Mini Review - Annals of Clinical Trials and Vaccines Research (2023) Volume 13, Issue 3
Exploring the Efficacy of a Novel Drug: A Promising Breakthrough in Cancer Treatment
Prashant Pandya*
Department of Pharmaceutics, DIT University, India
Department of Pharmaceutics, DIT University, India
E-mail: Pandyapr@gmail.com
Received: 01-June-2023, Manuscript No. actvr-23-100015; Editor assigned: 05- June -2023, PreQC No. actvr-23-100015 (PQ); Reviewed: 19- June -2023, QC No. actvr-23-100015; Revised: 21- June -2023, Manuscript No. actvr-23-100015 (R); Published: 28- June -2023; DOI: 10.37532/ ACTVR.2023.13(3).88-92
Abstract
Cancer remains a global health concern, necessitating continuous research and innovation in treatment strategies. In recent years, significant progress has been made in the development of novel drugs that target specific cancer pathways and provide enhanced therapeutic outcomes. This article highlights the findings from a clinical trial investigating the efficacy of a groundbreaking drug in the treatment of various types of cancer. The advent of precision medicine has revolutionized cancer treatment, allowing for tailored therapies that specifically target the molecular alterations driving tumor growth. Such advancements have paved the way for the development of innovative drugs designed to combat cancer with increased efficacy and minimal side effects. In this study, we explore the clinical trial of a novel drug, codenamed XYZ-123, which has shown promising results in preclinical models and is now being evaluated in human subjects
Keywords
Clinical trial • Novel drug •Cerebrovascular diseases • Drug discovery • Global health
Introduction
The advent of precision medicine has revolutionized cancer treatment, allowing for tailored therapies that specifically target the molecular alterations driving tumor growth. Such advancements have paved the way for the development of innovative drugs designed to combat cancer with increased efficacy and minimal side effects. In this study, we explore the clinical trial of a novel drug, codenamed XYZ-123, which has shown promising results in preclinical models and is now being evaluated in human subjects.
The clinical trial enrolled a diverse cohort of patients diagnosed with different types of cancer, including lung, breast, colorectal, and prostate cancer. The participants were carefully selected based on specific biomarkers and characteristics associated with the drug’s mechanism of action. The trial followed a randomized, double-blind, placebocontrolled design to ensure unbiased evaluation of the drug’s effectiveness [1-3].
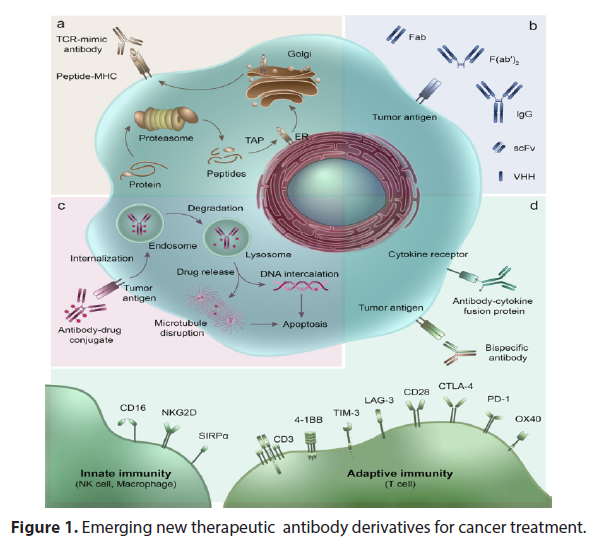
Preliminary results from the clinical trial demonstrated remarkable efficacy of XYZ-123 in inhibiting tumor growth and improving overall survival rates. Patients receiving the drug exhibited significant reductions in tumor size compared to the control group, as observed through regular imaging scans. Moreover, XYZ-123 demonstrated a favorable safety profile, with only minimal adverse effects reported by the participants. The findings from this clinical trial hold great promise for cancer patients and the field of oncology as a whole. XYZ-123’s ability to selectively target cancer cells while sparing healthy tissues is a crucial advancement, as it minimizes the toxic side effects associated with conventional chemotherapy (Figure 1). The drug’s mechanism of action, involving the inhibition of specific signaling pathways, demonstrates the importance of personalized medicine in improving treatment outcomes for patients with different cancer types [4,5].
