Commentary - Interventional Cardiology (2011) Volume 3, Issue 3
Focus on the COCTAIL study
- Corresponding Author:
- Francesco Prati
Interventional Cardiology, San Giovanni Hospital
Rome, Italy
Tel: +39 067 705 5330
E-mail: fprati@hsangiovanni.roma.it
Abstract
Keywords
abciximab, acute coronary syndrome, perfusion balloon, thrombus
Intracoronary thrombosis is the final event in the pathophysiologic process leading to acute coronary syndrome. Percutaneous interventions in this unfavorable clinical setting, and particularly in ST-elevation myocardial infarction (STEMI), can be hampered by the dislodgment of thrombus components and cholesterol debris from the lipid-rich plaques. This can lead to microvascular impairment and, in the worst scenario, to the noreflow phenomenon. Platelet–fibrin aggregates in the distal bed stimulate an inflammatory cascade that can perpetuate myocardial edema as well as vessel spasm and endothelial dysfunction. These processes may be associated with myocyte necrosis even after successful reperfusion of the epicardial artery [1]. In addition, following reperfusion, leukocyte transmigration and consequent free-radical injury to myocytes can occur, which can compound microvascular dysfunction with endothelial dysfunction, resulting in further extension of infarction.
In recent years, drug strategies such as glycoprotein IIb/IIIa inhibitors, filters and aspiration devices have been tested to prevent thrombus microembolization [2–5].
Glycoprotein IIb/IIIa inhibitors, infused through a systemic route, are shown to reduce percutaneous coronary intervention (PCI) complications, improving epicardial and tissue-level perfusion [6]. Intracoronary administration of the glycoprotein IIb/IIIa inhibitor, abciximab, potentially increases platelet glycoprotein IIb/ IIIa receptor occupancy in the infarct-related artery and can provide additional clinical benefit compared with the standard intravenous administration [7]. However, even this modality of guiding catheter delivery does not allow optimal contact between the drug and the vessel wall as the drug is rapidly washed away. Recently, the CICERO (Comparison of Intracoronary Versus Intravenous Abciximab in ST-Segment Elevation Myocardial Infarction) trial determined the effect of intracoronary versus intravenous administration of abciximab in STEMI patients undergoing primary PCI. The primary end point, the incidence of restored myocardial reperfusion, defined as complete ST-segment resolution, was similar in the intracoronary and intravenous groups. However, the secondary end points of myocardial reperfusion as assessed by myocardial blush grade and infarct size (in the subset of evaluable patients) were improved in the intracoronary group.
A novel promising solution to ameliorate the outcome of angioplasty in STEMI resides in the combined use of pharmacological and catheterbased invasive therapies.
The ClearWay™ RX therapeutic infusion catheter (Atrium Medical Corporation, Hudson, NH, USA) proved to be a valid solution to increase local concentration of the drug, prolonging its residency time. In vitro data suggests that at high concentrations (nearly 500-times the usual systemic dose), abciximab disaggregates existing clots.
The COCTAIL (ClearWay™ RX System to Reduce Intracoronary Thrombus in Patients with Acute Coronary Syndromes According to Optical Coherence Tomography after Abciximab Intracoronary Local Infusion) study was specifically designed to verify whether administration of abciximab by local intracoronary infusion through ClearWay RX was capable of reducing thrombus burden as compared with the modality of traditional guiding catheter infusion. The secondary objective was to address postintervention assessment of coronary microcirculatory function.
Study design & patient selection
The COCTAIL study was a randomized, openlabel, multicenter trial with blinded assessment of the study end points.
According to the study design, the study population consisted of patients with unstable angina/ non-STEMI in which an invasive approach was planned, and patients with STEMI undergoing primary PCI. Owing to safety reasons, patients with STEMI were not randomized if the infarctrelated artery was totally occluded. Patients were randomized if they had a significant lesion in the culprit artery indicative of local thrombosis or haziness suggestive of thrombus.
Randomized patients entered the final analysis if they had a thrombus score ≥50 according to the optical coherence tomography (OCT) independent core laboratory.
