | 0.93 ± 2.40 | 1.23 ± 2.92 | 1.53 ± 3.38 | 1.71 ± 3.84 |
| Bio-naïve group | 0.52 ± 1.46 | 0.77 ± 1.63 | 1.04 ± 2.12 | 1.38 ± 2.67 | 1.61 ± 3.09 |
| Bio-switch group | 0.82 ± 3.09 | 1.43 ± 3.97 | 1.94 ± 4.92 | 2.08 ± 5.29 | 2.06 ± 5.99 |
| MTX (+) group | 0.53 ± 1.40 | 0.85 ± 1.80 | 1.21 ± 2.69 | 1.46 ± 3.46 | 1.36 ± 3.63 |
| MTX (−) group | 0.71 ± 2.78 | 1.04 ± 3.07 | 1.24 ± 3.18 | 1.59 ± 3.34 | 2.02 ± 4.05 |
Table 2. Mean change from baseline in total sharp score in all patients, Bio-naïve, Bio-switch, MTX (+), and MTX (−) groups.
Clinical efficacy
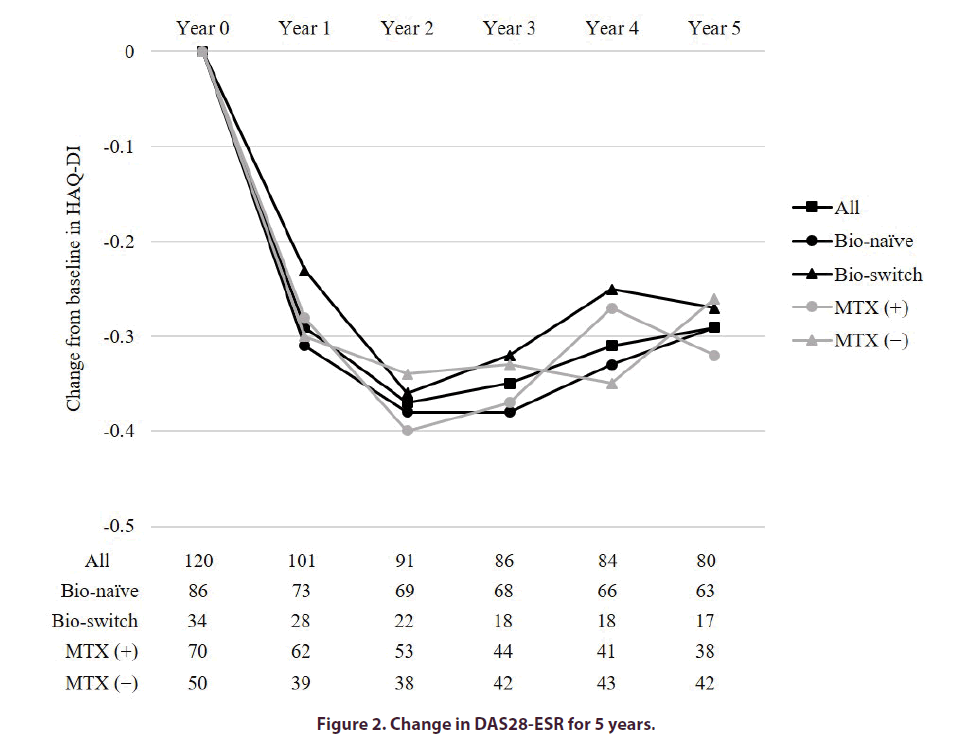
Figure 2 showed the percentage of patients who achieved remission in DAS28-ESR at baseline, and years 1, 2, 3, 4, and 5. In all patients, the remission rates of DAS28- ESR were 44.6 and 50.0% at years 1 and 5. In the Bio-naïve and Bio-switch groups, the remission rates of DAS28-ESR at year 1 and 5 were 45.2% and 42.9%, and 50.8% and 47.1%, respectively. The remission rates were not significantly different between the two groups at any of the time points. In the MTX(+) and MTX(−) groups, the remission rates of DAS28-ESR at years 1 and 5 were 45.2% and 43.6%, and 52.6% and 47.6%, respectively. The remission rates were not significantly different between the two groups at any of the time points.