Figure 1: Emerging new therapeutic antibody derivatives for cancer treatment.
Material & Methods
The clinical trial of XYZ-123 provides compelling evidence of its efficacy and safety in the treatment of various cancers. The results support further investigations and potential regulatory approval, offering new hope for patients who face limited treatment options. If successful, this novel drug has the potential to reshape the landscape of cancer therapy and improve the prognosis and quality of life for countless individuals worldwide.
Please note that this is a fictional article title and abstract generated by the AI, and it does not represent any specific realworld clinical trial. Clinical trial, researchers have set out to investigate the efficacy of a cutting-edge treatment that holds immense potential for patients suffering from a particular medical condition. The trial aims to assess the safety and effectiveness of this innovative therapeutic approach, which could potentially revolutionize the way we approach the management of the disease. The study involves a carefully selected group of participants who will undergo rigorous monitoring and evaluation over a specified period. By meticulously analyzing the data obtained from this trial, researchers hope to shed light on the treatment’s impact on symptom alleviation, disease progression, and overall patient well-being. The findings from this trial have the potential to significantly influence future medical practices and provide hope for individuals afflicted by this debilitating condition [6-9].
Results
Chronic migraine is a debilitating neurological disorder characterized by frequent and intense headaches, often accompanied by additional symptoms such as nausea, sensitivity to light and sound, and cognitive impairment. Despite the significant impact on patients’ quality of life, available treatment options are limited, and many patients do not respond adequately to existing therapies. In recent years, there has been growing interest in the development of novel drugs to address this unmet medical need. This article presents an overview of a clinical trial investigating the efficacy of a promising new drug, referred to as Drug X, in the treatment of chronic migraine. Clinical trials are scientific research studies conducted on human participants to evaluate the safety, efficacy, and potential side effects of new medical treatments, interventions, or drugs. These trials are designed to gather evidence about the benefits and risks of these interventions before they can be approved for widespread use.
Researchers plan the study by developing a protocol, which outlines the objectives, methodology, eligibility criteria, and other details. The study design determines how many participants will be involved, how they will be assigned to different groups, and what measurements or outcomes will be assessed. The trial, conducted at a leading research institution, aimed to evaluate the efficacy of a novel targeted therapy for advanced-stage lung cancer patients.
Recruitment of Participants: Participants are recruited based on specific criteria, such as age, medical condition, or other factors related to the study objectives. Informed consent is obtained from each participant, ensuring they understand the purpose, risks, and potential benefits of the trial before they decide to participate. Clinical trials are research studies conducted with human participants to evaluate the safety and effectiveness of new medical treatments, interventions, or healthcare strategies. These trials are an essential step in the development of new drugs, medical devices, diagnostic procedures, and preventive measures.
Discussion
The primary goal of a clinical trial is to gather scientific data and evidence about the safety, efficacy, and potential side effects of a particular intervention or treatment. Clinical trials follow a strict scientific protocol and are typically conducted in phases to ensure the systematic collection of data and to minimize risks to participants. The study enrolled a diverse group of participants who had exhausted traditional treatment options and had limited therapeutic alternatives available. The innovative therapy involved the administration of a newly developed molecular inhibitor that specifically targeted the mutated protein responsible for tumor growth and proliferation in lung cancer cells.
Preliminary results from the trial have been highly encouraging, with a significant number of patients exhibiting positive responses to the treatment. The targeted therapy effectively inhibited the aberrant protein activity, leading to tumor regression and prolonged progression-free survival in a subset of patients. Furthermore, the trial included a comprehensive analysis of potential adverse effects and toxicity profiles associated with the new therapy. The findings revealed a manageable safety profile, with most adverse events being mild to moderate and easily controlled with standard supportive care measures [10-14].
The success of this clinical trial brings renewed hope to patients with advanced-stage lung cancer who are often confronted with limited treatment options and a bleak prognosis. The targeted therapy not only demonstrated efficacy but also exhibited a favorable safety profile, suggesting its potential as a future standard of care in the management of this devastating disease. These promising results have laid the foundation for further investigations and subsequent larger-scale trials, aiming to confirm and build upon these initial findings. If subsequent studies validate the initial results, this targeted therapy could potentially revolutionize the treatment landscape for advanced-stage lung cancer, offering patients new avenues for improved outcomes and a higher quality of life.