▪ Randomization & treatment
Before PCI, patients were randomly assigned to receive local delivery of abciximab through the ClearWay RX catheter (CW group) or intracoronary infusion of abciximab via the guiding catheter (GC group), with the use of a randomization scheme devised and implemented by the study statistician.
The ClearWay RX therapeutic perfusion catheter is a microporous balloon catheter that acts as a low-pressure irrigating system for localized perfusion of therapeutic agents into the coronary vasculature. The device is a rapid exchange perfusion catheter, 0.014 inches guidewire compatible, and is available in 1.0–2.0-mm balloon diameters.
All patients underwent baseline angiography and OCT assessment before abciximab administration. Patients in the local delivery group received a bolus dose of abciximab (0.25 mg/kg) delivered with ClearWay RX, whereas patients in the control group received the same bolus dose of abciximab delivered through the guiding catheter.
Both groups received postprocedural infusion of abciximab for the ensuing 12 h at the recommended dosage, aspirin 81–325 mg orally as soon as possible, clopidogrel with a loading dose of 600 mg after the completion of PCI and unfractionated heparin following the standard protocol.
▪ End points & definitions
The primary end point was the change of the thrombus burden defined by the thrombus score, as detailed in the following sections. Secondary end points were postprocedural corrected thrombolysis in myocardial infarction frame count, myocardial blush grade, procedurerelated myocardial infarction (MI), and 30-day and 1-year rates of major adverse cardiac events (MACE), defined as the composite of death from any cause, reinfarction,or target lesion revascularization.
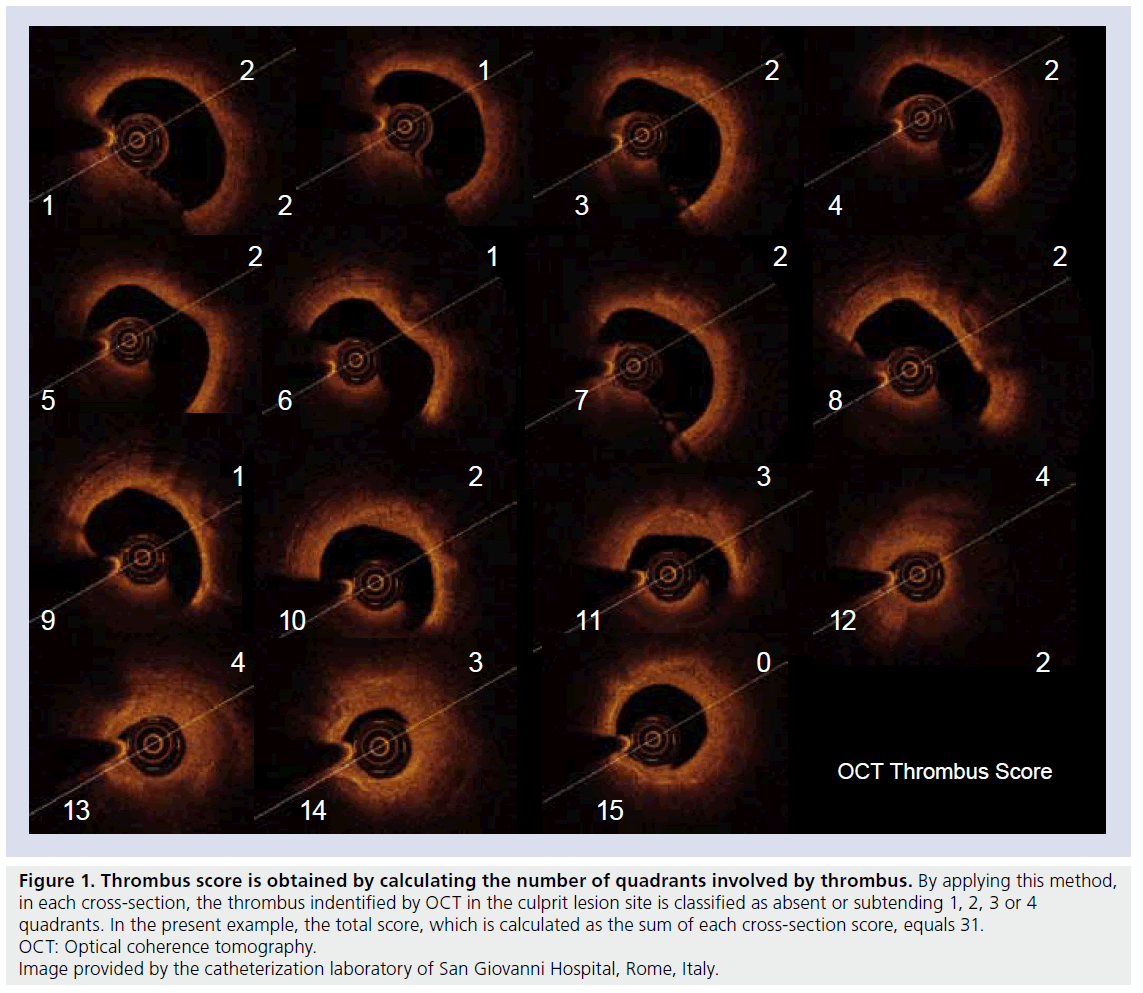
Procedure-related MI was diagnosed if the creatine kinase-myocardial band level increased to twice the upper limit of normal or twice the last non-normalized measurement. The OCT image acquisition was performed at baseline and after abciximab administration to document changes in the thrombus score, using the time domain M2 and M3 technology (LightLab Imaging, Inc., Westford, MA, USA) and adapting the nonocclusive technique of acquisition [8–10]. Thrombus score grading was based on the count of the number of quadrants involved by thrombus. By applying this method, in each cross-section, the thrombus indentified by OCT in the culprit lesion site was classified as absent or subtending 1, 2, 3 or 4 quadrants. Hence, the score was calculated as the sum of each cross- section score (Figure 1).