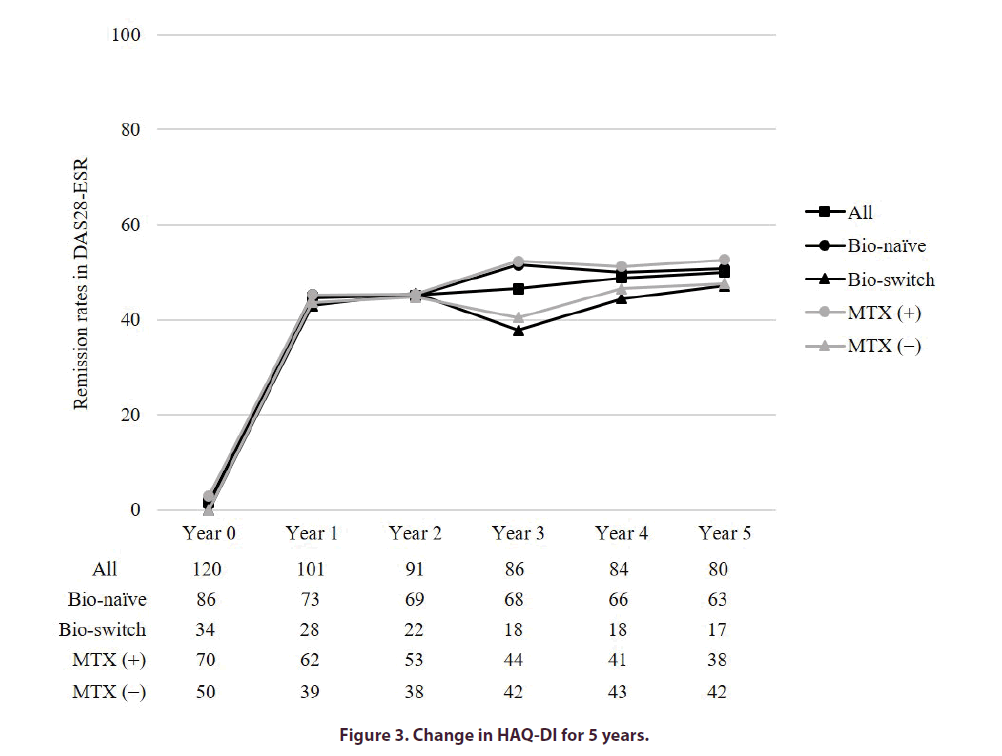
The HAQ-DI improved in all groups at any of the time points (Figure 3). At year 5, the rate of improvement in HAQ-DI ≥ 0.3 was 43.8%. Of note, the rate of deterioration of HAQ-DI ≥ 0.3 was 12.5%. Moreover, the rates of HAQ-DI ≤ 0.5 at baseline and year 5 were 40.0% and 58.8%, respectively.
Discussion
The present clinical observational study evaluated the long-term joint damage and clinical efficacy in Japanese patients treated with abatacept. The radiographic joint damage was suppressed over the 5-year period. Progression of joint damage did not differ between the Bio-naïve and Bio-switch groups, MTX(+) and MTX(−) groups. In the present study, the retention rate was 66.7% at year 5. Similarly to previous reports[11,13,17,19,20,27], the most common reason for discontinuation in this study was lack of efficacy (Table 3).
| Variables at baseline | Without progression | With progression | p value |
|---|---|---|---|
| (n=41) | (n=39) | ||
| Age, years | 66.4 ± 9.4 | 67.1 ± 9.5 | 0.745 |
| Sex, female, n (%) | 37 (90.2) | 48 (87.2) | 0.734 |
| Disease duration, years | 10.8 ± 12.5 | 9.2 ± 10.0 | 0.528 |
| RF positivity, n (%) | 29 (70.7) | 46 (84.6) | 0.183 |
| Anti-CCP Ab positivity, n (%) | 31 (75.6) | 35 (89.7) | 0.142 |
| Bio-naïve, n (%) | 30 (73.2) | 33 (84.6) | 0.227 |
| MTX use, n (%) | 26 (63.4) | 26 (66.7) | 0.817 |
| Glucocorticoid use, n (%) | 14 (34.1) | 21 (53.8) | 0.114 |
| CRP, mg/dL | 1.26 ± 1.80 | 1.89 ± 1.85 | 0.129 |
| MMP-3, ng/mL | 179.1 ± 159.4 | 256.6 ± 242.5 | 0.098 |
| DAS-ESR | 4.29 ± 1.00 | 4.85 ± 1.25 | 0.032 |
| TSS | 48.5 ± 63.9 | 63.2 ± 67.1 | 0.317 |
| HAQ-DI | 0.81 ± 0.70 | 1.01 ± 0.78 | 0.229 |
Values are presented as the mean ± standard deviation. Bio-naïve, abatacept of first biological disease-modifying antirheumatic drug; RF: Rheumatoid Factor; anti-CCP Ab: Anti-Cyclic Citrullinated Peptide Antibody; MTX: Methotrexate; CRP: C-Reactive Protein; MMP-3: Matrix Metalloproteinase-3; DAS28: Disease Activity Score in 28 joints; ESR: Erythrocyte Sedimentation Rate; TSS: Total Sharp Score using van der Heijde-modified total Sharp score; HAQ-DI: Health Assessment Questionnaire Disability Index
Table 3. Comparison between patients without and with radiographic progression for 5 years by univariate analysis.