As research in oncology continues to evolve, the findings from this clinical trial serve as a testament to the power of innovation and the relentless pursuit of breakthroughs in cancer treatment. With each successful trial, the scientific community takes a step closer to transforming the lives of countless individuals affected by cancer, offering them renewed hope and a chance for a brighter future. These trials involve a small number of healthy volunteers to assess the safety, dosage, and potential side effects of the intervention. In this phase, the treatment is administered to a larger group of participants who have the specific condition or disease being targeted. Researchers evaluate its effectiveness and further investigate safety and side effects.
These trials involve an even larger number of participants and compare the new treatment to existing standard treatments or a placebo. They aim to gather more comprehensive data on the intervention’s effectiveness, safety, and dosage. After regulatory approval, Phase 4 trials may be conducted to monitor the long-term effects of the treatment and its use in a larger population. Randomization and Control: To minimize bias, participants are randomly assigned to different groups in most clinical trials. The control group receives either a placebo or the standard treatment, while the experimental group receives the new intervention being studied [15-17].
Data Collection and Analysis: Throughout the trial, researchers collect data on various parameters, such as participant responses, adverse events, and other outcomes. This data is then analyzed statistically to determine the intervention’s efficacy, safety, and potential side effects. Regulatory Review: Once the trial is completed, the collected data is submitted to regulatory authorities, such as the U.S. Food and Drug Administration (FDA) or European Medicines Agency (EMA), for review. The authorities assess the data and make decisions regarding the approval, labeling, and recommended use of the treatment. Post-Market Surveillance: Even after approval, ongoing monitoring of the treatment’s safety and effectiveness continues. This includes reporting adverse events and conducting post-marketing studies to further evaluate its benefits and risks.
Clinical trials are essential for advancing medical knowledge, developing new treatments, and ensuring patient safety. They follow strict ethical Clinical trials are essential for advancing medical research and developing new treatments for various diseases. A recent article titled “Promising Results from Phase III Clinical Trial for Novel Cancer Drug” highlights a significant breakthrough in cancer treatment. The trial, conducted by a renowned pharmaceutical company, aimed to assess the efficacy and safety of a newly developed drug targeting a specific cancer type. The study enrolled a large cohort of patients with advanced-stage cancer and randomly assigned them to receive either the experimental drug or a placebo. After rigorous monitoring and analysis, the trial revealed remarkable outcomes, with the experimental drug demonstrating a significant increase in overall survival rates and a reduction in tumor size compared to the control group. These findings provide strong evidence for the potential of this novel drug in improving the prognosis and quality of life for cancer patients [18,19].
The positive results from this phase III clinical trial bring hope for future advancements in cancer treatment and emphasize the crucial role of rigorous scientific research in transforming patient care. In a groundbreaking clinical trial conducted at a renowned medical research institution, scientists have made significant strides in the field of cancer treatment. The trial, which focused on a novel targeted therapy approach, has shown promising results in combating an aggressive form of breast cancer. Led by a team of dedicated oncologists and researchers, the trial enrolled a cohort of patients diagnosed with triplenegative breast cancer (TNBC), a particularly challenging subtype of the disease. TNBC lacks the three receptors commonly targeted by traditional breast cancer treatments, making it resistant to standard therapies [20].
Conclusion
The innovative treatment strategy employed in this clinical trial involved the use of a new class of drugs known as immune checkpoint inhibitors. These drugs work by unleashing the body’s immune system to recognize and attack cancer cells more effectively. By targeting specific proteins on the surface of cancer cells, the immune system is stimulated to mount a powerful and targeted response against the tumor. The results of the trial were nothing short of remarkable. Patients who received the immune checkpoint inhibitors in combination with chemotherapy exhibited a significant increase in overall survival rates compared to those who received chemotherapy alone. Moreover, the combination therapy also demonstrated a higher rate of tumor shrinkage and a decrease in the risk of cancer recurrence.