Figure 1. Thrombus score is obtained by calculating the number of quadrants involved by thrombus. By applying this method,
in each cross-section, the thrombus indentified by OCT in the culprit lesion site is classified as absent or subtending 1, 2, 3 or 4
quadrants. In the present example, the total score, which is calculated as the sum of each cross-section score, equals 31.
OCT: Optical coherence tomography.
Image provided by the catheterization laboratory of San Giovanni Hospital, Rome, Italy.
Statistical analysis
We estimated that we had to enroll 40 patients to achieve a power of 80%, with a two-sided significance level of 0.05, to detect a 25% difference in the primary end point reduction in patients who underwent local intracoronary abciximab delivery through ClearWay RX as compared with those who underwent intracoronary bolus administration through the guiding catheter.
Categorical variables were presented as counts and percentages and compared by means of the chi-square test or Fisher exact test when at least 25% of values showed an expected cell frequency below five. Continuous variables were normally distributed and therefore presented as mean plus or minus standard deviation and compared by Student unpaired t test for between-group comparison and paired t test for within-group comparison. For all analyses, a two-sided p-value of ≤0.05 was considered statistically significant.
Results
▪ Study population
During the study period, 50 out of 87 screened patients at three sites in Europe matched the eligibility criteria and, before PCI, were randomly assigned to undergo local delivery of abciximab through ClearWay RX (n = 25) or intracoronary delivery of abciximab through the guiding catheter (n = 25).
Nine patients (five in the local delivery group and four in the standard delivery group) were excluded for the following reasons: inability to match segments, no appropriate OCT acquisition and insufficient thrombus score. In total, 47 stents have been deployed in the 41 patients that entered the final analysis. Three patients in the CW group and three in the GC group had two stents implanted overlapping to cover the target lesion.