The short-term results in terms of joint damage in patients with early RA who received abatacept plus MTX were 0.65 and 0.19 at 0yearsto 1yearsand 1yearsto 2 years, respectively [28]. In patients with RA who had an inadequate response to MTX, ΔTSS was 0.58 ± 3.22 at year 1[29]. The long-term results in terms of joint damage in patients treated with abatacept in Abatacept in Inadequate Responders to Methotrexate (AIM) trial were 0.80 ± 1.99, 0.41 ± 1.28, 0.37 ± 1.49, 0.34 ± 1.12, and 0.26 ± 1.40 at 0–1, 1–2, 2–3, 3–4, and 4–5 years, respectively, using the Genant-modified Sharp scoring method [22]. These results are similar to those of our study regarding the suppression of joint damage over time; however, they demonstrated greater effectiveness. The AIM trial had differences from the present study. The patients included in the previous report had a mean age of 51.5 years, were Bio-naïve, and all received treatment with MTX (mean dosage: 16.1 mg/week) [30]. In the present study, the mean dosage of MTX was 7.4 mg/week. Based on our results, at this dose of MTX, there was no difference between the MTX(+) and MTX(−) groups in terms of joint damage. In previous reports, factors associated with short-term joint damage during treatment with abatacept were disease duration, CRP, and SDAI at baseline, as well as SDAI remission at month 6 [17,31,32]. In the present study, the only factor associated with radiographic progression was DAS28-ESR. This result suggests that DAS28-ESR at baseline would be considered a long-term treatment goal for the prevention of radiographic progression in patients treated with abatacept.
In the present study, the remission rates of DAS28-ESR at year 1 and 5 were 44.6% and 50.0%, respectively. In Japanese patients with RA treated with abatacept, the remission rate of DAS28-CRP (<2.6) in subcutaneous and intravenous groups were 63.5% and 62.7% at week 76. The remission rate improved from week 24 to week 76 [14]. In present study, the remission rate of DAS28-CRP (<2.6) at year 1 and 5 were 69.3% and 72.5%. Although the retention rate was slightly different, abatacept had well effectiveness in long-term period. In our previous report, improvement in DAS28-ESR continued from week 24 to year 2 using last observation carried forward analysis [20]. Moreover, the present study showed that improvement in DAS28-ESR continued throughout the entire 5-year period. Similarly, the remission rates of DAS28-ESR were maintained in all patients, as well as in the Bio-naïve, Bio-switch, MTX(+), and MTX(−) groups. In the Bio-switch group of the present study, the remission rate of DAS28-ESR was 44.1% at year 5. The remission rate of DAS28-CRP (<2.6) was 22.3% at year 5 in patients with inadequate response to therapy with an anti-tumor necrosis factor [33]. Although these results differed in remission rates, they were similar in maintenance rates at year 5. Moreover, clinical results of abatacept were also not affected by MTX in the Orencia and Rheumatoid Arthritis (ORA) registry [34]. In PMS of Japan, the reduction rate in DAS28-ESR at week 24 was 22.5%, and MTX was not associated with improvement in DAS28-CRP in patients with moderate disease activity at baseline [15]. The patients examined in the present study had moderate disease activity of mean DAS28-ESR at baseline. Therefore, it is suggested that the clinical results were also not affected by MTX in this study.
Based on the results of the present study, the HAQ-DI improved for up to 2 years, and was maintained at the end of the investigation (5 years). In a previous report, progression of one point in the TSS score was related to 0.013 in HAQ-DI [35]. The remaining functional disability may be attributed to joint damage according to the TSS at baseline and progression of joint damage for 5 years.