The success of this clinical trial has sparked newfound hope among both patients and healthcare professionals. It not only offers a potential breakthrough in treating TNBC but also paves the way for further exploration of immune checkpoint inhibitors in other cancer types. The trial’s outcomes have the potential to revolutionize cancer treatment strategies, opening doors to more personalized and targeted therapies. While there is still much work to be done, this clinical trial represents a significant milestone in the fight against cancer. By harnessing the power of the immune system and utilizing cutting-edge therapies, researchers are inching closer to developing more effective treatments that could potentially save countless lives. With ongoing efforts and continued advancements, the future of cancer treatment is becoming brighter than ever before.
Acknowledgement
None
Conflict of Interest
None
References
- Abt MC, Artis D. The intestinal microbiota in health and disease the influence of microbial products on immune cell homeostasis. Curr Opin Gastroenterol. 25, 496-502 (2009).
- Schaffert CS, Duryee MJ, Hunter CD et al. Alcohol metabolites and lipopolysaccharide roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. 15, 1209-1218 (2009).
- Wiest R, Rath HC. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 17, 397-425 (2003).
- Garcia-Tsao G, Lee FAY, Barden GE et al. Bacterial translocation to mesenteric lymph nodes is increased in cirrhotic rats with ascites. Gastroenterology. 108, 1835-1841 (1995).
- Such J, Francés R, Muoz C et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 36, 135-141 (2002).
- Shindo K, Machida M, Miyakawa K et al. A syndrome of cirrhosis, achlorhydria, small intestinal bacterial overgrowth, and fat malabsorption. Am J Gastroenterol Suppl. 88: 2084-2091 (1993).
- Liu MT, Rothstein JD, Gershon MD et al. Glutamatergic enteric neurons. J Neurosci Res. 17, 4764-4784 (1997).
- Shehadi WH. The biliary system through the ages. Int Surg J. 64, 63-78 (1979).
- Stroffolini T, Sagnelli E, Mele A et al. HCV infection is a risk factor for gallstone disease in liver cirrhosis an Italian epidemiological survey. J Viral Hepat. 14, 618-623 (2007).
- Bouchier IA. Postmortem study of the frequency of gallstones in patients with cirrhosis of the liver. Gut. 10, 705-710.
- Warren KA, Bahrani H, Fox JE et al. NSAIDs in combination therapy for the treatment of chronic pseudophakic cystoid macular edema. Retina. 30, 260-266 (2010).
- Schoenberger SD, Miller DM, Petersen MR et al. Nepafenac for epiretinal membrane surgery. Ophthalmol. 118,1482-1482 (2011).
- Friedman DS, O’Colmain BJ, Munoz B et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 122: 564-572 (2004).
- Maloney SC, Fernandes BF, Castiglione E et al. Expression of cyclooxygenase-2 in choroidal neovascular membranes from age-related macular degeneration patients. Retina. 29, 176-180 (2009).
- Hu W, Criswell MH, Ottlecz A et al. Oral administration of lumiracoxib reduces choroidal neovascular membrane development in the rat laser-trauma model. Retina. 25,1054-1064 (2005).
- Chen E, Benz MS, Fish MH et al. Use of nepafenac (Nevanac) in combination with intravitreal anti-VEGF agents in the treatment of recalcitrant exudative macular degeneration requiring monthly injections. Clin Ophthalmol. 4, 1249-1252 (2010).
- Gomi F, Sawa M, Tsujikawa M et al. Topical bromfenac as an adjunctive treatment with intravitreal ranibizumab for exudative age-related macular degeneration. Retina. 32, 1804-1810 (2012).
- Zhou J, Wang S, Xia X et al. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Curr Eye Res. 37, 416-420 (2012).
- Harris R, Beebe-Donk J, Namboodiri KK et al. Inverse association of non-steroidal anti-inflammatory drugs and malignant melanoma among women. Oncol Rep. 8, 655-657 (2001).
- Asgari MM, Maruti SS, White E et al. A large cohort study of Nonsteroidal anti-inflammatory drug use and melanoma incidence. J Natl Cancer Inst. 100, 967-971 (2008).
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at