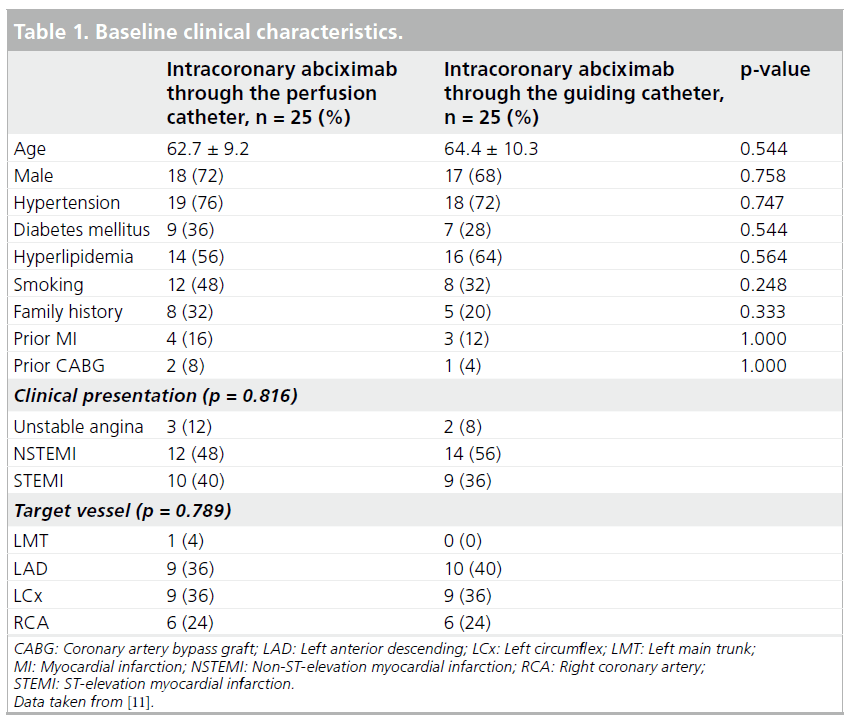
The baseline clinical characteristics, including age and cardiovascular risk factors, were well- balanced in the two arms of the study (Table 1).
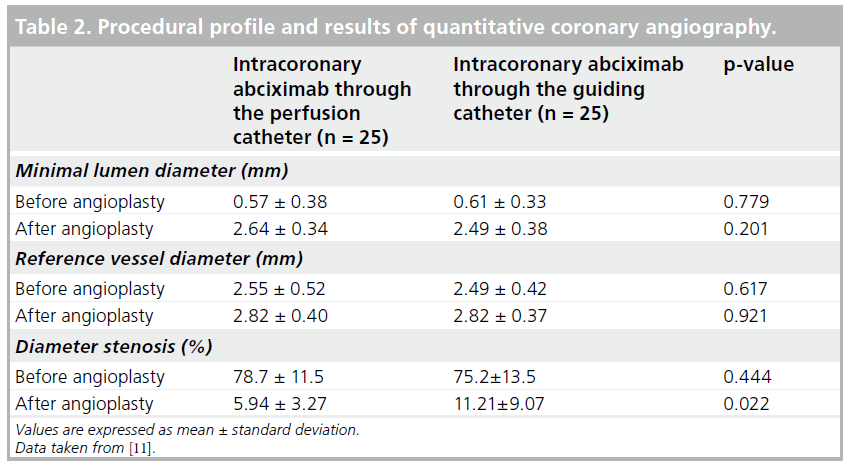
A STEMI was the clinical presentation in 40% of patients in the CW group and 36% of patients in the GC group. Minimal lumen diameter, reference vessel diameter and stenosis diameter before intervention did not differ between the study groups. After the intervention, the mean residual diameter stenosis was significantly lower in the CW group (5.9 ± 3.3 vs 11.2 ± 9.1%; p = 0.022) and MLD tended to be increased (2.64 ± 0.34 vs 2.49 ± 0.38 mm; p = 0.20) (Table 2).
▪ Thrombus burden
Thrombus score changes were assessed in 41 of the 50 randomized patients (82%). Nine patients (five in the CW group and four in the GC group) were excluded at the core laboratory analysis due to a suboptimal acquisition.
In the overall study population, thrombus scores at baseline and after the procedure were 98.3 ± 50.0 and 77.1 ± 48.9, respectively. The absolute thrombotic burden defined by the thrombus score was similar at baseline between patients in the CW group and those in the GC group (106.8 ± 50.0 vs 90.5 ± 48.4; p = 0.272). After abciximab administration, the thrombus score was 68.8 ± 44.8 in the CW group and 85.4 ± 52.7 in GC group (p = 0.393). As a result, the absolute change of the thrombus score after the procedure was significant in the CW group and not significant in the GC group. Accordingly, the mean percentage change of the thrombus score (primary end point) was significantly higher among patients of the CW group compared with those of the GC group (33.8 vs 3.9%; p = 0.002).