This study had several limitations. Firstly, the patients of the present study had moderate disease activity at baseline. Therefore, our results may differ from those of previous randomized controlled trials. However, our data are close to the patient characteristics of daily practice in PMS [15]. We suggest that long-term results are useful in daily practice. Secondly, the data was analyzed using observed case analysis. Therefore, there is a possibility for differences in these results if all patients were able to continue treatment for 5 years. Moreover, this study did not include a control group owing to its clinical observational design. Therefore, the present study could not compare abatacept with other DMARDs.
In conclusion, we analyzed the efficacy of treatment with abatacept in Japanese patients with RA for 5 years in daily clinical practice. The findings of the present study suggested that improvement in joint damage, disease activity, and physical function are maintained in the long-term. Long-term clinical results are important in the treatment of abatacept. These results are useful for the long-term use of abatacept in patients with RA.
Authors’ contributions
All authors have contributed to the concept and design of the study, interpretation of the data and revising the manuscript, and have approved the final draft.
Conflict of interest
TM received honoraria for lectures from AbbVie, Astellas, Bristol- Myers, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Janssen, Mochida, Pfizer, Takeda, and Tanabe-Mitsubishi. KY received honoraria for lectures from AbbVie, Astellas, Ayumi, Bristol-Meyers, Eisai, Hisamitsu, Mochida, and Takeda. KI received honoraria for lectures from AbbVie, Astellas, Bristol-Myers, Chugai, Eisai, Eli Lilly, Janssen, Takeda, Tanabe-Mitsubishi, and UCB. The otfher authors declare that they have no conflicts of interest. The sponsors were not involved in the study design; collection, analysis, and interpretation of data; writing of the article; and/or decision to submit the results for publication.
Funding
None
References
- Kvien TK, Uhlig T. Quality of life in rheumatoid arthritis. Scand. J. Rheumatol. 34, 333–341 (2005).
- Odegard S, Finset A, Kvien TK et al. Work disability in rheumatoid arthritis is predicted by physical and psychological health status: a 7-year study from the Oslo RA register. Scand. J. Rheumatol. 34, 441–447 (2005).
- Yelin E. Work disability in rheumatic diseases. Curr. Opin. Rheumatol. 19, 91–96 (2007).
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14, 397–440 (1996).
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell co-stimulation. Annu. Rev. Immunol. 14, 233–258 (1996).
- Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J. Am. Soc. Nephrol. 13, 559–575 (2002).
- Linsley PS, Greene JL, Brady W et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1, 793–801 (1994).
- Chambers CA, Allison JP. CTLA-4-the costimulatory molecule that doesn’t: regulation of T-cell response by inhibition. Cold. Spring. Harb. Symp. Quant. Biol. 64, 303–312 (1999).
- Kremer JM, Westhovens R, Leon M et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA-4Ig. N. Engl. J. Med. 349, 1907–1915 (2003).
- Kremer JM, Dougados M, Emery P et al. Treatment of rheumatoid arthritis with the selective costimulation modulator abatacept: twelve-month results of a phase IIb, double-blind, randomized placebo-controlled trial. Arthritis. Rheum. 52, 2263–2271 (2005).
- Takeuchi T, Matsubara T, Nitobe T et al. Phase II dose-response study of abatacept in Japanese patients with active rheumatoid arthritis with an inadequate response to methotrexate.Mod. Rheumatol. 23, 226–235 (2013).
- Matsubara T, Inoue H, Nakajima T et al. Abatacept in combination with methotrexate in Japanese biologic-naive patients with active rheumatoid arthritis: a randomised placebo-controlled phase IV study.RMD. Open. 4, e000813 (2018).
- Iwahashi M, Inoue H, Matsubara T et al. Efficacy, safety, pharmacokinetics and immunogenicity of abatacept administered subcutaneously or intravenously in Japanese patients with rheumatoid arthritis and inadequate response to methotrexate: a Phase II/III, randomized study. Mod. Rheumatol. 24, 885–891 (2014).
- Amano K, Matsubara T, Tanaka T et al. Long-term safety and efficacy of treatment with subcutaneous abatacept in Japanese patients with rheumatoid arthritis who are methotrexate inadequate responders.Mod. Rheumatol. 25, 665–671 (2015).
- Harigai M, Ishiguro N, Inokuma S et al. Postmarketing surveillance of the safety and effectiveness of abatacept in Japanese patients with rheumatoid arthritis. Mod. Rheumatol. 26, 491–498 (2016).
- Takahashi N, Kojima T, Terabe K et al. Clinical efficacy of abatacept in Japanese rheumatoid arthritis patients. Mod. Rheumatol. 23, 904–912 (2013).
- Kubo S, Saito K, Hirata S et al. Abatacept inhibits radiographic progression in patients with rheumatoid arthritis: a retrospective analysis of 6 months of abatacept treatment in routine clinical practice. The ALTAIR study. Mod. Rheumatol. 24, 42–51 (2014).
- Takahashi N, Kojima T, Kaneko A et al. Use of a 12-week observational period for predicting low disease activity at 52 weeks in RA patients treated with abatacept: a retrospective observational study based on data from a Japanese multicentre registry study. Rheumatol (Oxford). 54, 854–859 (2015).
- Kojima T, Takahashi N, Kaneko A et al. Predictive factors for achieving low disease activity at 52 weeks after switching from tumor necrosis factor inhibitors to abatacept: results from a multicenter observational cohort study of Japanese patients. Clin. Rheumatol. 35, 219–225 (2016).
- Mochizuki T, Yano K, Ikari K et al. The efficacy of abatacept in Japanese patients with rheumatoid arthritis: 104 weeks radiographic and clinical results in clinical practice. Mod. Rheumatol. 26, 499–506 (2016).
- Kremer JM, Russell AS, Emery P et al. Long-term safety, efficacy and inhibition of radiographic progression with abatacept treatment in patients with rheumatoid arthritis and an inadequate response to methotrexate: 3-year results from the AIM trial. Ann. Rheum. Dis. 70, 1826–1830 (2011).
- Kremer JM, Peterfy C, Russell AS et al. Longterm safety, efficacy, and inhibition of structural damage progression over 5 years of treatment with abatacept in patients with rheumatoid arthritis in the abatacept in inadequate responders to methotrexate trial. J. Rheumatol. 41, 1077–1087 (2014).
- Arnett FC, Edworthy SM, Bloch DA et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis. Rheum. 31, 315–324 (1988).
- Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheum. 62, 2569–2581 (2010).
- Prevoo ML, Van ‘t Hof MA, Kuper HH et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis. Rheum. 38, 44–48 (1995).
- Inoue E, Yamanaka H, Hara M et al. Comparison of Disease Activity Score (DAS)28-erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann. Rheum. Dis. 66, 407–409 (2007).
- Harigai M, Ishiguro N, Inokuma S et al. Safety and effectiveness of abatacept in Japanese non-elderly and elderly patients with rheumatoid arthritis in an all-cases post-marketing surveillance. Mod. Rheumatol. 29, 747–755 (2019).
- Bathon J, Robles M, Ximenes AC et al. Sustained disease remission and inhibition of radiographic progression in methotrexate-naive patients with rheumatoid arthritis and poor prognostic factors treated with abatacept: 2-year outcomes. Ann. Rheum. Dis. 70, 1949–1956 (2011).
- Weinblatt ME, Schiff M, Valente R et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis. Rheum. 65, 28–38 (2013).
- Kremer JM, Genant HK, Moreland LW et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 144, 865–876 (2006).
- Murakami K, Sekiguchi M, Hirata S et al. Predictive factors for structural remission using abatacept: Results from the ABROAD study. Mod. Rheumatol. 29, 406–412 (2019).
- Yamazaki H, Hirano F, Takeuchi T et al. Simplified Disease Activity Index remission at month 6 is an independent predictor of functional and structural remissions at month 12 during abatacept treatment in patients with rheumatoid arthritis: A multi-center, prospective cohort study in Japan. Mod. Rheumatol. 27, 787–794 (2017).
- Genovese MC, Pacheco-Tena C, Covarrubias A et al. Longterm Safety and Efficacy of Subcutaneous Abatacept in Patients with Rheumatoid Arthritis: 5-year Results from a Phase IIIb Trial. J. Rheumatol. 45, 1085–1092 (2018).
- Gottenberg JE, Ravaud P, Cantagrel A et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the 'Orencia and Rheumatoid Arthritis' registry. Ann. Rheum. Dis. 71, 1815–1819 (2012).
- Smolen JS, Aletaha D, Grisar JC et al. Estimation of a numerical value for joint damage-related physical disability in rheumatoid arthritis clinical trials. Ann. Rheum. Dis. 69, 1058–1064 (2010).