▪ Myocardial reperfusion, 30-day & 1-year clinical outcome
In the CW group, the postprocedural corrected thrombolysis in myocardial infarction frame count was shorter compared with the GC group (15.3 ± 10.2 vs 21.1 ± 9.9; p = 0.049). A myocardial blush grade of 0 or 1 occurred in no patients of the CW group and in 5.9% patients of the GC group. Postprocedural mean myocardial blush grade did not differ between groups (2.78 ± 0.43 vs 2.59 ± 0.62; p = 0.303). However, procedurerelated MI was detected in 10% of patients in the CW group and 43% of patients in the GC group (p = 0.018).
There were no clinical events at 30 days in either group. Conversely, at 1 year, MACE were observed in 5.9% of patients in the CW group versus 27.2% of those in the GC group (p = 0.046), driven by a higher rate of target lesion revascularization in the GC group compared with the CW group (21.6 vs 5.9%; p = 0.126).
Conclusion
The COCTAIL study showed that local intracoronary delivery of abciximab by means of a dedicated low-pressure, microporous balloon catheter (ClearWay RX) significantly reduces thrombus burden with the potential to improve coronary microcirculation and reduce major event rates.
Future perspective
Clinical results of primary angioplasty are still hampered by the consequences of thrombus embolization. The TAPAS study provided a clear demonstration that technical solutions alternative to coronary angioplasty with stenting can ameliorate the clinical outcome of primary angioplasty. A question to be addressed is whether clot aspiration will be the only solution to be pursued in the coming years. The COCTAIL study showed that other technical strategies may have a role.
Unpublished OCT data from our center reveal that thrombus aspiration remove 45% of the baseline thrombus burden, measured applying the thrombus score, that has been validated in the COCTAIL study. These preliminary data indicate that thrombo-aspiration may potentially remove a greater proportion of thrombus in comparison with what was observed after abciximab administration through the ClearWay RX. In fact, this latter technical solution led to a 35% reduction in the thrombus burden.
In the coming years, comparative studies between thrombo-aspiration, local intracoronary administration of glycoprotein IIb/IIIa inhibitors and the multimodal approach combining both techniques of thrombo-aspiration and local intracoronary administration of glycoprotein IIb/ IIIa inhibitors will identify the best transcatheter therapy to treat patients with myocardial infarction. It is likely that therapies of local delivery will lead to a smaller thrombus removal but this relative draw-back will be possibly offset by the mechanical action that abciximab can exert in the microcirculatory district, to improve distal myocardial flow.
The landmark studies on the upstream IV administration of glycoprotein IIb/IIIa inhibitors unexpectedly provided negative data. For any anti-thrombotic drug there is a price to be paid as the stronger the effect, the higher the risk of causing hemorrhagic complications. Upstream IV strategies seem to enhance the hemorrhagic risk and in the future researchers and the industry will probably focus on the best strategy to be applied in the catheter laboratory to restore coronary patency.
Furthermore, the advent of novel drug in the clinical arena will further improve treatment of STEMI. It is reasonable to foresee a reduction in the incidence of acute/subacute and late stent thrombosis due to a more aggressive thrombus removal and by the adoption of more efficient thienopyridines such as prasugrel and ticagrelor.
Information resources
▪ Prati F, Capodanno D, Pawlowski T et al.: Local delivery versus intracoronary infusion of abciximab in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 3(9), 928– 934 (2010).
▪ Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI: Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. JACC Cardiovasc. Interv. 2, 1035–1046 (2009).
▪ Topol EJ, Byzova TV, Plow EF: Platelet GPIIb- IIIa blockers. Lancet 353, 227–231 (1999).
▪ Moser M, Bertram U, Peter K, Bode C, Ruef J: Abciximab, eptifibatide, and tirofiban exhibit dose-dependent potencies to dissolve platelet aggregates. J. Cardiovasc. Pharmacol. 41, 586–592 (2003).
▪ Vlaar PJ, Svilaas T, van der Horst IC et al.: Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet 371, 1915–1920 (2008).
▪ Bartorelli AL, Trabattoni D, Galli S, Grancini L, Cozzi S, Ravagnani P: Successful dissolution of occlusive coronary thrombus with local administration of abciximab during PTCA. Catheter Cardiovasc. Interv. 48, 211–213 (1999).
Acknowledgements
The authors are thankful to the CLI Foundation for their contribution to the development of the COCTAIL study.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Executive summary
Mechanism of action of abciximab
▪ Abciximab can tackle the consequences of thrombus dislodgments during coronary interventions for acute coronary syndromes. These include microvascular and endothelial dysfunction resulting in further extension of infarction.
▪ Abciximab antagonizes platelet-fibrin aggregates, leukocyte transmigration and consequent free-radical injury to myocytes.
Limitations of intravenous administration of abciximab
▪ Insufficient local concentration and residency time of the drug.
Mechanism of abciximab administration through ClearWay™ RX
▪ ClearWay RX is a microporous polytetrafluoroethylene balloon catheter, which acts as a low pressure (1 atm) irrigating infusion system able to deliver abciximab at high concentrations directly to the site of thrombus.
▪ During inflation, abciximab is infused through the microporous balloon pores at a dose of 0.25 mg/kg delivered with the infusion catheter, while blood flow is occluded, maximizing drug availability without substantial dilution by the systemic circulation.
▪ Containment of the treatment zone provides extended residence time to help local drug bioavailability, concentration and dose.
▪ Controlled infusion at 1 atm throughout the entire length of the targeted treatment zone provides increased residence time and uptake.
Advantages deriving from abciximab administration through ClearWay RX in acute coronary syndromes as observed in the COCTAIL study
▪ Significant reduction in thrombus burden.
▪ Improvement in microcirculatory index.
▪ Significant reduction in procedural-related myocardial infarction.
▪ Significant reduction in 1-year MACE rates.
▪ Potential improvement of clinical outcome.
References
- Bolognese L, Falsini G, Liistro F, Angioli P, Ducci K: Epicardial and microvascular reperfusion with primary percutaneous coronary intervention. Ital. Heart J. 6, 447–452 (2005).
- Gick M, Jander N, Bestehorn HP et al.: Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation 112, 1462–1469 (2005).
- Stone GW, Webb J, Cox DA et al.: Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA 293, 1063–1072 (2005).
- Ishii H, Ichimiya S, Kanashiro M et al.: Impact of a single intravenous administration of nicorandil before reperfusion in patients with ST-segment-elevation myocardial infarction. Circulation 112, 1284–1288 (2005).
- Vlaar PJ, Svilaas T, van der Horst IC et al.: Cardiac death and reinfarction after 1 year in the Thrombus Aspiration During Percutaneous Coronary Intervention in Acute Myocardial Infarction Study (TAPAS): a 1-year follow-up study. Lancet 371, 1915–1920 (2008).
- De Luca G, Suryapranata H, Stone GW et al.: Abciximab as adjunctive therapy to reperfusion in acute ST-segment elevation myocardial infarction: a meta-analysis of randomized trials. JAMA 293, 1759–1765 (2005).
- Romagnoli E, Burzotta F, Trani C et al.: Angiographic evaluation of the effect of intracoronary abciximab administration in patients undergoing urgent PCI. Int. J. Cardiol. 105, 250–255 (2005).
- Prati F, Cera M, Ramazzotti V, Imola F, Giudice R, Albertucci M: Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervention 3, 365–370 (2007).
- Prati F, Cera M, Ramazzotti V et al.: From bench to bedside: a novel technique of acquiring OCT images. Circ. J. 72, 839–843 (2008).
- Prati F, Regar E, Mintz GS et al.: Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur. Heart J. 31, 401–415 (2010).
- Prati F, Capodanno D, Pawlowski T et al.: Local delivery versus intracoronary infusion of abciximab in patients with acute coronary syndromes. JACC Cardiovasc. Interv. 3(9), 928–934 (2010).